Abstract
Subpopulations of bone marrow-derived cells can be induced to assume a number of endothelial properties in vitro. However, their ability to form a functional vascular barrier has not been demonstrated. We report that human CD14+ peripheral blood monocytes cultured under angiogenic conditions develop a number of phenotypic and functional properties similar to brain microvascular endothelial cells. These cells express the tight junction proteins zonula occludens 1 (ZO-1) and occludin and form a barrier with a transcellular electrical resistance (TCER) greater than 100 ohm cm2 and low permeability to 4 kDa and 20 kDa dextrans. The TCER of the cellular barrier is decreased by bradykinin and histamine. We also demonstrate that these cells associate with repairing vasculature in areas of brain and skin injury. Our data suggest that CD14+ peripheral blood monocytes participate in the repair of the vascular barrier after brain injury.
Introduction
Bone marrow-derived cells are important in the formation of new blood vessels in both physiologic and pathologic processes. Several populations of hematopoietic cells assume an endothelial phenotype when cultured under proangiogenic conditions. These include CD34+, Sca1+, CD133+, and CD14+ cells.1-19 Additionally, these cells incorporate into growing vasculature in experimental models.3,20-29 This expanding body of evidence suggests a critical link between the ability of hematopoietic cells to adopt endothelial characteristics and the processes of blood vessel growth and repair.
Although there are certain common features of all endothelial cells, there is great specificity of function and heterogeneity of phenotype between different populations.30-32 A specialized form of endothelium makes up the microvasculature of the central nervous system and participates in the formation of the blood-brain barrier (BBB). The BBB is a complex microanatomical structure designed to closely regulate the influx and efflux of substances from the circulation to the brain parenchyma, and vice versa. Several cell types, including endothelial cells, astrocytes, pericytes, and possibly neurons participate in the formation and maintenance of the BBB.33-40 Some of the features of brain microvascular endothelial cells that differentiate them from endothelial cells in other locations include the presence of numerous tight junctions, a decrease in the amount of pinocytosis, and the presence of enzyme systems involved in the transport of molecules in and out of the central nervous system (CNS).33,39,41-43
In vitro systems for the culture of brain microvascular endothelial cells have been developed in which many of the properties of BBB endothelial cells can be maintained. Cultured brain microvascular endothelial cells can form a tight cellular barrier that develops a high transendothelial electrical resistance, impedes the passage of larger molecules across the cellular layer, and has enzymatic activities associated with in vivo BBB endothelial cells.33,38,44-49 These in vitro systems also maintain some of the regulatory properties of the in vivo BBB such as an increase in barrier permeability mediated by vasoactive substances such as bradykinin and histamine.50-52
We hypothesized that if cells of hematopoietic origin could express endothelial cellular markers, they may also be able to carry out other important endothelial functions. To that end, we now demonstrate that a population of peripheral blood-derived monocytes adopt endothelial characteristics, including the formation of a regulated vascular barrier, and migrate to damaged vasculature at sites of brain injury. These data suggest an important role for monocytes in the repair of the cerebral vasculature.
Materials and methods
Cell culture
Leukopacks from consenting healthy donors were obtained from the National Institutes of Health (NIH) blood bank or from the New Brunswick-Affiliated Hospitals blood bank. Mononuclear cells were isolated using Histopaque-1077 density separation (Sigma, St Louis, MO). CD14+ cells were isolated using anti-CD14 magnetic beads according to the manufacturer's instructions (Miltenyi, Auburn, CA). CD14+ cells were then plated at a density of 1 to 2 × 107 cells/80 cm2 and cultured in endothelial basal media (EBM-2) with microvascular endothial-cell medium-2 (EGM-2-MV) supplements (hydrocortisone, 5% fetal bovine serum [FBS], vascular endothelial growth factor [VEGF], human basic fibroblast growth factor [hFGF-b], insulin-like growth factor 1 [IGF-1], human epidermal growth factor [hEGF], and ascorbic acid) (Cambrex Bio Science, Walkersville, MD) for 10 to 14 days at 37°C with 5% CO2 in a humidified atmosphere. Cells were then replated on 24-mm diameter or 12-mm diameter Corning Costar Transwell-Clear inserts, pore size 0.4 microns; catalog nos. 3450 (24 mm) and 3460 (12 mm) (Corning, Corning, NY), that had been coated with a 5-ng/mL solution of fibronectin (Sigma, St Louis, MO), on chambered microscope slides, or in tissue culture flasks. The endothelial-like monocytic cells (ELMCs) were cultured in M199 (Mediatech, Herndon, VA) with 10% fetal calf serum (HyClone, Logan, UT) plus 50 ng/mL VEGF, 1 ng/mL FGF-b, and 1 ng/mL IGF-1 (R&D Systems, Minneapolis, MN). Cells were grown an additional 10 to 14 days in this medium prior to cell staining, fluorescence-activated cell-sorting (FACS) analysis, or enzymatic analysis. Throughout this process the media was changed every 3 to 5 days. Whole human bone marrow was obtained from All Cells (Berkeley, CA) and plated at a density of 2 × 107 cells per 80 cm2. The human bone marrow cells were cultured in conditions identical to those used to generate ELMCs as described. Mouse brain microvascular endothelial cells were isolated from Cr:NIH(S) (Swiss) mice (National Cancer Institute [NCI], Frederick, MD) as previously described.38,44 Human umbilical vein endothelial cells (HUVECs) and human aorta endothelial cells (HAECs) were purchased from Cambrex (Bioscience) and were used at passages 5 to 11.
Transcellular resistance experiments
After culturing cells on Transwell inserts, the transcellular electrical resistance (TCER) was measured using the EVOM voltohmmeter (World Precision Instruments, Sarasota, FL). For experiments showing the effect of vasoactive substances, ELMCs with an established barrier were used. Bradykinin (Sigma) to a concentration of 100 μM or histamine (Sigma) to a concentration of 100 μM was added to both the upper and lower chambers and the TCER measured over time. DMSO was the vehicle for these drugs and was used as a negative control. The final DMSO concentration was 0.5%.
Diffusion of labeled dextran
Cells were cultured to confluence on Transwell inserts. The media in both the upper and lower chambers of the Transwell was replaced with prewarmed Hanks balanced salt solution. FITC-labeled dextran of either 4 kDa or 20 kDa (Sigma) was added to the upper chamber of the Transwell to a concentration of 0.5 mg/mL, and samples were taken of the buffer in the lower chamber over time. Fluorescence measurements were made using a Victor 2 1420 multilabel counter (Perkin Elmer, Boston, MA) and the concentration of dextran determined. Known concentrations of FITC-labeled dextrans were used to construct concentration curves for fluorescence for both 4-kDa and 20-kDa FITC-dextrans. The concentration of FITC-dextran in the upper and lower chambers was determined by fluorescence measurements of the chamber solutions using these curves. Permeability coefficients were calculated as previously described.38 Briefly, the permeability surface product (PS) was determined using the equation ΔCl/Δt where ΔCl is the incremental clearance volume and Δt is time. ΔCl is determined by multiplying the fraction of compound transferred from donor to acceptor compartment at a time point by the volume of the donor chamber. The permeability coefficient (P) was calculated using the equation PS/S = P where S is the filter surface area. To correct for the contribution of the filter, P was also determined for a fibronectin-coated filter and the permeability coefficient for the cell layer alone was calculated using the equation 1/Pc = 1/Pt - 1/Pf where Pc is the permeability of the cell layer, Pt is the permeability of the total system, and Pf is the permeability of the filter.
Enzymatic assays
Cells in culture were rinsed 3 times with phosphate-buffered saline (PBS) and then scraped from the culture surface in a minimal volume of ice-cold lysis buffer (0.1% Triton X-100 in PBS). The cells were then stored at -80°C until enzyme activity was assayed. Just prior to assay, cells were rapidly thawed at 37°C and cell debris was spun down at 10 000g for 10 minutes at 4°C. The supernatant was used in the enzyme assays. γ-glutamyl transpeptidase (γ-GT) and alkaline phosphatase (ALP) activities were determined using diagnostic assay kits (Sigma). Protein concentration was determined using the Bio-Rad DC Protein Assay (Bio-Rad, Hercules, CA). Enzyme activities were expressed in units per milligram of protein. Human peripheral blood monocytes used in these experiments were more than 95% CD14 positive.
Immunohistochemistry and FACS analysis
The following antibodies were used: rabbit anti-von Willebrand factor (VWF; DAKO, Carpinteria, CA), rabbit anti-ZO-1 and anti-occludin (Zymed, South San Francisco, CA), mouse anti-CD14, anti-CD11b, anti-CD31, and rat anti-CD31 (BD Pharmingen, San Diego, CA), and labeled goat anti-rabbit, goat anti-rat, and goat anti-mouse secondary antibodies (Molecular Probes, Eugene, OR). Biotin-labeled Ulex europaeus aggluttin-1 (UEA-1) was purchased from Sigma, and Texas red avidin was purchased from Vector Laboratories (Burlingame, CA). Cultured cells on glass slides or cytospin preparations of freshly isolated monocytes were fixed for 20 minutes at -20° C in 5% acetic acid in ethanol, blocked with 10% goat serum and 0.1% Triton X-100 in PBS then incubated at 4°C for 4 to 8 hours in 1:100 (ZO-1, occludin) or 1:200 (VWF) of the primary antibody. Cells were washed and incubated in 1:1000 of the secondary antibody for 1 hour at 4°C, washed and mounted with Vectashield with DAPI (Vector Laboratories). For FACS analysis of CD14, CD11b, CD31, and occludin cells were removed from culture using nonenzymatic cell dissociation buffer (Invitrogen, Carlsbad, CA), incubated with primary antibody at a concentration of 1 μg/106 cells for 30 minutes on ice, and washed and incubated with secondary antibody at a concentration of 1 μg/106 cells. For FACS analysis of von Willebrand factor expression cells were removed from culture plates and fixed with 1% paraformaldehyde at 4°C for 20 minutes prior to staining. For tissue sections, mice were deeply anesthetized using a combination of ketamine (100 mg/kg) and xylazine (4 mg/kg) delivered intraperitoneally. The chest was opened and a small nick was made in the right atrium. The mice were then perfused through the left ventricle with 40 mL PBS. The brains were removed immediately and frozen in isopentane on dry ice. Brains were mounted in Tissue-Tec (Sakura Finetek USA, Torrence, CA) and 30-μM sections were cut and placed on glass slides. The tissue sections were then fixed on the slides in 100% methanol at -20°C for 15 minutes. The procedure for staining was as described for cultured cells. FACS analysis was performed and compared to corresponding isotype controls. Fluorescent microscopy was performed using a Zeiss LSM 510 confocal microscope; for Figures 4, 5, and 6A-C, the microscope was equipped with Plan-Apochromat 63×/1.4 oil-immersion and Plan-Neofluar 10×/0.3 objective lenses (Zeiss, Heidelberg, Germany) and Zeiss LSM Image Browser imaging software was used to acquire images. For Figure 6D-E, images were obtained through a Nikon C1 confocal system using a Nikon Plan Fluor 10×/0.3 objective lens and EZC1 software (Nikon, Melville, NY). Light microscopy was performed using a Nikon Eclipse TE2000 microscope equipped with a Nikon Plan Fluor 4×/0.3 objective lens and a SPOT digital camera running SPOT 4.01 imaging software (Diagnostic Instruments, Sterling Heights, MI). Adobe Photoshop CS version 8.0 (Adobe Systems, San Jose, CA) was used to perform subsequent image processing.
DiI-Ac-LDL labeling
Uptake of acetylated low-density lipoprotein (Ac-LDL) was performed on living cells with DiI-labeled Ac-LDL (Molecular Probes). DiI Ac-LDL was added to a concentration of 10 μg/mL to the media, and cells were returned to the tissue culture incubator for 4 hours. The cells were then washed 3 times with PBS and Ac-LDL uptake was visualized using fluorescence microscopy.
Bone marrow transplantation model
FVB/N-TgN(TIE2LacZ)182Sato mice express β-galactosidase driven by the endothelial-specific TIE2 promoter. These mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were killed and bone marrow was collected from the femurs for use in the transplantation studies. Sublethally irradiated (350 cGy) severe combined immunodeficient (SCID)/NCr mice (NCI) were injected with 5 × 106 bone marrow cells via tail vein. One month after transplantation mice were used in brain injury studies. Mice were anesthetized and under stereotaxic guidance a 30-gauge needle was inserted 2.5 mm into the brain parenchyma 2 mm lateral to and 1 mm anterior to the bregma. Mice were killed 3 to 5 days later, and sections through the injury site were analyzed. Injuries in 5 mice that received transplants were analyzed. For evaluation of FITC-albumin permeability, Cr:NIH (Swiss) mice were used. Zero to 12 days after stab injury, mice were deeply anesthetized and injected with 100 μL of a 10 mg/mL solution of FITC-labeled dextran (Sigma) via tail vein. Ten minutes after tail vein injection, mice were perfused through the left ventricle with 40 mL PBS. The brains were removed, frozen, fixed, and stained as described in “Immunohistochemistry and FACS analysis.”
Incorporation of ELMCs into repairing vasculature after brain injury
Cold injury was performed on SCID mice as described previously.53 Briefly, mice were anesthetized using a combination of ketamine (100 mg/kg) and xylazine (4 mg/kg) intraperitoneally. The skull was then exposed. Under stereotaxic guidance a metal rod 4 mm in diameter that had been cooled to -80°C in dry ice was applied to the skull 2 mm lateral to and 1 mm anterior to the bregma. The probe was applied for 30 seconds. ELMCs, freshly isolated human CD14+ peripheral blood mononuclear cells, or CD14-depleted human peripheral blood mononuclear cells were labeled with Cell Tracker DiI (Molecular Probes) according to the manufacturer's instructions. Twenty-four hours after injury 106 DiI-labeled human cells were injected into each mouse via tail vein. Mice were killed at either 5 days or 4 weeks after the injury, and tissue sections were analyzed.
Incorporation of ELMCs into repairing vasculature after skin injury
A skin biopsy was performed on SCID mice. Briefly, mice were anesthetized using a combination of ketamine (100 mg/kg) and xylazine (4 mg/kg) intraperitoneally. The back was then shaved. A circular skin biopsy punch was used to make an injury on the back. ELMCs were labeled with Cell Tracker DiI according to the manufacturer's instructions. Twenty-four hours after injury 106 DiI-labeled human cells were injected into each mouse via tail vein. Mice were killed 5 days after the injury, and tissue sections were analyzed.
Animal experiments
All procedures followed in the animal experiments were in accordance with NIH or UMDNJ/Robert Wood Johnson Medical School institutional guidelines and had been approved by the appropriate institutional animal care and use committee.
Statistical analysis
Results are expressed as means ± SEM. Statistical analysis was assessed using the Student t test. The lower limit of significance was taken as a P value less than .05.
Results
CD14+ peripheral blood monocytes form a cellular layer with high electrical resistance in vitro
Various models have been used to study the permeability characteristics of brain microvascular endothelial cells.38,45-50,54-56 One well-defined system, the Transwell (Corning), consists of a filter dividing upper and lower chambers. Cells can be cultured on the filter and the movement of drugs and other molecules from upper (apical) to lower (basolateral) chamber can be measured. The electrical resistance of a cell layer, or TCER, can also be measured.38,47,50 We used this model system to identify a population of human peripheral blood-derived cells with the capacity to generate a cellular barrier with a high TCER similar to brain microvascular endothelial cells. Cells from human bone marrow and peripheral blood were cultured in endothelial growth media with microvascular supplements (EGM-2; Cambridge Bioscience) for 10 days and then transferred to the Transwell system. The cells were then grown in the presence of various angiogenic cytokines and other growth factors. Cultures were screened for the formation of a tight cellular barrier using TCER measurements. Human peripheral blood mononuclear cells consistently formed a cellular barrier with a high TCER when grown in the presence of VEGF, bFGF, and IGF-1. By contrast, human bone marrow cells cultured under the same conditions adopted a mesenchymal morphology and did not generate a high TCER.
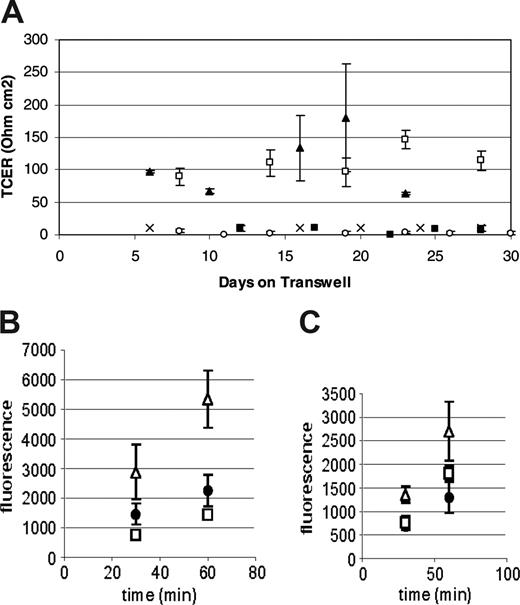
Preliminary immunohistochemical data suggested that the cells responsible for this TCER phenotype were of the monocyte/macrophage lineage (data not shown). To confirm these observations we performed subsequent experiments using CD14+ monocytes. In multiple experiments, monocytes were consistently able to produce a TCER of over 100 ohm cm2 compared with CD14-depleted human peripheral blood mononuclear cells, which did not form a confluent monolayer and did not generate resistances over 20 ohm cm2 (Figure 1A). The TCER seen using peripheral blood mononuclear cells was similar to that seen with mouse brain endothelial cells when used in the same system. Additionally, HUVECs as well as HAECs did not form a TCER under similar culture conditions (Figure 1A). Based on these observations we have referred to the CD14+ human peripheral blood cells grown under these conditions as ELMCs.
The ELMC barrier has low permeability to high-molecular-weight dextrans
The barrier formed by the ELMCs also has a low permeability to larger molecules. FITC-labeled dextrans (4 kDa or 20 kDa) were applied to the upper chamber of the Transwells on which ELMCs, HUVECs, or mouse brain endothelial cells were grown, and samples of the lower chamber were taken at 30 and 60 minutes. The permeability of ELMCs for 4-kDa dextran (Pc = 3.8 ± 1.1 × 10-6 cm/s) was significantly lower than the permeability of HUVECs for 4-kDa dextran (5.5 ± 1.3 × 10-6 cm/s; P < .05). This was also true for 20-kDa dextran (ELMCs Pc = 2.23 ± 0.1 × 10-6 cm/s vs HUVECs Pc = 3.8 ± 0.4 × 10-6 cm/s; P < .05). The permeability of ELMCs to both 4-kDa and 20-kDa dextran was similar to mouse brain endothelial cells (Figure 1B-C).
Human peripheral blood monocytes form a low permeability barrier in culture. (A) CD14+ human peripheral blood monocytes cultured on Transwells form a high-resistance barrier similar to mouse brain microvascular endothelial cells (MBECs), while CD14-depleted human peripheral blood mononuclear cells do not. HUVECs and HAECs do not develop a high transcellular resistance under the same culture conditions. (n = 3-4 for each cell type) P < .05 (CD14+, □; MBEC, ▴; HAEC, ○; HUVEC, ▪; and CD14-depleted, ×). (B-C) The permeability of ELMCs to FITC-labeled 4-kDa (B) and 20-kDa (C) dextran is lower than the permeability of HUVECs and similar to the permeability of MBECs. Cells were cultured on transwell inserts and the movement of FITC-labeled 4-kDa and 20-kDa dextrans through the cell layer was monitored and used to calculate permeability coefficients (HUVEC, ▵; ELMC, •; and MBEC, □) (n = 3). The permeability surface product (PS) was determined using the equation ΔCl/Δt where ΔCl is the incremental clearance volume and Δt is time. The permeability coefficient (P) was calculated using the equation PS/S=P where S is the filter surface area. To correct for the contribution of the filter, P was also determined for a fibronectin-coated filter and the permeability coefficient for the cell layer alone was calculated using the equation 1/Pc = 1/Pt - 1/Pf where Pc is the permeability of the cell layer, Pt is the permeability of the total system, and Pf is the permeability of the filter. The permeability of ELMCs for 4-kDa dextran (Pc = 3.8 ± 1.1 × 10-6 cm/s) was significantly lower than the permeability of HUVECs for 4-kDa dextran (Pc = 5.5 ± 1.3 × 10-6 cm/s; P < .05). This was also true for 20-kDa dextran (ELMC Pc = 2.23 ± 0.1 × 10-6 cm/s vs HUVEC Pc = 3.8 × 0.4 × 10-6 cm/s; P < .05). This experiment was performed twice with similar results. Data are expressed as mean ± SEM.
Human peripheral blood monocytes form a low permeability barrier in culture. (A) CD14+ human peripheral blood monocytes cultured on Transwells form a high-resistance barrier similar to mouse brain microvascular endothelial cells (MBECs), while CD14-depleted human peripheral blood mononuclear cells do not. HUVECs and HAECs do not develop a high transcellular resistance under the same culture conditions. (n = 3-4 for each cell type) P < .05 (CD14+, □; MBEC, ▴; HAEC, ○; HUVEC, ▪; and CD14-depleted, ×). (B-C) The permeability of ELMCs to FITC-labeled 4-kDa (B) and 20-kDa (C) dextran is lower than the permeability of HUVECs and similar to the permeability of MBECs. Cells were cultured on transwell inserts and the movement of FITC-labeled 4-kDa and 20-kDa dextrans through the cell layer was monitored and used to calculate permeability coefficients (HUVEC, ▵; ELMC, •; and MBEC, □) (n = 3). The permeability surface product (PS) was determined using the equation ΔCl/Δt where ΔCl is the incremental clearance volume and Δt is time. The permeability coefficient (P) was calculated using the equation PS/S=P where S is the filter surface area. To correct for the contribution of the filter, P was also determined for a fibronectin-coated filter and the permeability coefficient for the cell layer alone was calculated using the equation 1/Pc = 1/Pt - 1/Pf where Pc is the permeability of the cell layer, Pt is the permeability of the total system, and Pf is the permeability of the filter. The permeability of ELMCs for 4-kDa dextran (Pc = 3.8 ± 1.1 × 10-6 cm/s) was significantly lower than the permeability of HUVECs for 4-kDa dextran (Pc = 5.5 ± 1.3 × 10-6 cm/s; P < .05). This was also true for 20-kDa dextran (ELMC Pc = 2.23 ± 0.1 × 10-6 cm/s vs HUVEC Pc = 3.8 × 0.4 × 10-6 cm/s; P < .05). This experiment was performed twice with similar results. Data are expressed as mean ± SEM.
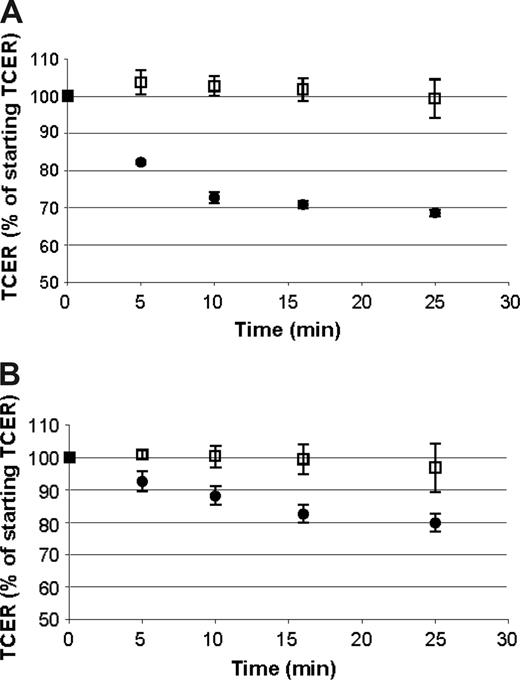
The permeability of cellular barriers, including the BBB, can be modulated by a number of vasoactive compounds.52 To determine if the barrier formed by ELMCs could be modulated in a similar way, we evaluated the effect of histamine and bradykinin on the TCER of ELMCs. Confluent ELMC cultures on Transwells that had developed a high resistance barrier were prepared. Histamine, bradykinin, or vehicle was applied to both the upper and lower chambers, and the TCER was measured over time. Bradykinin caused a decrease in the TCER by 20% over 30 minutes and in histamine a decrease of 30% over 30 minutes (Figure 2). The magnitude of the decrease in TCER is comparable to the reported effect of bradykinin and histamine in other in vitro BBB systems.51,52
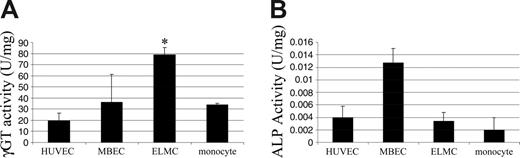
The activity of several enzyme systems is elevated in BBB-derived endothelial cells compared with endothelial cells from other locations.32,33,38,47,55 Thus, we measured the activity of γ-glutamyl transpeptidase and alkaline phosphatase in ELMCs in order to determine if ELMCs had elevated expression of these BBB-associated enzymes. γ-Glutamyl transpeptidase activity was approximately 3-fold higher in ELMCs compared with monocytes or HUVECs, and was similar to the level of γ-glutamyl transpeptidase activity seen in mouse brain endothelial cells (Figure 3A). By contrast, ELMCs did not have increased alkaline phosphatase activity compared with freshly isolated monocytes or HUVECs, and had significantly lower alkaline phosphatase activity than mouse brain endothelial cells (Figure 3B).
The cellular barrier formed by ELMCs is responsive to bradykinin and histamine. A decrease in TCER is seen with the application of histamine (A) or bradykinin (B) to endothelial-like monocytic cells cultured on Transwells (bradykinin or histamine, •; vehicle, □). Experiments were done 3 times each; n = 3. TCER values for samples after the application of bradykinin or histamine were significantly lower than the controls at each time point (P < .05). Data are expressed as mean ± SEM.
The cellular barrier formed by ELMCs is responsive to bradykinin and histamine. A decrease in TCER is seen with the application of histamine (A) or bradykinin (B) to endothelial-like monocytic cells cultured on Transwells (bradykinin or histamine, •; vehicle, □). Experiments were done 3 times each; n = 3. TCER values for samples after the application of bradykinin or histamine were significantly lower than the controls at each time point (P < .05). Data are expressed as mean ± SEM.
ELMCs have high γ-glutamyl transpeptidase activity. γ-glutamyl transpeptidase (γ-GT) activity is significantly higher in ELMCs than HUVECs or freshly isolated monocytes (*P < .05). However, ELMCs do not have increased alkaline phosphatase (ALP) activity compared with freshly isolated monocytes or HUVECs. γ-GT (A) and ALP (B) activity of freshly isolated monocytes, ELMCs, HUVECs, and MBECs were compared (n = 3). Results are expressed as enzyme activity per microgram of protein. These experiments were performed 4 times with similar results.
ELMCs have high γ-glutamyl transpeptidase activity. γ-glutamyl transpeptidase (γ-GT) activity is significantly higher in ELMCs than HUVECs or freshly isolated monocytes (*P < .05). However, ELMCs do not have increased alkaline phosphatase (ALP) activity compared with freshly isolated monocytes or HUVECs. γ-GT (A) and ALP (B) activity of freshly isolated monocytes, ELMCs, HUVECs, and MBECs were compared (n = 3). Results are expressed as enzyme activity per microgram of protein. These experiments were performed 4 times with similar results.
Human peripheral blood monocytes express endothelial markers when cultured with proangiogenic cytokines
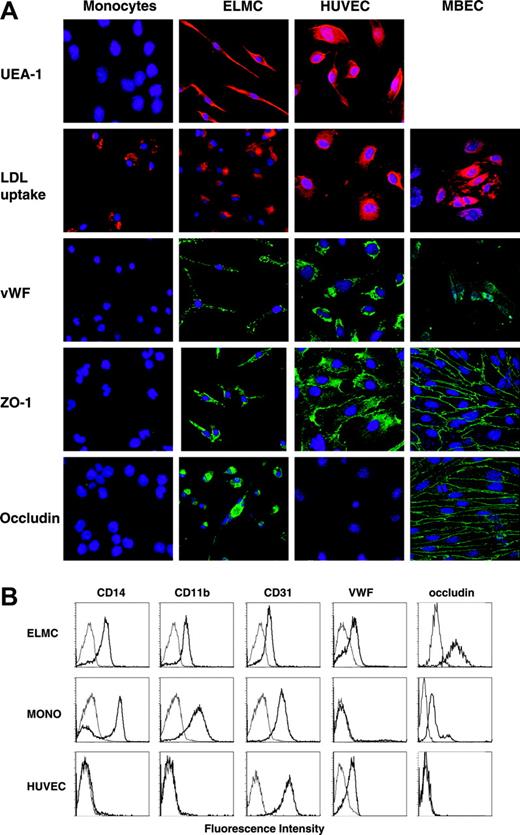
In order to better define the cellular phenotype of the ELMCs once they had formed an intact barrier, we examined their immunophenotype. ELMCs express the endothelial-specific marker von Willebrand factor, and were stained with the endothelial-specific lectin UEA-1 (Figure 4). In addition, the cells continued to express markers common to cells of both the monocytic and endothelial lineages such as acetylated LDL uptake and CD31 staining (Figure 4). They also retained the expression of the monocyte markers CD14 and CD11b (Figure 4). ELMCs also express the tight junction proteins ZO-1 and occludin, whereas freshly isolated monocytes did not (Figure 4). Taken together, these data demonstrate that ELMCs have an immunophenotype intermediary between BBB-derived endothelial cells and monocytes.
Immunohistochemical and immunocytochemical analysis of ELMCs. (A) Freshly isolated human peripheral blood CD14+ monocytes, ELMCs, HUVECs, and MBECs were evaluated for uptake of DiI Ac-LDL, expression of VWF, ZO-1, and occludin by immunohistochemistry. ELMCs, HUVECs, and freshly isolated monocytes were also evaluated for binding of the endothelial-specific lectin UEA-1. (B) FACS analysis of freshly isolated monocytes, ELMCs, and HUVECs for expression of CD14, CD11b, CD31, VWF, and occludin was performed.
Immunohistochemical and immunocytochemical analysis of ELMCs. (A) Freshly isolated human peripheral blood CD14+ monocytes, ELMCs, HUVECs, and MBECs were evaluated for uptake of DiI Ac-LDL, expression of VWF, ZO-1, and occludin by immunohistochemistry. ELMCs, HUVECs, and freshly isolated monocytes were also evaluated for binding of the endothelial-specific lectin UEA-1. (B) FACS analysis of freshly isolated monocytes, ELMCs, and HUVECs for expression of CD14, CD11b, CD31, VWF, and occludin was performed.
Bone marrow-derived cells incorporate into the repairing vasculature in the injured brain. Stab injury to the brain was performed on Swiss mice. Zero to 12 days after injury the mice were injected intravenously with FITC-labeled albumin and perfused with PBS 10 minutes after injection. Sections through the area of brain injury show leakage of FITC-labeled albumin from the vasculature that is no longer evident by day 12 (A). Bone marrow transplantations were performed on SCID mice using marrow from FVB/N-TgN (Tie2-LacZ)182 Sato mice that express β-galactosidase specifically in endothelial cells. One month after transplantation, stab injury of the brain was performed. Three to 5 days after the injury the animals were killed and sections through the injured area were stained with anti-β-galactosidase (green) and the endothelial marker CD31 (red). Fluorescence microscopy shows incorporation of β-galactosidase-expressing cells into the repairing vasculature. Scale bars represent 20 mm (B) and 100 mm (C).
Bone marrow-derived cells incorporate into the repairing vasculature in the injured brain. Stab injury to the brain was performed on Swiss mice. Zero to 12 days after injury the mice were injected intravenously with FITC-labeled albumin and perfused with PBS 10 minutes after injection. Sections through the area of brain injury show leakage of FITC-labeled albumin from the vasculature that is no longer evident by day 12 (A). Bone marrow transplantations were performed on SCID mice using marrow from FVB/N-TgN (Tie2-LacZ)182 Sato mice that express β-galactosidase specifically in endothelial cells. One month after transplantation, stab injury of the brain was performed. Three to 5 days after the injury the animals were killed and sections through the injured area were stained with anti-β-galactosidase (green) and the endothelial marker CD31 (red). Fluorescence microscopy shows incorporation of β-galactosidase-expressing cells into the repairing vasculature. Scale bars represent 20 mm (B) and 100 mm (C).
Bone marrow-derived cells localize to the repairing vasculature after brain injury
It has been demonstrated that bone marrow-derived cells can incorporate into areas of new vasculature formation in both pathologic and physiologic conditions.2,3,20-29 A bone marrow transplantation was performed on SCID mice using donor marrow from FVB/N-TgN (Tie2-LacZ)182Sato mice. These mice express β-galactosidase driven by the Tie2 promoter specifically in endothelial cells. A stab injury was performed on the mice 1 month following transplantation. Three to 5 days after the injury, the mice were killed and sections of the injured brain were stained for β-galactosidase and endothelial markers. Bone marrow-derived cells were present, and incorporated into the vasculature in the area of injury (Figure 5). Thus, hematopoietic cells appear to participate in repair of the vasculature in the brain.
Labeled ELMCs localize to vasculature at sites of injury
To more directly demonstrate the homing of ELMCs to the repairing cerebral vasculature, 2 distinct injury models were used. Initial experiments used a stab injury model in SCID mice. Twenty-four hours after injury DiI-labeled human ELMCs were injected via tail vein and 3 to 5 days later the animals were killed and brains sectioned and stained for endothelial markers. DiI-labeled cells localized to repairing vasculature at sites of brain injury and were seen in a perivascular location (Figure 6A-C).
ELMCs participate in vascular repair after brain injury. Stab brain injury was performed on SCID mice and DiI-labeled ELMCs were administered to mice via tail vein 24 hours after injury. Three to 5 days later the mice were killed and sections through the area of injury were stained for the endothelial markers CD105 and ZO-1. DiI-labeled ELMCs (red) and vascular endothelial cells stained with CD105 (green) in panels A and B and ZO-1 (green) (C) are shown. Labeled ELMCs can be seen in a perivascular location. Scale bars are 20 mm (A, B) and 10 mm (C). ELMCs are located at the periphery of the injury site in a cold brain injury. Cold injury was performed on SCID mice. DiI-labeled human ELMCs, freshly isolated CD14+ monocytes, or CD14-depleted peripheral blood mononuclear cells were injected via tail vein 24 hours after injury. Mice were killed 5 days after injury and sections were stained for glial fibrillary acidic protein (GFAP) (D). Hematoxylin and eosin (H&E) sections of the corresponding injury site are shown for comparison (D). Red-labeled uncultured monocytes and ELMCs can be seen at the periphery of the injury site. CD14-depleted cells are not present. Reactive astrocytes around the injury site are stained with GFAP (green). ELMCs will localize to injuries at non-CNS sites. Skin punch biopsy was performed on the backs of SCID mice. DiI-labeled ELMCs were administered to mice via tail vein 24 hours after injury. Five days after injury the mice were killed and sections through the area of injury were obtained. DiI-labeled ELMCs (red) (E). Nuclei are labeled with DAPI.
ELMCs participate in vascular repair after brain injury. Stab brain injury was performed on SCID mice and DiI-labeled ELMCs were administered to mice via tail vein 24 hours after injury. Three to 5 days later the mice were killed and sections through the area of injury were stained for the endothelial markers CD105 and ZO-1. DiI-labeled ELMCs (red) and vascular endothelial cells stained with CD105 (green) in panels A and B and ZO-1 (green) (C) are shown. Labeled ELMCs can be seen in a perivascular location. Scale bars are 20 mm (A, B) and 10 mm (C). ELMCs are located at the periphery of the injury site in a cold brain injury. Cold injury was performed on SCID mice. DiI-labeled human ELMCs, freshly isolated CD14+ monocytes, or CD14-depleted peripheral blood mononuclear cells were injected via tail vein 24 hours after injury. Mice were killed 5 days after injury and sections were stained for glial fibrillary acidic protein (GFAP) (D). Hematoxylin and eosin (H&E) sections of the corresponding injury site are shown for comparison (D). Red-labeled uncultured monocytes and ELMCs can be seen at the periphery of the injury site. CD14-depleted cells are not present. Reactive astrocytes around the injury site are stained with GFAP (green). ELMCs will localize to injuries at non-CNS sites. Skin punch biopsy was performed on the backs of SCID mice. DiI-labeled ELMCs were administered to mice via tail vein 24 hours after injury. Five days after injury the mice were killed and sections through the area of injury were obtained. DiI-labeled ELMCs (red) (E). Nuclei are labeled with DAPI.
To more completely assess the time course and microanatomical location of the ELMCs after injury, a cold injury model was employed. One day after cold injury, labeled human ELMCs, freshly isolated monocytes, or freshly isolated CD14-depleted cells were injected via tail vein. Mice were killed at either 5 days or 4 weeks after injury. Five days after injury, labeled ELMCs were present at the injury site. Most cells were located at the periphery of the injury, with only rare labeled cells seen in areas of uninjured brain (Figure 6D). Labeled cells could be found in both a perivascular location and in positions more distant from vessels. A similar pattern was seen with uncultured human CD14+ cells (Figure 6D). However, only rare labeled CD14-depleted cells were apparent at the injury site. At 4 weeks after injury neither labeled ELMCs nor newly isolated CD14+ cells were present at the injury site, suggesting that these cells are present transiently during vessel repair.
In order to determine the distribution of injected ELMCs to other organs, the liver, spleen, and lungs of experimental animals were collected. Labeled ELMCs were present in the spleen but were not observed in the lungs or liver (data not shown).
The ability of ELMCs to form a high-resistance barrier suggests that they may have a specific role in brain vasculature. We therefore investigated the ability of ELMCs to home to a site of injury outside of the brain. Skin biopsies were performed on SCID mice and 1 day after biopsy labeled ELMCs were injected via tail vein. The mice were killed 5 days after injury and the skin injury site was sectioned. Labeled ELMCs could also be seen in the area of the skin injury (Figure 6E). This suggests that the ELMC phenotype is not specific to the central nervous system.
Discussion
Recent studies have documented the critical role of hematopoietic cells in vascular growth and repair. Bone marrow-derived endothelial-like cells incorporate into vasculature following ischemic injury to various organs, including the heart,57-60 limb,3,7,20,61,62 retina,21 and brain,29 and also participate in the formation of tumor vasculature.2,3,20,22,25 The magnitude of this contribution has been estimated to be between 2% and 20%.1,26,62
Two broad categories of circulating cells with the capacity to assume endothelial traits have been described.1,63 Cells expressing hematopoietic progenitor markers such as CD34 and CD133 with a high proliferative capacity and the ability to differentiate into mature endothelial cells have been variously categorized as endothelial progenitor cells (EPCs)13,15,24 and outgrowth endothelial cells.63 EPCs express endothelial-specific proteins such as vascular endothelial (VE)-cadherin and do not express myeloid markers such as CD45 and CD14. They also have the capacity to incorporate into growing vasculature.7 Cells of the monocyte/macrophage lineage also express endothelial markers when cultured in the presence of proangiogenic cytokines.6-8,12,16,19,63 However, as our data as well as those of others show, they maintain expression of monocyte markers such as CD14 and CD45.6,19,63,64 The continued expression of myeloid proteins implies that the cells do not trans-differentiate into mature endothelial cells, but rather take on specific endothelial characteristics while remaining inherently monocytic cells. These findings suggest an expanded functional repertoire for monocytes.
Several lines of evidence implicate endothelial-like monocytes in angiogenesis. In addition to expressing endothelial markers, monocytes cultured with proangiogenic cytokines localize to injured vasculature after intravenous injection7 and produce angiogenic growth factors that may act as a stimulus for endothelial cell proliferation.16 They may also play an important role in “drilling” tunnels for new vasculature65,66 and are able to adhere to injured vessel walls and accelerate re-endothelialization.67 Our data add participation in a physiologically regulated blood/tissue barrier to the possible role of monocytes and macrophages in vessel growth.
The ability of CD14+ cells to produce a barrier with a low permeability is unexpected. Although comparable to the in vitro barrier formed by mouse brain microvascular endothelial cells, there are important differences between these 2 cell types. ELMCs form a stringent barrier in the presence of concentrations of VEGF that would be expected to disrupt the BBB39,51 and have a lower level of alkaline phosphatase expression. Other in vitro BBB studies use manipulations such as serum starvation and coculture with astrocytes to obtain significantly higher TCER.34,35,38,46,47 The response of the ELMC barrier to similar conditions remains an area for further study. Additionally, ELMCs localize to sites of injury both inside and outside of the CNS, suggesting that the role of ELMCs in vascular repair is not limited to the brain.
The potential pitfalls of an immunocompromised murine model necessitate that the in vivo data be interpreted with caution. However, taken together, the localization of ELMCs to injury sites in a time frame corresponding to reformation of the BBB, their presence in a perivascular location, and in vitro evidence demonstrating the ability of these cells to form a cellular barrier suggests that they are involved in repair of the vasculature after injury. The homing of unmanipulated CD14+ cells to injury sites implies that peripheral blood monocytes localize to injury sites initially and are then exposed to proangiogenic stimuli and develop endothelial-like characteristics. The absence of injected ELMCs at the injury site after 1 month suggests that their function is transient.
In summary, we show that peripheral blood monocytes form a cellular barrier in vitro with a high TCER and low permeability to larger molecules that is responsive to physiologic stimuli such as bradykinin and histamine. We also provide evidence that monocytes may function in vivo in this capacity by demonstrating that cells from both transplanted bone marrow and exogenously cultured ELMCs localize to repairing vasculature. Thus, a previously unrecognized function of monocytes (or a monocyte subpopulation) may be their participation in the formation of a blood-tissue barrier, a function that has traditionally been within the exclusive domain of endothelial cells. More work is required to better define the importance of this phenotype in normal physiology.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2004-11-4403.
Supported by a grant from the New Jersey Commission on Cancer Research (grant award no. 05-2002-CCR-EO to J.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal