Tumor necrosis factor-α (TNF-α) and thrombopoietin (TPO) have been shown to induce the differentiation and proliferation of CD34+ cells toward dendritic cells (DCs) in the presence of multiacting cytokines. We hypothesized that the costimulation of TPO and TNF-α generates megakaryocytic progenitors and DCs together from human CD34+ cells and that the interaction of these cells may indicate a physiologic and/or a pathologic role of DCs in megakaryopoiesis. When highly purified human CD34+ cells were cultured for 7 days with TPO alone, the generated cells expressed megakaryocytic markers, such as CD41, CD42b, and CD61. The addition of TNF-α with TPO remarkably decreased the number of megakaryocytic progenitor cells without affecting the cell yield. Almost half of the cells thus generated expressed CD11c, and most of them were positive for CD4 and CD123. Furthermore, CD11c+ cells were found to capture damaged CD61+ cells and to induce autologous T-cell proliferation, although the cytokine productions were low. We also confirmed an engulfment of CD61+ cells and their fragment by CD11c+ cells in bone marrow cells from patients with hemophagocytic syndrome. These findings suggest that DCs generated under megakaryocytic and inflammatory stimuli are involved in megakaryopoiesis and the subsequent immune responses to self-antigens.
Introduction
One of the earliest mediators of the acute phase response of infection, inflammation, and/or tissue damage is tumor necrosis factor-α (TNF-α).1 TNF-α, a proinflammatory cytokine, is mainly produced by activated macrophages and lymphocytes,2,3 and it has a multifunctional effect on various cell types.4-6 For example, TNF-α acts as a positive and negative regulator on myeloid-cell proliferation and differentiation during hematopoiesis.7-10 It is also well documented that TNF-α enhances the proliferation of human CD34+ cells while also promoting the development of dendritic cells (DCs) in the presence of stem-cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and/or interleukin-3 (IL-3).11,12
Thrombopoietin (TPO) is a primary and sole factor for megakaryopoiesis,13 because the genetic elimination of c-mpl or tpo leads to profound thrombocytopenia in mice due to a greatly reduced number of megakaryocytic progenitors and mature megakaryocytes.14 TPO also acts in synergy with IL-3 and SCF on hematopoietic stem cells to induce cell-cycle progression and to increase the both primitive and committed hematopoietic progenitor cells of all lineages.15-17 Furthermore, TPO cooperates with FLT3 ligand (FLT3-L) and SCF in the generation of DC precursors from human CD34+ cells,18-20 and human DCs generated from CD34+ cells following incubation with SCF, GM-CSF, and TNF-α either with or without TPO express the TPO receptor c-mpl.21 As a result, both TPO and TNF-α enhance the proliferation of CD34+ cells and differentiation toward DCs in the presence of multipotent cytokines such as SCF, FLT3-L, and IL-3.
We recently showed that nonerythroid cells were cogenerated from human CD34+ cells during erythroid differentiation in the presence of IL-3/SCF/erythropoietin with TNF-α and expressed DC phenotypes. The CD11c+ DCs physically and selectively associate with developing damaged and immature self-erythroid cells and then phagocytose them.22 This phenomenon may not be limited to the erythroid lineage. We hypothesized that TNF-α in the course of the inflammatory response by viral and microbial infection facilitates DC development during hematopoiesis, thus leading to phagocytosis of damaged self-progenitor cells by DCs. To confirm this hypothesis, we examined the effect of TNF-α and TPO on CD34+ cells.
We herein provide evidence that TNF-α inhibits the generation of megakaryocytic progenitors from CD34+ cells in the presence of TPO while inversely increasing the number of CD4+ CD11c+ CD123+ nonmegakaryocytic cells that show a DC phenotype. We also found that DCs physically associate with immature megakaryocytic cells during differentiation and then phagocytose damaged cells. Next, DCs acquire an ability to induce autologous T-cell proliferation in vitro However, they produce cytokines, which are more tolerogenic than immunogenic, and the T cells activated by them also do not produce a significant amount of immune cytokines. Interestingly, DCs with an immature phenotype in bone marrow from hemophagocytic syndromes are also found to capture CD61+ cells. These findings may suggest the possibility that the phagocytosis of damaged cells by DCs under daily life conditions with either a weak or strong inflammatory response could thus play a pivotal role in regulating the immune responses against hematopoietic progenitor cells.
Materials and methods
Reagents
Bovine serum albumin (BSA), Iscove modified Dulbecco medium (IMDM), and propidium iodide (PI) were purchased from Sigma (St Louis, MO). Fetal calf serum (FCS), penicillin, and streptomycin were obtained from Flow Laboratories (McLean, VA). Insulin (porcine sodium, activity 26.3 U/mg; United States Pharmacopoeia) was obtained from Calbiochem of Behring Diagnostics (La Jolla, CA). Fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies (mAbs) specific for CD4 (SK7), CD8 (SK1), CD19 (4G7), CD20 (L27), and CD34 (8G12) and phycoerythrin (PE)-labeled antibody for CD34 (8G12) were purchased from Becton Dickinson (Mountain View, CA). The PE-CD120a (TNFR-I: 16803.1) and PE-CD120b (TNFR-II: 22235) were obtained from R&D Systems (Minneapolis, MN), and PE-CD11c (B-ly6) and PE-CD83 (HB15a) were from Immunotech (Marseilles, France). PE-CD14 (TUK4), FITC-CD14 (TUK4), PE-CD41 (5B12), PE-CD42b (AN51), mouse anti-human glycophorin A (GPA: JC159), normal mouse serum, goat anti-rabbit IgG conjugated to peroxidase-labeled dextran polymer, diaminobenzidine (DAB)-substrate chromogen system, and fuchsin-substrate chromogen system were from DAKO Japan (Kyoto, Japan). Cyanin-labeled CD4, FITC-CD86 (FUN-1), PE-conjugated CD61 (VI-PL2), CD80 (L307.4), CDw123 (IL-3Rα; 9F5), c-mpl (BAH-1), major histocompatibility complex (MHC) class I (-A, -B, -C) (B9.12.1) and MHC class II (-DR) (B8.12.2), and unconjugated antimannose receptor were purchased from PharMingen (San Diego, CA). Anti-single-stranded DNA (anti-ssDNA) antibody was donated by Dr T. Sugiyama (Akita University, Japan).23 Anti-CD3 (OKT3) was purified from ascites.
TNF-α was purchased from R&D Systems (Minneapolis, MN). TPO, IL-3, and SCF were kind gifts from the Kirin Brewery (Tokyo, Japan) and granulocyte colony-stimulating factor (G-CSF) from Chugai Pharmaceutical (Tokyo, Japan). Vitamin B12 and folic acid were purchased from Sankyo Pharmaceutical (Tokyo, Japan). 3H-thymidine was purchased from Perkin Elmer (Yokohama, Japan).
Cell preparations
Human CD34+ cells and CD34- cells were purified from healthy volunteers, who had signed informed consent forms approved by the Akita University School of Medicine Committee for the Protection of Human Subjects, and stored in liquid nitrogen until use as previously described.24 The thawed CD34+ cells were suspended in IMDM containing 30% FCS and 100 U/mL DNAse and then were washed twice with IMDM containing 20% FCS. Next, the cells were seeded in 50 mL polystyrene flasks (Corning Costar, Cambridge, MA) at 2 × 105 to 5 × 105 cells per milliliter in IMDM containing 5% heat-inactivated pooled human AB plasma, 1% BSA, 10 μg/mL insulin, 10 μg/mL vitamin B12, 15 μg/mL folic acid, 50 nM β-mercaptoethanol, 50 U/mL penicillin, and 50 U/mL streptomycin in the presence or absence of 100 ng/mL TPO and/or at the indicated doses of TNF-α for the various periods at 37°C in a 5% CO2/5% O2 incubator.
Bone marrow aspirate was obtained from 3 patients (patients 1 to 3; Table 1) with hemophagocytic syndrome who had signed informed consent forms approved by the Akita University School of Medicine Committee for the Protection of Human Subjects. The diagnosis was made according to the diagnostic criteria for hemophagocytic lymphohistiocytosis.25 Smear preparations of bone marrow were air dried and stored at -80°C.
Characteristics of each of 3 patients with hemophagocytic syndrome*
. | Patient no. . | . | . | ||
|---|---|---|---|---|---|
. | 1 . | 2 . | 3 . | ||
| Sex/age, y | M/70 | M/58 | F/67 | ||
| Underlying diseases | DIC‡ | HIV/AIDS§ | DLBCL∥ | ||
| Clinical findings | |||||
| Nonremitting high fever | + | + | + | ||
| Hepatosplenomegaly | + | + | + | ||
| Peripheral blood | |||||
| White cells (neutrophils), × 109/L | 2.7 (1.6) | 0.8 (0.5) | 1.0 (0.6) | ||
| Hemoglobin, g/L | 85 | 65 | 61 | ||
| Platelet, × 109/L | 96 | 31 | 6 | ||
| Biochemistry¶ | |||||
| Triglycerides, mg/dL | ND | 120 | 106 | ||
| Fibrinogen, mg/dL | 97 | 78 | 481 | ||
| Ferritin, μg/L | 1746 | ND | 2861 | ||
| sIL-2R, U/mL | 3870 | ND | 6280 | ||
| FDP, μg/mL | 53.2 | 38.6 | 8 | ||
| Hemophagocytosis in bone marrow† | + | + | + | ||
| Outcome | Remission | Rapidly fatal | Remission | ||
. | Patient no. . | . | . | ||
|---|---|---|---|---|---|
. | 1 . | 2 . | 3 . | ||
| Sex/age, y | M/70 | M/58 | F/67 | ||
| Underlying diseases | DIC‡ | HIV/AIDS§ | DLBCL∥ | ||
| Clinical findings | |||||
| Nonremitting high fever | + | + | + | ||
| Hepatosplenomegaly | + | + | + | ||
| Peripheral blood | |||||
| White cells (neutrophils), × 109/L | 2.7 (1.6) | 0.8 (0.5) | 1.0 (0.6) | ||
| Hemoglobin, g/L | 85 | 65 | 61 | ||
| Platelet, × 109/L | 96 | 31 | 6 | ||
| Biochemistry¶ | |||||
| Triglycerides, mg/dL | ND | 120 | 106 | ||
| Fibrinogen, mg/dL | 97 | 78 | 481 | ||
| Ferritin, μg/L | 1746 | ND | 2861 | ||
| sIL-2R, U/mL | 3870 | ND | 6280 | ||
| FDP, μg/mL | 53.2 | 38.6 | 8 | ||
| Hemophagocytosis in bone marrow† | + | + | + | ||
| Outcome | Remission | Rapidly fatal | Remission | ||
sIL-2R indicates soluble interleukin-2 receptor; FDP, fibrin degeneration products; DIC, disseminated intravascular coagulation syndrome; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; DLBCL, diffuse large B-cell lymphoma; ND, not determined.
According to the diagnostic guidelines developed by the Histiocyte Society, the diagnosis of hemophagocytic lymphohistiocytosis (HLH) requires the presence of all 5 major criteria: (1) fever, (2) splenomegaly, (3) cytopenia involving 2 or more cell lines, (4) hypertriglyceridemia or hypofibrinogenemia, (5) hemophagocytosis. Either criterion (a) low or absent natural killer cell activity or a combination of criteria, (b) ferritin above 500 mg/L, and (c) sIL-2R above 2400 U/mL may substitute for 1 of the major criteria.25
Details are shown in Table 3
Chronic DIC after surgery for aortic abdominal aneurysm
Diagnosed as AIDS at acute onset of Pneumocystis carinii pneumonia. Cytomegalovirus antigenemia assay was also positive
DLBCL with bone marrow invasion. Some cases of DLBCL have a prominent background of reactive T cells and histiocytes
Reference ranges for normal values are 30 to 150 mg/dL for triglycerides, 160 to 360 mg/dL for fibrinogen, below 234 μg/L for ferritin, 135 to 483 U/mL for sIL-2R, and less than 10 μg/mL for FDP
Flow cytometry
The cells collected from the cultures were washed twice with phosphate-buffered saline (PBS) containing 3% FCS, 2 mM EDTA, and 0.05% NaN3 (staining medium) and stained with FITC- and PE-labeled mAbs, and they then were analyzed using a FACS Calibur (Becton Dickinson). To determine the TNF receptor (TNFR) expression, the cells were incubated at 37°C for 2 hours without TNF-α before staining with specific mAbs, because TNFRs were down-regulated by TNF-α in the culture medium (data not shown).
Enzymatic immunohistochemistry
The cells were spun onto slides using a Cytospin 3 (Shandon Lipshaw, Pittsburgh, PA), and smear specimens of bone marrow were fixed in 100% methanol, dried, and examined by enzymatic immunohistochemistry as described.26 After blocking endogenous peroxidase activity with 3% hydrogen peroxide, the preparations were incubated with mouse mAbs, followed by goat anti-mouse IgG conjugated to peroxidase-labeled dextran polymer, and then were visualized with a DAB-substrate chromogen system. For double staining, the preparations were further incubated with rabbit antihuman antibody, followed by goat anti-rabbit IgG conjugated to alkaline phosphatase-labeled dextran polymer using a fuchsin-substrate chromogen system. ssDNA-specific rabbit polyclonal IgG antibodies were used to recognize hexadeoxynucleotides with various base sequences in apoptotic cells,23 and the stained specimens were incubated with goat anti-rabbit IgG conjugated to peroxidase-labeled dextran polymer. Specimens that were incubated with normal serum with secondary antibody served as negative controls.
Confocal microscopy
For confocal microscopy, cells generated after 5 days of culture with TPO and TNF-α were fixed using PermaFluor Aqueous Mounting Medium (Thermo Shandon, Pittsburgh, PA), permeabilized with BD FACS Permeabilizing Solution (Becton Dickinson), and then stained with PE-CD11c and FITC-CD61 as described previously.22 Cells engulfing CD61+ cells were observed using a CLSM 510 confocal laser scanning microscope (Carl Zeiss Microscope Systems, Oberkochen, Germany) equipped with a 40 ×/65 μm (FITC) or 40 ×/72 μm (PE) oil-immersion objective lens at zoom 5. Fluorochromes were excited using an argon laser at 488 nm for FITC and helium neon at 543 nm for PE. Detector slits were configured to minimize cross talk between channels. Z-sliced optical sections were collected with an optimal interval of 0.45 mm and processed using Carl Zeiss software and Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA).
T-cell proliferation assays
Autologous T lymphocytes were prepared as a negative fraction from a nylon-fiber syringe27 of thawed CD34- cells. Cells generated in the culture with TPO plus TNF-α or TNF-α alone for 7 days were used as a stimulator after γ-irradiation at 30 Gy (3000 rad) and then were cocultured with autologous responder T cells (1 × 103 cells per well) in RPMI 1640 medium containing 10% heat-inactivated human AB serum in round-bottom 96-well microculture plates. Anti-CD3 mAb was added into some cultures as positive controls. 3H-thymidine (1 μCi per well [37 κBq/well]) incorporations for the last 18 hours of 5 days of culture were measured by a liquid scintillation counter (TopCount NXT; PerkinElmer LAS, Boston, MA), and the results were expressed as the mean ± SD of triplicate cultures.
Cytokine measurement
Culture supernatants were obtained from autologous mixed lymphocyte reactions (MLRs) and culture of CD34+ cells at 4 × 105 in 2 mL complete medium with TPO (100 ng/mL) and TNF-α (100 ng/mL) for 7 days in 24-well flat-bottomed Falcon tissue culture plates (Becton Dickinson, Franklin Lakes, NJ). Cytokine activities were assessed with cytometric bead array system (Pharmingen) according to the manufacturer's protocol.
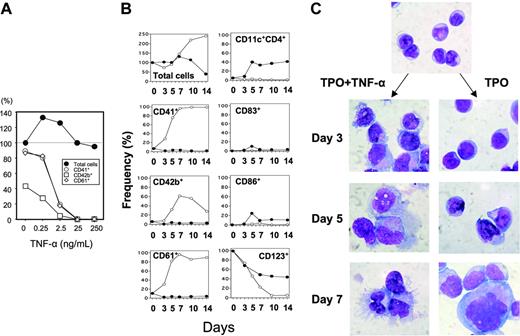
TNF-α inhibits the generation of megakaryocytic progenitors but increases the number of nonmegakaryocytic cells from CD34+ cells supported by TPO. (A) Human CD34+ cells at 7.5 × 105/mL were cultured with 100 ng/mL TPO in the presence of various concentrations of TNF-α ranging from 0 to 250 ng/mL. Seven days later, the total numbers of recovered cells (•) were counted, and CD41 (○), CD42b (□), and CD61 (⋄) expressions were examined by a cytofluorometer. The cell yields were represented as the percent relative to the total number of cells without TNF-α. Marker-positive cells were also shown as the percent of the total number of cells in an individual group. The result shown is representative of 3 independent experiments. (B) CD34+ cells were cultured as in panel A in the presence (•) or absence (○) of 100 ng/mL TNF-α. Cell counts and phenotype analyses were performed at various time points. The results shown are representative of 3 independent experiments. (C) Morphologic differences in the cells differentiated by TPO with and without TNF-α as in panel B. Cytospin specimens were stained with May-Grünwald-Giemsa solution at the indicated time points (× 1000).
TNF-α inhibits the generation of megakaryocytic progenitors but increases the number of nonmegakaryocytic cells from CD34+ cells supported by TPO. (A) Human CD34+ cells at 7.5 × 105/mL were cultured with 100 ng/mL TPO in the presence of various concentrations of TNF-α ranging from 0 to 250 ng/mL. Seven days later, the total numbers of recovered cells (•) were counted, and CD41 (○), CD42b (□), and CD61 (⋄) expressions were examined by a cytofluorometer. The cell yields were represented as the percent relative to the total number of cells without TNF-α. Marker-positive cells were also shown as the percent of the total number of cells in an individual group. The result shown is representative of 3 independent experiments. (B) CD34+ cells were cultured as in panel A in the presence (•) or absence (○) of 100 ng/mL TNF-α. Cell counts and phenotype analyses were performed at various time points. The results shown are representative of 3 independent experiments. (C) Morphologic differences in the cells differentiated by TPO with and without TNF-α as in panel B. Cytospin specimens were stained with May-Grünwald-Giemsa solution at the indicated time points (× 1000).
Results
TNF-α inhibits the generation of megakaryocytic progenitors while increasing the nonmegakaryocytic cells from CD34+ cells supported by TPO
Human CD34+ cells (98.5% ± 1.0% purity; mean ± SD) were cultured with TPO in the presence of various concentrations of TNF-α ranging from 0 to 250 ng/mL. Seven days later, the total number of recovered cells was counted, and CD41, CD42b, and CD61 expressions, specific markers for the megakaryocytic lineage, were examined using a cytofluorometer. The total number of cells increased 1.4-fold during the 7 days of culture with TPO alone, and 89%, 43%, and 87% of the generated cells expressed CD41, CD42b, and CD61, respectively, thus indicating that most of them consisted of megakaryocytic progenitors. The addition of TNF-α significantly decreased the number of CD41+, CD42b+, and CD61+ cells in a dose-dependent manner while conversely increasing CD41-, CD42b-, and CD61- cells (Figure 1A). A low dose of TNF-α (0.25 ng/mL) tended to increase the cell yield but reduced the proportion of CD41+, CD42b+, and CD61+ cells. Then half-maximal dose of TNF-α to inhibit the generation of megakaryocytic cells ranged from 0.25 to 2.5 ng/mL.
Phenotypic analyses of nonmegakaryocytic cells. (A) Cells generated after 7 days cultured as described for Figure 1 were assessed for their phenotype with cytofluorometer using various combinations of mAbs. (B) The purification of CD11c+ CD123+ cells and their morphology. Cytospin specimens of sorted CD11c+ cells were stained with May-Grünwald-Giemsa solution (arrow). Original magnification, × 1000.
Phenotypic analyses of nonmegakaryocytic cells. (A) Cells generated after 7 days cultured as described for Figure 1 were assessed for their phenotype with cytofluorometer using various combinations of mAbs. (B) The purification of CD11c+ CD123+ cells and their morphology. Cytospin specimens of sorted CD11c+ cells were stained with May-Grünwald-Giemsa solution (arrow). Original magnification, × 1000.
Characterization of nonmegakaryocytic cells
To understand the kinetics of nonmegakaryocytic-cell development in our system, purified CD34+ cells were cultured for 14 days with 100 ng/mL TPO in the presence or absence of 100 ng/mL TNF-α, and the surface phenotypes were monitored at the indicated time points (Figure 1B). The number of total cells recovered from the cultures with and without TNF-α were comparable to each other for 7 days. During this period, the proportions of CD41+, CD42b+, and CD61+ cells dramatically increased only when TNF-α was not added. The cell yield substantially increased in the culture with TPO alone but decreased with TPO plus TNF-α thereafter. Very few cells expressed CD41, CD42b, and CD61 in the presence of TNF-α throughout the culture period, thus suggesting that TNF-α affects CD34+ cell development by inhibiting the generation of megakaryocytic progenitors. Interestingly, CD11c+ cells were inversely generated as early as at day 5, and some of them expressed CD86 and CD83. Then morphologic changes of cells also showed the development of cells with DC features, such as dendrites and eccentric nuclei (Figure 1C).
To further characterize the DC-like cells in detail, the surface phenotypes were monitored on day 7 (Figure 2A). All cells became positive for MHC class II in the presence of TNF-α. A significant number of CD11c+ cells and CD86+ cells coexpressed c-mpl and TNFRII. In addition, CD61+ cells expressed not only c-mpl but also TNFRII. None of the TNFRI+, CD3+, CD8+, CD19+, CD20+, and CD56+ cells was present in either of the culture conditions (data not shown).
CD123 (IL-3 receptor-α), a marker of plasmacytoid DCs or interferon-producing cells (IPCs),28 was detected in 70% ± 19% of the total cells and 97% of CD11c+ cells (Figure 2A). Chen et al20 demonstrated that TPO and FLT3-L allow human CD34+ cells to differentiate into CD11c- CD123high IPCs, CD11c+ immature DCs, and CD14+ monocytes. Therefore, we examined the CD123 expression on CD11c+ DCs using purified cells gated on CD4, which is expressed on DCs.28 CD4+ cells consisted of 92% of bulk cells at day 7, and all of them were positive for CD123 (Figure 2B). The sorted CD11c+ CD123+ cells showed a uniform picture characterized by abundant dendrites and eccentric nuclei.
DCs develop in aggregates along with megakaryocytic cells in the presence of TPO and TNF-α. The aggregates formed were collected by gentle pipetting after 5 days of culture with TPO in the presence or absence of TNF-α as in Figure 2. The cytospin specimens were stained with megakaryocyte-specific and DC-associated markers. Original magnification, × 40.
DCs develop in aggregates along with megakaryocytic cells in the presence of TPO and TNF-α. The aggregates formed were collected by gentle pipetting after 5 days of culture with TPO in the presence or absence of TNF-α as in Figure 2. The cytospin specimens were stained with megakaryocyte-specific and DC-associated markers. Original magnification, × 40.
Megakaryocytic cells and DCs are closely associated together during their development in the presence of TNF-α
DCs are known to develop from proliferating precursor cells in aggregates. We stained cells with megakaryocyte-specific and DC-associated markers to visualize the localization of megakaryocytic progenitors and DCs (Figure 3). The CD34+ cells proliferated as single cells without forming aggregates in the absence of TNF-α, and they expressed CD41 and CD61. In the presence of TNF-α, the aggregates were found to consist of CD11c+, CD83+, and CD86+ cells, and they contained fragments that were positive for CD41 or CD61 (Figure 3, arrows).
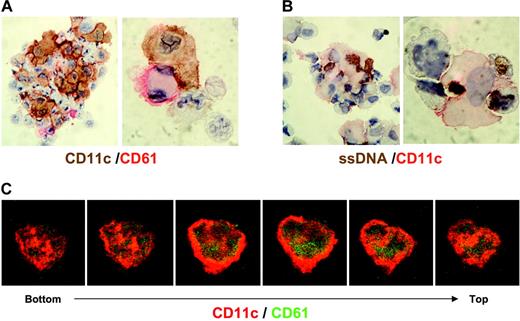
Physical association and phagocytosis of CD61+ cells by CD11c+ cells. Five days after culture with 100 ng/mL TPO and 2.5 ng/mL TNF-α, the cells were cytospun and stained with anti-CD11c (brown) and anti-CD61 (red) (A) or with anti-CD11c (red) and anti-ssDNA (brown) (B); original magnification, × 1000. (C) Cells placed on coverslips were fix-permeabilized, stained with FITC-CD61 (green) and PE-CD11c (red), and then were observed by laser scanning confocal microscopy. A serial 3-dimensional reconstruction of the cells demonstrates the phagocytosis of CD61+ cells (green) by CD11c+ cells (red).
Physical association and phagocytosis of CD61+ cells by CD11c+ cells. Five days after culture with 100 ng/mL TPO and 2.5 ng/mL TNF-α, the cells were cytospun and stained with anti-CD11c (brown) and anti-CD61 (red) (A) or with anti-CD11c (red) and anti-ssDNA (brown) (B); original magnification, × 1000. (C) Cells placed on coverslips were fix-permeabilized, stained with FITC-CD61 (green) and PE-CD11c (red), and then were observed by laser scanning confocal microscopy. A serial 3-dimensional reconstruction of the cells demonstrates the phagocytosis of CD61+ cells (green) by CD11c+ cells (red).
Immature DCs are known to be capable of capturing apoptotic and necrotic cells,29,30 thus leading to antigen presentation, which thus induces immunity and tolerance.31,32 Therefore, it is possible that developing immature DCs in aggregates engulf damaged megakaryocytic progenitor cells. To address this question, CD34+ cells were cultured for 5 days with 100 ng/mL TPO and 2.5 ng/mL TNF-α, half-maximal dose of inhibition, to maintain the shape of megakaryocytic progenitor cells. Cytospin specimens of the aggregated cells were stained with CD11c in combination with anti-CD61 (Figure 4A) or anti-ssDNA (Figure 4B). CD11c+ cells were often shown to be associated with CD61+ megakaryocytic progenitor cells in aggregates, and many ssDNA+ cells were present in the aggregates. When the aggregates were dispersed with gentle pipetting, large parts of CD11c+ cells associate with (63% ± 5%) or engulfed (15% ± 4%) CD61+ cells. In addition, ssDNA was also found in the cytoplasm of CD11c+ cells, which associated with ssDNA+ cells. These observations indicate that DCs capture dying megakaryocytic progenitors. This was also confirmed with confocal microscopy, which showed that the CD11c+ cells phagocytosed CD61+ cells (Figure 4C).
Autologous T-cell activation by DCs generated in the presence of TPO and TNF-α
We hypothesized that DCs phagocytosing self-megakaryocytic cells might thus modify self-proteins and, as a result, they can present antigen to autologous T cells. We therefore assessed autologous T-cell proliferation using cells generated during a 7-day culture with TPO plus TNF-α or TNF-α alone (Figure 5). CD3-dependent T-cell proliferation induced by these DCs served as positive controls. TNF-α alone was able to induce DC development from CD34+ cells, although the cell yield was much lower than the culture with TPO and TNF-α (data not shown). DCs generated in the culture with TPO plus TNF-α, but not TNF-α alone, were found to potently activate autologous T cells, although T-cell proliferation was much lower than that induced by anti-CD3 mAb.
When T cells were cocultured with DCs at a 2:1 ratio, low but significant amounts of IL-2 (6.5 ± 1.7 pg/mL) and IL-6 (12.5 ± 2.4 pg/mL) were detected. However, no IFN-γ, IL-4, and IL-10 were detected. In contrast, large amounts of IFN-γ (825 ± 70 pg/mL), TNF-α (233 ± 21 pg/mL), and IL-10 (416 ± 68 pg/mL) as well as IL-6 (193 ± 35 pg/mL) were produced when T cells were stimulated via CD3 with DCs, although no IL-2 activity was detected at this time point.
Autologous T-cell proliferation by DCs generated in the presence of TPO and TNF-α. The cells prepared by culture for 7 days with 100 ng/mL TNF-α in the presence (▪) or absence (□) of 100 ng/mL TPO were used at graded doses as stimulator cells after γ-irradiation. In some MLRs, anti-CD3 mAb was added. Proliferation was assessed by adding 3H-thymidine (1 μCi per well [37 κBq/well]) during 72 to 90 hours of culture. Results represent the mean ± SD of triplicate cultures.
Autologous T-cell proliferation by DCs generated in the presence of TPO and TNF-α. The cells prepared by culture for 7 days with 100 ng/mL TNF-α in the presence (▪) or absence (□) of 100 ng/mL TPO were used at graded doses as stimulator cells after γ-irradiation. In some MLRs, anti-CD3 mAb was added. Proliferation was assessed by adding 3H-thymidine (1 μCi per well [37 κBq/well]) during 72 to 90 hours of culture. Results represent the mean ± SD of triplicate cultures.
Cytokine secretion by developing cells during the culture
Cytokines secreted during the culture are believed to have an important autocrine and paracrine effect on developing cells both in vivo and in vitro. As a result, we assessed the cytokine activities in the media of CD34+ cell culture in the presence of TPO and/or TNF-α (Table 2).
Cytokine secretion profile of the generated cells
. | Concentration, pg/mL . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine combination (s) . | INF-γ . | IL-6 . | IL-8 . | IL-10 . | IL-12p70 . | ||||
| TPO | < 5 | < 5 | 302 ± 18 | < 5 | < 5 | ||||
| TPO + TNF-α | < 5 | 5.5 ± 0.8 | > 5000 | < 5 | < 5 | ||||
| TNF-α | < 5 | 7.0 ± 0.8 | 3152 ± 74 | < 5 | < 5 | ||||
. | Concentration, pg/mL . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine combination (s) . | INF-γ . | IL-6 . | IL-8 . | IL-10 . | IL-12p70 . | ||||
| TPO | < 5 | < 5 | 302 ± 18 | < 5 | < 5 | ||||
| TPO + TNF-α | < 5 | 5.5 ± 0.8 | > 5000 | < 5 | < 5 | ||||
| TNF-α | < 5 | 7.0 ± 0.8 | 3152 ± 74 | < 5 | < 5 | ||||
Purified human CD34+ cells were cultured for 7 days with or without TPO and TNF-α, and the supernatants were collected. The cytokines secreted during culture were measured using a cytometric beads array system.
TPO alone induced a small amount of IL-8, whereas TNF-α induced the secretion of IL-6 and IL-8 but not that of IL-1β, IL-10, or IL-12p70. The combination of TPO and TNF-α further enhanced IL-8, but not IL-6, secretion. These results suggest that TNF-α is a primary factor to induce the other proinflammatory cytokines and that TPO synergizes with TNF-α for only IL-8 secretion.
Hemophagocytosis by CD11c+ cells in vivo is confirmed
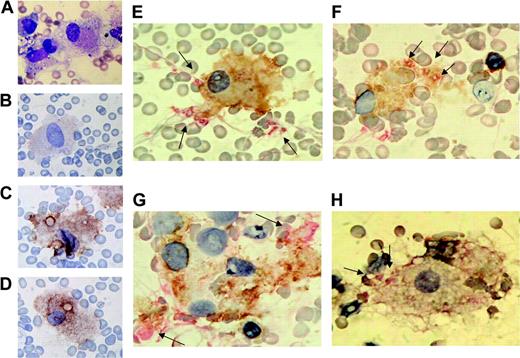
Our observation clearly shows that developing DCs under hematopoietic and inflammatory conditions phagocytose codifferentiated progenitor cells with damage by inflammatory cytokines. We therefore attempted to corroborate that our observation in vitro also takes place in situ. To do so, we stained specimens of bone marrow smears from patients with hemophagocytic syndrome with DC markers, such as CD11c, CD83, CD86, and mannose receptor, and the megakaryocytic marker CD61 or the erythroid marker GPA. As shown in Figure 6, hemophagocytic cells (Figure 6A) were weakly positive for CD83 (Figure 6B), while they were strongly positive for CD86 (Figure 6C), mannose receptor (Figure 6D), and CD11c (Figure 6E-F). Double staining with anti-CD11c and anti-CD61 showed that CD11c+ cells phagocytose platelets (Figure 6E-F,H) and CD61+ substrates (Figure 6G) in vivo. Our previous report demonstrated that nonerythroid cells generated from CD34+ cells treated with erythropoietin, SCF, IL-3, and TNF-α expressed DC phenotype and that CD11c+ DCs phagocytosed immature erythroid cells.22 We therefore stained bone marrow cells from the patients with CD11c+ and erythroid marker, GPA (Figure 7A-C). As a result, CD11c+ cells in the patients' bone marrow were consistently shown to capture erythroid as well as megakaryocytic cells. In addition, staining with α-naphthyl butyrate (α-NB) of hemophagocytic cells showed a similar perinuclear distribution of granules in immature DCs generated from purified normal human CD34+ cells (Figure 7D-E). Such a distribution pattern of α-NB-positive granules was different from pancellular staining in macrophages.
Hemophagocytosis by CD11c+ cells in bone marrow cells from patients with hemophagocytic syndrome. Bone marrow smear preparations from patients with hemophagocytic syndrome were stained with May-Grünwald-Giemsa staining (patient 1) (A), CD83 staining (patient 1) (B), CD86 staining (patient 1) (C), mannose receptor staining (patient 1) (D), or CD11c (brown)/CD61 (red) double staining for patient 1 (E), patient 2 (F-G), and patient 3 (H). The arrows indicate CD61+ platelets or substrates.
Hemophagocytosis by CD11c+ cells in bone marrow cells from patients with hemophagocytic syndrome. Bone marrow smear preparations from patients with hemophagocytic syndrome were stained with May-Grünwald-Giemsa staining (patient 1) (A), CD83 staining (patient 1) (B), CD86 staining (patient 1) (C), mannose receptor staining (patient 1) (D), or CD11c (brown)/CD61 (red) double staining for patient 1 (E), patient 2 (F-G), and patient 3 (H). The arrows indicate CD61+ platelets or substrates.
Capture of erythroid progenitor cells expressing GPA by CD11c+ cells from bone marrow of hemophagocytic syndrome. Bone marrow smear preparations from patients with hemophagocytic syndromes were stained with CD11c (red)/GPA (brown) for patient 1 (A) or CD11c (brown)/GPA (red) for patient 2 (B) and patient 3 (C). The arrows indicate GPA-positive erythroid cells. Staining with α-NB of hemophagocytic cells is also shown in patient 3 (D) and DCs generated from purified normal human CD34+ cells in the presence of TPO and TNF-α (day 5 [E]; day 7 [F]).
Capture of erythroid progenitor cells expressing GPA by CD11c+ cells from bone marrow of hemophagocytic syndrome. Bone marrow smear preparations from patients with hemophagocytic syndromes were stained with CD11c (red)/GPA (brown) for patient 1 (A) or CD11c (brown)/GPA (red) for patient 2 (B) and patient 3 (C). The arrows indicate GPA-positive erythroid cells. Staining with α-NB of hemophagocytic cells is also shown in patient 3 (D) and DCs generated from purified normal human CD34+ cells in the presence of TPO and TNF-α (day 5 [E]; day 7 [F]).
To obtain more detailed information about hemophagocytic cells in these patients, we clarified the frequency of monocytic cells macrophages by May-Grünwald-Giemsa staining and CD11c+ cells associated with/capturing dying hematopoietic progenitor cells by immunohistochemical staining (Table 3). The frequencies of hemophagocytic cells were in the range of 3.8% ± 13.6% of total bone marrow cells, which consisted of 35.8% ± 15.0% of monocytic cells/macrophages and 31.0% ± 11.6% of CD11c+ cells. In addition, CD11c+ cells were composed of 84.4% ± 62.2% of hemophagocytic cells, and they captured megakaryocytic as well as erythroid cells. These data strongly suggest the possibility that hemophagocytic cells in the bone marrow of patients, at least in part, are DCs.
Frequencies of CD11c+ hemophagocytic cells in the bone marrow of patients with hemophagocytic syndrome
. | Patient no. . | . | . | ||
|---|---|---|---|---|---|
. | 1 . | 2 . | 3 . | ||
| Cellularity | Hypo | Normo | Hypo | ||
| Frequencies, % | |||||
| By May-Grünwald-Giemsa staining | |||||
| Monocytic cells/macrophages in total bone marrow | 35.8 | 15.0 | 26.0 | ||
| Hemophagocytes in total bone marrow | 13.6 | 3.8 | 5.2 | ||
| CD11c+ cells by immunohistochemical staining | |||||
| In total bone marrow | 31.0 | 11.6 | 27.8 | ||
| In hemophagocytes | 84.4 | 62.2 | 70.3 | ||
| Associated with/capturing CD61+ cells/substrates | 36.1 | 42.0 | 9.7 | ||
| Associated with/capturing GPA+ cells/substrates | 94.4 | 53.6 | 91.4 | ||
. | Patient no. . | . | . | ||
|---|---|---|---|---|---|
. | 1 . | 2 . | 3 . | ||
| Cellularity | Hypo | Normo | Hypo | ||
| Frequencies, % | |||||
| By May-Grünwald-Giemsa staining | |||||
| Monocytic cells/macrophages in total bone marrow | 35.8 | 15.0 | 26.0 | ||
| Hemophagocytes in total bone marrow | 13.6 | 3.8 | 5.2 | ||
| CD11c+ cells by immunohistochemical staining | |||||
| In total bone marrow | 31.0 | 11.6 | 27.8 | ||
| In hemophagocytes | 84.4 | 62.2 | 70.3 | ||
| Associated with/capturing CD61+ cells/substrates | 36.1 | 42.0 | 9.7 | ||
| Associated with/capturing GPA+ cells/substrates | 94.4 | 53.6 | 91.4 | ||
GPA indicates glycophorin A; hypo, hypocellular marrow; normo, normocellular marrow.
Discussion
This study demonstrated that TNF-α inhibits the generation of megakaryocytic progenitor cells from human CD34+ cells in the presence of TPO and inversely increases nonmegakaryocytic cells with feature of DCs. These cells expressed the typical surface markers of DCs, such as CD11c, CD4, and CD86. They were closely associated with codeveloping immature megakaryocytic progenitor cells and then captured them. Interestingly, the cells thus generated were capable of inducing autologous MLRs. Like DCs generated by TPO and TNF-α, capture of CD61+ cells by CD11c+ cells in bone marrow was also observed in patients with hemophagocytic syndrome, thus indicating that similar phenomena can take place in vivo. These findings suggest that megakaryocytic and inflammatory costimuli on hematopoietic stem/progenitor cells may induce hemophagocytosis of megakaryocytic cells in a physiologic situation.
We documented that TNF-α inhibits the generation of megakaryocytic progenitor cells from human CD34+ cells in the presence of TPO in a dose-dependent fashion with a half-maximal dose ranging from 0.25 to 2.5 ng/mL. Although considerable advances have been made during the past 2 decades in our understanding of the biology and the clinical role of the TNF superfamily, the role of TNF-α in megakaryopoiesis remains controversial. TNF-α stimulates colony formation by a megakaryoblastic leukemia cell line (CMK) established from a patient with Down syndrome,33 and it also induces megakaryocytic differentiation of the HIMeg-1 cells,34 a cell line derived from a patient with chronic myeloid leukemia capable of monocytic and megakaryocytic differentiation. In contrast, TNF-α has been well documented to inhibit the megakaryopoiesis of normal progenitor cells. Lu et al35 reported that TNF-α suppressed colony-forming-unit megakaryocytes derived from human CD34+ cells in the presence of IL-11, IL-3, SCF, and TPO. TNF-α almost completely abrogated the growth of human CD34+ CD38- progenitor cells in response to TPO alone as well as SCF/FLT3/TPO.36 It is very likely that the differences between various cell lines and primary cells account for some of the discrepancy between observations made by different researchers. The mechanism by which TNF-α inhibits normal megakaryocytic differentiation remains unclear.
Although TPO has been shown to be the primary regulator of platelet production,13 many studies have demonstrated that TPO supports the proliferation and long-term expansion of primitive CD34+ cells in synergy with FLT3-L, SCF, and/or IL-3.15-17 We further demonstrated that TPO and TNF-α permit CD34+ cells to differentiate into CD4+ CD11c+ CD123+ DCs and CD4+ CD11c- CD123+ cells. The finding that TPO alone did not induce the generation of CD11c+ immature DCs suggests that TNF-α thus plays a critical role in synergy with TPO. The coexpression of TNFR-II and c-mpl on CD11c+ cells suggests that TPO and TNF-α act in synergy with the downstream signaling pathways of both receptors.
In suspension cultures, a rapid increase in the proportion of CD11c+, CD83+, or CD86+ cells was observed as early as day 5. Although CD34+ cells can also differentiate into monocytes/macrophages, it takes 10 to 14 days in vitro.37 Day 5 CD11c+ DCs were capable of capturing codeveloping megakaryocytic progenitors. Most CD11c+ cells coexpressed CD4 and CD123. Therefore, the CD4+ CD11c+ CD123+ cells are most likely DCs in an immature stage. The remaining nonmegakaryocytic cells consisted of CD4+ CD11c- CD123+ cells. However, a dot plot analysis showed a continuous CD11c expression in CD123+ cells on day 5 (Figure 2). The proportion of CD11c+ CD4+ cells reached a plateau by day 5, while that of CD123+ cells gradually decreased by day 7 to the equivalent with CD11c+ CD4+ cells. Therefore, some CD11c- CD123+ cells may not be in a transitional stage to CD11c+ CD123+ cells. Blom et al28 have shown that CD34+ CD45RA+ CD123+ cells develop into mature DCs in cultures with SCF or FLT3-L through the differentiation pathway of plasmacytoid DC (CD11c- CD123+) precursor cells. More recently, Chen et al20 showed that TPO cooperates with FLT3-L in the generation of plasmacytoid DC precursors from human CD34+ cells. DCs derived from plasmacytoid precursor cells are known to lack CD11c and CD1a.38 The possibility that CD11c- CD123+ cells are capable of differentiating plasmacytoid DCs upon transfer to the culture with FLT3-L and TPO remains to be elucidated. However, the CD4+ CD11c+ CD123+ DCs are possibly myeloid lineage DCs, because they do not produce a large amount of IFN-α in response to CpG-ODN (data not shown).
The potent effects of TNF-α on generation of DCs and, in turn, the inhibition of TPO-induced megakaryopoiesis are also of particular interest, because the CD11c+ DCs closely associated with codeveloping megakaryocytic progenitor cells and also phagocytosed them (Figure 4). Moreover, those DCs induced autologous MLRs, whereas CD11c+ cells generated by TNF-α alone did not (Figure 5). As previously reported,39,40 phagocytosis of necrotic but not apoptotic cells induces DC maturation. Under in vitro culture conditions, developing DCs possibly captured both apoptotic and necrotic cells, thus leading to DC maturation. Indeed, the proportions of CD11c+ cells were comparable with each other, but a slight increase in the expression of costimulatory molecules was noted in DCs induced by TPO plus TNF-α. However, DCs generated with TNF-α alone and TNF-α and TPO showed comparable amounts of IL-6 and IL-8, although the latter produced more IL-8 than the former (Table 2).
An infection with a virus, such as Epstein-Barr virus,41 mumps virus,42 dengue virus,43 and hepatitis A virus,44 has been suggested to result in fatal hemophagocytic syndrome, which is associated with an overproduction of IFN-γ, TNF-α, IL-1, and IL-6,41,45 which are known to be potent activators of macrophages. Well-differentiated macrophages have been observed to phagocytose hematopoietic cells in the bone marrow.46 We herein also show that CD11c+ CD83+ CD86+ cells in patients with hemophagocytic syndrome contained CD61+ cells and their fragments and GPA-positive cells intracellularly (Figures 6, 7; Table 3), thus suggesting the involvement of DCs in hemophagocytosis.
Recent studies indicate that DCs are involved in the counterregulation of potential autoimmune T-cell responses47 and that CD11c+ DCs that have not been activated by pathogen-related or endogenous inflammatory stimuli can significantly contribute to peripheral tolerance by inducing the inactivation and/or deletion of specific T cells.48 On the other hand, DCs expressing endogenous self-peptides or pulsed ex vivo with immunogenic self-peptides can induce severe autoimmune disease.47,49 The data presented in this work demonstrate that TNF-α has strong effects on cytokine secretion by developing cells, most probably immature DCs, including the up-regulation of early proinflammatory cytokines (IL-6 and IL-8). On the other hand, TNF-α-treated DCs have been reported to be semimature and induced regulatory cells upon inoculation in vivo.50,51 Therefore, limited immune response or induction of regulatory T cells may prevent T cell-mediated autoimmune diseases in a steady state as well as in pathologic situations.
In conclusion, this is the first report showing that in the presence of TNF-α the nonmegakaryocytic cells with typical feature of DCs are cogenerated from human CD34+ cells during megakaryocytic differentiation by TPO. The CD4+ CD11c+ CD123+ DCs are physically associated with and phagocytose either developing or dying immature megakaryocytic cells. A similar phenomenon showing engulfment of CD61+ fragment by CD11c+ cells was also observed in the bone marrow cells from patients with hemophagocytic syndrome. Therefore, it may be conceivable that DCs with phagocytic activity during the development in bone marrow may play a crucial role in the maintenance of tolerance for self-substances derived from hematopoietic progenitor cells.
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-08-3155.
Supported in part by Grants-in-Aid (14370075,17590978, and 17925036) and funds from the Center of Excellence Program of the Ministry of Education, Science, Technology, Sports, and Culture of Japan and a research grant from the Idiopathic Disorders of Hematopoietic Organs Research Committee of the Ministry of Health, Labour and Welfare of Japan.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sanford B. Krantz, MD, for helpful comments, and H. Kataho for excellent technical assistance.



![Figure 5. Autologous T-cell proliferation by DCs generated in the presence of TPO and TNF-α. The cells prepared by culture for 7 days with 100 ng/mL TNF-α in the presence (▪) or absence (□) of 100 ng/mL TPO were used at graded doses as stimulator cells after γ-irradiation. In some MLRs, anti-CD3 mAb was added. Proliferation was assessed by adding 3H-thymidine (1 μCi per well [37 κBq/well]) during 72 to 90 hours of culture. Results represent the mean ± SD of triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/4/10.1182_blood-2005-08-3155/2/m_zh80040691350005.jpeg?Expires=1766009967&Signature=Cftv28vuFXSyjDVR1d-fbc-9~0G~bqucLlOcf6t-cLRva6mr9OE-iglRGDQ7ir7MWlT7o4aZHRhCUQHWqhG0JBpqJ38XNfoOZfHFyl83bz0-fXiewIX5iNcAyLHiWQ6Cnctx2WZIz5sIf2YGf5dPjs6NZ~lUn3k7eXT8~QsRw34fG3gEU-hKGIedjh0Pl6gbbSJz9xduUyHjFGjM5VpXKUFrUpcd0gtF3x3xswDl80zuTiBeR6CBm3O4p6EPsZ~8W00dIVGlSgoEyupUYLM8ml4UzKT64nJcbdd~kLePhP0rk4Xu5xbjXZUqSM38Qay6wDR~9Gy0tsXmSteVDjcf5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Capture of erythroid progenitor cells expressing GPA by CD11c+ cells from bone marrow of hemophagocytic syndrome. Bone marrow smear preparations from patients with hemophagocytic syndromes were stained with CD11c (red)/GPA (brown) for patient 1 (A) or CD11c (brown)/GPA (red) for patient 2 (B) and patient 3 (C). The arrows indicate GPA-positive erythroid cells. Staining with α-NB of hemophagocytic cells is also shown in patient 3 (D) and DCs generated from purified normal human CD34+ cells in the presence of TPO and TNF-α (day 5 [E]; day 7 [F]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/4/10.1182_blood-2005-08-3155/2/m_zh80040691350007.jpeg?Expires=1766009967&Signature=0VuBv~2vRSboo7gl5MGuPOr3h0v6bJJheEnpfQ1jBvU60I7KhwdHCZiuONDn8ILOPj4y0cazDcVsYOsuG8HY5eS7cFL2c9IQnWsxwm81TL84oou0oPWc2GE8dvjhjnhOfgXGCqRuxMDp0fNY0VqCxoBTXjHHyKpQBxljtEBMKSujbaa8bLN0mXCOBNlo8UrQj9ApUA4mdPhey52dCzwkLldrymGowIqQXjykncufGjsufGqEmcUX0YMkkANfq5ySqAXCAcpVE2QT3zCIy1COAha4IEkNVvFacnwaYsspA08~4m8f9QkpLpBj2yFAPUSTftTTdyCQclCOV4XnQA~qqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal