Comment on Inoue et al, page 1405
Previously thought to function only in collagen-induced platelet activation, Inoue and colleagues report that the GPVI/FcRγ chain complex is a crucial component in relaying activation signals following platelet exposure to the extracellular matrix protein laminin.
Interaction of resting platelets with exposed components of the subendothelial matrix is an important “sentry” event that takes place early on at sites of vascular injury. Depending on the nature and extent of damage, platelets can encounter a variety of fibrous proteins, including laminin, fibronectin, and collagen, as well as the collagen-bound adhesive protein von Willebrand factor (VWF). To be able to respond to such a diverse array of extracellular matrix components, platelets have been endowed with a host of receptors, each specific for different matrix constituents. Thus, the GPIb complex and the integrin αIIbβ3 bind to different regions on immobilized VWF, while the integrins α5β1, αIIbβ3, α2β1, and α6β1 interact with fibronectin, collagen, and laminin, respectively.
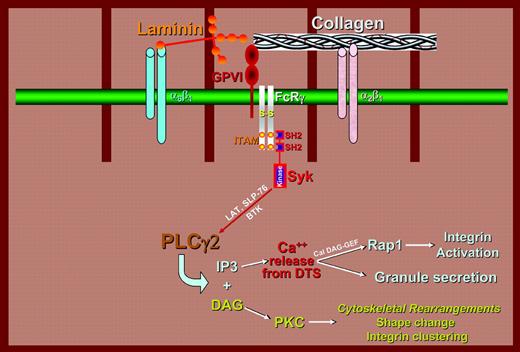
Receptors for 2 different extracellular matrix proteins use the GPVI/FcRγ chain complex for signal transduction. Upon binding of laminin to integrin α6β1 (blue, left) or of collagen to integrin α2β1 (pink, right), tyrosine residues within the ITAMs of the FcRγ chain signaling subunit of the GPVI/FcRγ chain complex become phosphorylated, recruit the Syk tyrosine kinase, and assemble a signaling complex that activates phospholipase Cγ2 (PLCγ2). Activation of PLCγ2 ultimately results in cytokskeletal changes, granule secretion, and activation of cell-surface integrins involved in platelet-matrix and platelet-platelet interactions. This pathway was previously thought to operate exclusively in response to collagen and collagen-related snake venom peptides.
Receptors for 2 different extracellular matrix proteins use the GPVI/FcRγ chain complex for signal transduction. Upon binding of laminin to integrin α6β1 (blue, left) or of collagen to integrin α2β1 (pink, right), tyrosine residues within the ITAMs of the FcRγ chain signaling subunit of the GPVI/FcRγ chain complex become phosphorylated, recruit the Syk tyrosine kinase, and assemble a signaling complex that activates phospholipase Cγ2 (PLCγ2). Activation of PLCγ2 ultimately results in cytokskeletal changes, granule secretion, and activation of cell-surface integrins involved in platelet-matrix and platelet-platelet interactions. This pathway was previously thought to operate exclusively in response to collagen and collagen-related snake venom peptides.
How the information that “contact has occurred” is transmitted to the platelet interior is an increasingly active and important area of platelet signal transduction research.1 GPIb/VWF interactions serve mainly to tether the platelet to the damaged vessel wall, although relatively weak activation signals also emanate from the GPIb complex that potentiate downstream platelet activation. Responses to collagen, on the other hand, are considerably more vigorous and complex, being mediated by 2 different receptors: the integrin α2β1 and a platelet-specific, heterotrimeric receptor composed of the Ig-superfamily member, GPVI, in noncovalent association with a homodimer of the FcRγ chain.2 Upon binding collagen, signals emanating from α2β1 and the GPVI/FcRγ chain complex reinforce each other, ultimately leading to conformational changes in the major platelet integrin, αIIbβ3, which in its activated state associates with bridging proteins like fibrinogen and VWF. Although the nature of α2β1-mediated signals is not as well understood, numerous studies using GPVI-deficient mice, FcRγ chain-deficient mice, and antibodies that either block the interaction of GPVI with collagen or deplete GPVI from the platelet surface, have shown that engagement of the GPVI/FcRγ chain complex works via Src family kinase-mediated phosphorylation of specific immunoreceptor tyrosine-based inhibitory motifs (ITAMs) that are present on the cytoplasmic domain of the FcRγ chain. These ITAMs, when phosphorylated, serve as docking sites for the protein-tyrosine kinase Syk, which, when recruited and activated, initiates the rapid assembly of a series of adaptor proteins and protein-tyrosine kinases that are crucial for platelet activation responses, including cytoskeletal rearrangements that mediate platelet shape change and spreading and coalescence and secretion of intracellular granules, as well as activation of additional adhesion receptors that are required for adhesion strengthening and thrombus formation to occur.
In 2003, Gruner and colleagues3 reported in this journal the somewhat surprising observation that platelets from α2β1-deficient mice adhere quite normally to the wall of a ligation-injured murine carotid artery, and that their adhesion was only partially reduced when αIIbβ3 was additionally blocked by a monoclonal antibody. When αIIbβ3 was inhibited in murine platelets missing all 3 β1 integrins (α2β1, α5β1, and α6β1), however, adhesion and thrombus formation were absent, implicating α5β1 and/or α6β1 as integrins capable of mediating platelet-vessel wall interactions under conditions of arterial shear. Interestingly, GPVI was found to be indispensable for platelet adhesion and aggregation to occur, strongly suggesting that it might be involved in regulating platelet interactions with the vessel wall.
In this issue of Blood, Inoue and colleagues report the novel observation that the GPVI/FcRγ chain complex, in addition to its crucial role as a collagen receptor, is also an important component of platelet activation following interaction of α6β1 with laminin—a prominent component of the basal lamina that underlies epithelial and endothelial cell monolayers and also surrounds individual muscle cells. The interaction of GPVI with laminin was found to be too weak by itself to support adhesion; however, in conjunction with α6β1, expression of the GPVI/FcRγ chain complex strongly potentiated formation of lamellipodia, and to a lesser extent filopodia, during the platelet spreading process. Interestingly, platelet activation by laminin was found to enlist the signaling, rather than the adhesive, properties of the GPVI/FcRγ chain complex, as both platelet spreading and tyrosine phosphorylation of several key downstream components of the GPVI/FcRγ chain-mediated signaling were markedly reduced in FcRγ chain-deficient platelets following their interaction with immobilized laminin.
Similar to the interplay between α2β1 and the GPVI/FcRγ chain complex following platelet interactions with collagen, the chicken-and-egg question as to whether (1) α6β1 first binds laminin and presents it to the GPVI/FcRγ chain for signaling to the cell interior, or (2) GPVI-laminin interactions first activate the α6β1 integrin to facilitate platelet spreading, remains controversial. Both probably occur. Finally, since the GPVI/FcRγ chain has now been identified as a central component of both collagen- and laminin-mediated platelet activation, it may be of future interest to determine whether the threshold for cellular activation in response to these prominent components of the extracellular matrix might be regulated by the same inhibitory signaling receptor.4 ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal