Abstract
Erythropoietin (Epo) stimulation of its receptor's downstream signaling pathways and optimum function of GATA-1 transcription factor are both essential for normal erythroid cell development. Epo-receptor (EpoR) signaling and GATA-1 regulate proliferation, survival, differentiation, and maturation of erythroid cells. Whether any signal that is generated by EpoR targets GATA-1 or affects GATA-1 transcriptional activity is not known. Here, we demonstrate that stimulation of EpoR results in phosphorylation of GATA-1 at serine 310 (S310) in primary fetal liver erythroid progenitors and in cultured erythroid cells. We show that phosphorylation of GATA-1 is important for Epo-induced maturation of fetal liver erythroid progenitor cells. The PI3-kinase/AKT signaling pathway is identified as a mediator of Epo-induced phosphorylation of GATA-1. AKT serine threonine kinase phosphorylates GATA-1S310 in vitro and in erythroid cells and enhances GATA-1 transcriptional activity. These data demonstrate that EpoR signaling phosphorylates GATA-1 and modulates its activity via the PI3-kinase/AKT signaling pathway.
Introduction
Erythropoietin receptor (EpoR) signaling and GATA-1 transcription factor are both required for normal erythroid cell development. Whether EpoR signaling has any effect on GATA-1 function is not known.
The terminal proliferation, differentiation, and maturation of erythroid progenitor cells requires EpoR signaling.1-3 Epo- and EpoR-deficient mice die during embryogenesis with severe anemia because of a lack of postprogenitor maturation of erythroid cells. Binding of Epo to its receptor triggers the phosphorylation and activation of EpoR-bound JAK2 tyrosine kinase, resulting in activation of several downstream signaling pathways that include the phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (PKB or AKT), the STAT5-Bcl-XL, and the ERK/MAPK (reviewed in Ghaffari et al4 ). Under optimum conditions, signaling by cytokine receptors other than EpoR or by exogenously expressed cytoplasmic oncoproteins can also support erythroid differentiation of EpoR-deficient fetal liver progenitor cells.3,5,6 However, erythroid cells produced under these conditions may not undergo a fully normal maturation process or may appear functionally abnormal,6,7 suggesting a specific role for EpoR signaling in inducing erythroid cell differentiation and maturation. In addition, the lack of EpoR signaling in Epo and EpoR mutant embryos cannot be fully compensated for by signaling through cytokine receptors other than EpoR, further illustrating the specificity of EpoR signaling in vivo.
Several studies suggest that the PI3-kinase/AKT signaling pathway may have an important role in supporting erythropoiesis.6,8-10,72 AKT serine threonine kinase of PKB family is a major effector downstream of PI3-kinase, playing fundamental roles in the regulation of cell cycle, survival, differentiation, and intermediary metabolism as demonstrated by the phenotype of mice bearing targeted disruption of AKT genes11-15 (reviewed in Brazil et al16 ). In erythroid cells, AKT is rapidly phosphorylated and activated in response to Epo.17-22
Another key regulator of erythroid development that plays a central role in red cell gene expression is GATA-1 transcription factor. GATA-1 belongs to a family of transcription factors with 2 conserved zinc finger DNA-binding motifs.23 This family currently comprises 6 members that exhibit distinct but overlapping expression pattern and serve essential functions in developing and mature blood cells (GATA-1, -2, and -3) and in nonhematopoietic tissues (GATA-4, -5, and -6). GATA-1 is expressed abundantly in erythroid cells. Functional GATA-binding motifs are found in the regulatory regions of virtually all erythroid-expressed genes encoding globins, heme biosynthetic enzymes, red blood cell transcription factors, and membrane proteins.23 In particular by binding to promoter and enhancer sequences GATA-1 regulates the transcription of EpoR and GATA-1 itself. In addition to erythroid cells, GATA-1 is also expressed in megakaryocytes, eosinophils, mast cells, multipotential hematopoietic cells, and at low levels in Sertoli cells.
Loss of GATA-1 results in fatal embryonic anemia.24 Gene targeting in mice and in vitro differentiation studies of GATA-1- embryonic stem (ES) cells have demonstrated that GATA-1-deficient cells are arrested at a proerythroblast stage and undergo rapid apoptosis.25,26 Like Epo and EpoR, GATA-1 is essential for the survival of erythroid precursors, and their terminal differentiation into red blood cells. GATA-1 serves an antiapoptotic function by regulating the expression of Bcl-XL that is required for erythroid cell viability.27 In fact, by regulating the transcription of many cell-cycle genes,28,29 GATA-1 coordinates a proliferation arrest and an active differentiation program during erythroid maturation. However, GATA-1, like EpoR, is not required for the determination of erythroid lineage from hematopoietic stem cells or for the development of erythroid progenitors.
The cumulative data on EpoR signaling, GATA-1 transcription factor, and erythropoiesis suggest a coordinated cell-specific sequence of events that are initiated by activation of EpoR and its downstream signaling pathways that leads to differentiation and maturation of committed erythroid progenitor cells.27,30,31 Existence of such an orchestrated sequence of events that is triggered by Epo at the cell surface suggests that EpoR signaling affects GATA-1 transcriptional activity. Here, we provide the first direct evidence for an Epo-generated signal that modulates GATA-1 function in erythroid cells.
Material and methods
Cells
E14 wild-type fetal liver cells were isolated as previously described.6,32 The human embryonic kidney 293T cells and murine erythroleukemic (MEL) cells were maintained in Dulbecco Modified Eagle Media (DMEM) containing 10% fetal calf serum (FCS). The murine erythroleukemic HCD57 cells were cultured in Iscoves Modified Dulbecco Media (IMDM) with 20% FCS, 2 U/mL Epo (Amgen, Thousand Oaks, CA), and 10-4 M β-mercaptoethanol. G1E and G1E R2H cells were cultured in IMDM media with 15% FCS, 2 U/mL Epo, 50 ng/mL Steel Factor (SF) (PeproTech, Rocky Hill, NJ) and 1.4 × 10-4 M monothioglycerol (Sigma, St Louis, MO).
Retroviral constructs and plasmids:
The GATA-1 S310A, A310S, S310D, and S310E mutations were created by the Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All GATA-1 WT and mutant cDNAs were cloned into BglII and XhoI sites, upstream of the IRES in the bicistronic retroviral vector MSCV-IRES-GFP (MIG). The desired inserts were also cloned into BamHI and XhoI sites in pGEX 4T1 (Amersham, Piscataway, NJ) and BamHI, KpnI sites in pEBG vector. To generate pMX-Flag-Myr-AKT-IRES-CD2, AKT was inserted by blunt-end ligation into the BglII site of pBI CD2 (a gift of Dr Jonathan Bogan, Yale University School of Medicine). All inserts were verified by DNA sequencing and Western blot analysis. MIG-Flag-Myr-AKT (MIG-AKT*, myristylated constitutively active AKT) and MIG-HA-AKT DN were obtained from Dr Zhou Songyang (Baylor College of Medicine, Waco, TX).
Protein purification
GST-tagged fusion proteins were expressed in Escherichia coli strain BL21 (DE3), purified using the GST purification module (Amersham) according to manufacturer's protocol. The quality of purified fusion proteins was verified by Coomassie Blue staining of the gel.
Flow cytometry
Fetal liver TER 119- cells, enriched for hematopoietic progenitors, were fluorescence-activated cell sorting (FACS) sorted (Becton Dickinson, Franklin Lakes, NJ) and immunostained using TER 119-biotin, CD71-PE antibodies, and streptavidin-APC (BD Pharmingen, San Diego, CA) as previously described.6 CELLQuest (BD Biosciences, San Jose, CA) was used for FACS analysis.
Retroviral transduction
High-titer retroviral supernatants were generated as previously described.6,32 Titers of 2 to 8 × 106/mL were routinely obtained. In brief, TER 119- wild-type (E14) fetal liver cells were resuspended in viral supernatants (2 × 105 cells/mL) at a multiplicity of infection of 5 to 10 and plated on 60-mm retronectin (fibronectin fragments; Takara Biomedicals, Osaka, Japan)-coated dishes in the presence of 100 ng/mL each of interleukin 6 (IL-6) and SF (PeproTech) overnight. Cells were then washed once and resuspended in α-MEM (StemCell Technologies, Vancouver, BC, Canada) containing 15% FCS and the same growth factors with or without Epo for another 24 hours. These conditions allow for the relative expansion of erythroid progenitor cells.6 G1E cells and G1E-R2H were retrovirally transduced using spin infection: 5 × 105 cells/well were seeded in 6-well plates and spun down at 228 g for 5 minutes to obtain a monolayer. Retroviral supernatant was added, cells were spun for 4 hours at 914 g in the presence of polybrene (5 μg/mL), Epo (2 U/mL), and SF (50 ng/mL), washed the next day, and resuspended in their media. Greater than 85% of cells were routinely positive for GFP by FACS analysis, and in most cases further FACS sorting was not required.
Cytospin and cytoplasmic staining
Cells were washed in PBS with 2% FCS, resuspended in PBS containing 1% BSA at a concentration of 3 × 105 cells/mL, and subjected (100 μL/slide) to cytospin for 2 minutes at 600 rpm (Cytospin3; Shandon, ThermoElectron, Waltham, MA). Air-dried slides were stained with Wright Giemsa as previously described.6
Kinase assay
293T cells (5 × 105/well) were transfected with 5 μg AKT constructs, washed 24 hours later, serum starved, and lysed after an additional 24 hours. The proteins were immunoprecipitated with 2 μg anti-Flag (M2) or anti-HA antibody. The AKT immunocomplexes were recovered by binding to protein G-Sepharose beads (Roche, Indianapolis, IN), washed with the lysis buffer (50 mM Tris-NaCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1% NP40, 1.5 mM Na3VO4, 1 mM PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin) and with the kinase buffer (20 mM HEPES, 15 mM MgCl2, and 6 mM DTT). The in vitro kinase reaction33 was performed at 30°C for 1 hour in the presence of 50 μM ATP, 10 μCi (0.37 MBq) [γ-32P]ATP, and 500 ng bacteria-purified glutathione S-transferase (GST) fusion proteins of GATA-1 WT or mutants, purified recombinant (400 ng of each) constitutively active AKT (AKT*), kinase inactive AKT, or constitutively active SGK protein (Upstate Biotechnology, Lake Placid, NY). Samples were resolved by SDS-PAGE, and 32P-labeled proteins were detected by autoradiography.
Western blot analysis
Nuclear extracts were prepared as previously described.34 Samples were boiled in SDS buffer, resolved by SDS-PAGE, and transferred to nitrocellulose membranes that were incubated with the indicated antibodies: (1) anti-GATA-1 (N6) (Santa Cruz Biotechnology, Santa Cruz, CA), (2) anti-GATA-1pS310 antibody, (3) anti-AKT (pS473) (Cell Signaling, Beverly, MA), (4) anti-AKT1 (C-20) (Santa Cruz Biotechnology), (5) anti-topoisomerase I (Santa Cruz Biotechnology), (6) anti-Flag (M2) (Sigma), (7) anti-HA (Roche), and (8) anti-β-actin (Santa Cruz Biotechnology). Rabbit polyclonal anti-phosphoS310 GATA-1 (GATA-1pS310) antibody was produced at Zymed Laboratories (San Francisco, CA) with 2 rounds of peptide-specific affinity purification using affinity gel coupled first with phosphorylated and then with unphosphorylated peptide and tested for specificity. Kinase inhibitors (Calbiochem, La Jolla, CA) were added 1 hour prior to and during the Epo stimulation.
Real-time PCR analysis
Total RNA was isolated using RNeasy Mini Kit (Qiagen) and DNase treated (RNase-Free DNase Set; Qiagen). First-strand cDNA was generated using random hexamers (SuperScript First-Strand Synthesis System for reverse transcriptase-polymerase chain reaction [RT-PCR]; Invitrogen, Carlsbad, CA) from 5 concentrations of total RNA to examine the linear range of standard curve (plot of log initial amount versus Ct) for each primer. On average 200 ng RNA was used for cDNA synthesis. cDNAs were subjected to PCR amplification with serial dilutions to check the amplification efficiency. Real-time PCR was performed in duplicates on LightCycler 2.0 (Roche) using SYBR Green Taq ReadyMix (Sigma) under the following conditions: 95°C for 1 minute and 40 cycles of 95°C for 15 seconds, 60°C for 20 seconds, and 72°C for 30 seconds. Gene-specific primers were designed to span intron-exon boundary by Primer Express 2.0 (ABI Instruments, Foster City, CA) and then subjected to blast analysis to ensure the primer specificity. Melting curve analysis was also performed to check the specificity of product. Relative quantification of gene expression between multiple samples was achieved by normalization against endogenous β-actin and Hprt using the LightCycler Relative Quantification Software (Roche). Primer sequences can be obtained on request. The protocol was approved by the Microarray Shared Facility of Mount Sinai School of Medicine.
Results
GATA-1 is a putative AKT target
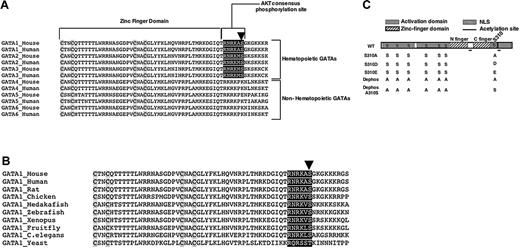
Recent findings support an important role for AKT in erythroid development.10,72 To delineate the AKT signaling pathway supporting erythroid cell maturation, we searched for potential downstream targets of AKT that may be involved in erythroid differentiation. GATA-1 contains a consensus AKT phosphorylation sequence RXRXXS/T35 surrounding serine residue 310 (S310) (Figure 1A). This sequence is also present in the other hematopoietic GATAs (GATA-2 and -3) but not in nonhematopoietic GATAs (GATA-4, -5, and -6) (Figure 1A). This consensus sequence is highly conserved in GATA-1 from yeast to human (Figure 1B).
Interestingly, S310 was previously identified by Crossley and Orkin36 as 1 of the 7 residues in GATA-1 extracted from murine erythroleukemic (MEL) cells that were constitutively phosphorylated (Figure 1C). Among these sites S310 is unique in that more of GATA-1S310 becomes phosphorylated in response to chemically induced differentiation of MEL cells.36 These results suggest that phosphorylation of GATA-1S310 may play a role in the erythroid differentiation process.
Putative AKT consensus phosphorylation site in GATA-1. (A-B) Alignment of the C-terminal zinc finger domain of GATA family of transcription factors. Highly conserved putative AKT consensus phosphorylation sequence surrounding S310 in hematopoietic GATAs (A) and among different species (B) are highlighted in black. Conserved cysteine residues in the C-terminal zinc finger domain are highlighted in gray. Arrow shows the highly conserved serine residue. (C) Schematic of wild-type (WT) GATA-1 protein. The serine (S) to alanine (A), aspartic acid (D), glutamic acid (E), and alanine to serine mutations in GATA-1 constructs are indicated. Nuclear localization sequence (NLS).
Putative AKT consensus phosphorylation site in GATA-1. (A-B) Alignment of the C-terminal zinc finger domain of GATA family of transcription factors. Highly conserved putative AKT consensus phosphorylation sequence surrounding S310 in hematopoietic GATAs (A) and among different species (B) are highlighted in black. Conserved cysteine residues in the C-terminal zinc finger domain are highlighted in gray. Arrow shows the highly conserved serine residue. (C) Schematic of wild-type (WT) GATA-1 protein. The serine (S) to alanine (A), aspartic acid (D), glutamic acid (E), and alanine to serine mutations in GATA-1 constructs are indicated. Nuclear localization sequence (NLS).
Mutation of GATA-1 phosphoacceptor sites creates a dominant-negative protein that blocks maturation of fetal liver erythroid progenitors
The effect of phosphorylation of GATA-1 on erythroid cell differentiation was first investigated. We reasoned that if phosphorylation is required for GATA-1 transactivation activity, phosphoacceptor-deficient mutants of GATA-1 should function as dominant-negative proteins and block Epo-induced differentiation of wild-type fetal liver erythroid progenitors.
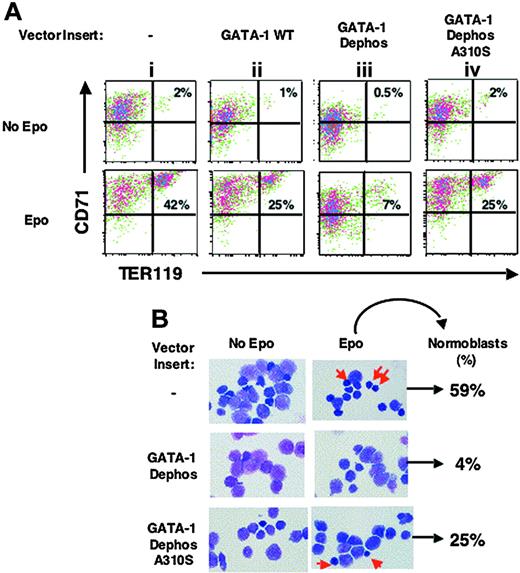
In the absence of Epo, erythroid differentiation was not significantly induced by the expression of any of the retroviral GATA-1 constructs (Figure 2A, top; < 2% of induction of CD71+TER 119+ cells). However, even when Epo was present, overexpression of GATA-1 Dephos blocked significantly erythroid differentiation (Figure 2A, compare iii with ii, in Epo). Overexpression of GATA-1 Dephos A310S, where S310 is added back to GATA-1 Dephos, alleviated the dominant-negative effect of GATA-1 Dephos in cells cultured in Epo (Figure 2A, compare iv with iii). The phenotype of GATA-1 WT- and Dephos A310S-transduced cells was similar (Figure 2A, plots ii and iv). As previously reported,37 ectopic expression of the wild-type GATA-1 had some inhibitory effect on erythroid differentiation of primary cells expressing endogenous GATA-1 (Figure 2A, plots i-ii). A comparison of the results obtained with GATA-1 Dephos and GATA-1 Dephos A310S suggest that mutation of S310 to alanine has a dominant-negative effect on the Epo-induced differentiation. In support of this conclusion, fetal liver progenitors transduced with GATA-1 Dephos A310S and cultured in the presence of Epo formed a significantly higher number of fully mature erythroblasts (normoblasts) than cells expressing GATA-1 Dephos (Figure 2B, 25% versus 4%). Indeed, in the presence of Epo, Dephos A310S-transduced fetal liver progenitor cells formed greater than 40% the number of mature erythroblasts that were formed by control-transduced cells (Figure 2B, 25% versus 59%). In the absence of Epo, retrovirally transduced cells did not form a significant number of normoblasts in culture (< 3% of transduced GFP+ cells; Figure 2B). In this system GATA-1S310A was significantly inhibitory only when cells were cultured in the absence of serum, suggesting that a serum factor may mask the potential effect of single S310 mutation on erythroid differentiation of wild-type fetal liver progenitor cells (data not shown).
Phosphorylation of GATA-1 is required for fetal liver erythroid cell differentiation. (A) TER 119- fetal liver progenitor cells were transduced with an empty retroviral vector (MIG) or vectors containing the indicated inserts and cultured in the presence or absence of Epo; 36 hours later live GFP+ cells were examined for expression of red cell differentiation markers, percentages of CD71+TER 119+ differentiated cells (top right quadrant) in the absence or presence of Epo are shown. Representative of at least 3 independent experiments. (B) Representative field of Wright-Giemsa staining of live GFP+ cells (× 1000) and of differential count: the percentage of highly mature erythroblasts (normoblasts shown by arrows) formed in Epo-stimulated cultures in indicated GFP+-transduced cell populations is shown. Images were captured with a Nikon E600 microscope (Garden City, NJ) with a 100×/0.3 numeric aperture oil immersion lens and an RT slider SPOT 2.3.1 camera (Diagnostic Instuments, Sterling Heights, MI) using SPOT advanced software (version 3.5.9).
Phosphorylation of GATA-1 is required for fetal liver erythroid cell differentiation. (A) TER 119- fetal liver progenitor cells were transduced with an empty retroviral vector (MIG) or vectors containing the indicated inserts and cultured in the presence or absence of Epo; 36 hours later live GFP+ cells were examined for expression of red cell differentiation markers, percentages of CD71+TER 119+ differentiated cells (top right quadrant) in the absence or presence of Epo are shown. Representative of at least 3 independent experiments. (B) Representative field of Wright-Giemsa staining of live GFP+ cells (× 1000) and of differential count: the percentage of highly mature erythroblasts (normoblasts shown by arrows) formed in Epo-stimulated cultures in indicated GFP+-transduced cell populations is shown. Images were captured with a Nikon E600 microscope (Garden City, NJ) with a 100×/0.3 numeric aperture oil immersion lens and an RT slider SPOT 2.3.1 camera (Diagnostic Instuments, Sterling Heights, MI) using SPOT advanced software (version 3.5.9).
Taken together these results suggest that (1) multiple serine residues within the 7 mutated in GATA-1 Dephos are required for optimal Epo-induced differentiation and maturation of fetal liver erythroid cells; (2) lack of S10 alone blocks significantly differentiation of fetal liver erythroid progenitor cells in the presence of Epo.
Epo induces phosphorylation of GATA-1S310, a putative AKT target, in a PI3-kinase-dependent manner
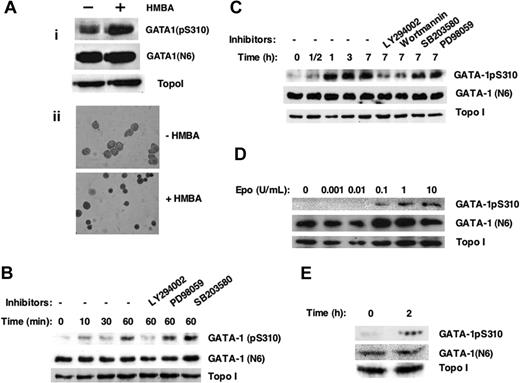
Next, to investigate whether GATA-1S310 is a direct target of EpoR signaling an anti-GATA-1pS310 specific antibody was raised (Figure 3). As anticipated,36 the anti-GATA-1pS310 antibody detected more of the phosphorylated form of GATA-1S310 in nuclear extracts (Figure 3Ai) of differentiated versus undifferentiated MEL cells (Figure 3Aii). Epo-induced phosphorylation of GATA-1S310 was detected within 10 minutes in nuclear extracts of the Epo-dependent erythroleukemic HCD57 cells (Figure 3B). GATA-1 phosphorylation increased with time (Figure 3B-C). In addition, phosphorylation of GATA-1S310 was inhibited specifically by PI3-kinase inhibitors Wortmannin and LY294002 but not by specific inhibitors for the p38 MAP kinase (SB203580) or ERK/MAP kinase (PD98059) (Figure 3B-C), indicating that phosphorylation of GATA-1S310 was dependent on activation of PI3-kinase but not MAP kinase. Phosphorylation levels of GATA-1S310 were dependent on the concentrations of Epo (Figure 3D). Importantly, GATA-1S310 was also phosphorylated in response to Epo stimulation of fetal liver erythroid progenitor cells (Figure 3E). Together, these data demonstrate that Epo induces phosphorylation of GATA-1 on S310 via a PI3-kinase signaling pathway.
Activated AKT phosphorylates GATA-1 in vitro
Given that Epo stimulation of erythroid cells results in phosphorylation of GATA-1S310 (Figure 3) and S310 is a putative target of AKT serine threonine kinase (Figure 1), it was investigated whether AKT phosphorylates GATA-1. Recombinant active AKT phosphorylated bacteria-purified GST-GATA-1 in vitro (Figure 4A). Serum and glucocorticoid-inducible kinase (SGK) is highly related within its kinase domain to AKT, shares with AKT the consensus substrate recognition sequence RXRXX(S/T),35 and like AKT is activated downstream of PI3-kinase.38 However, active SGK did not phosphorylate GATA-1 (Figure 4A). Thus, AKT's ability to phosphorylate GATA-1S310 seems to be specific.
GATA-1S310 is phosphorylated in response to Epo stimulation of primary fetal liver and cultured erythroid cells. (A) Western blot analysis of nuclear extracts of MEL cells induced to differentiate in the presence or absence of 5 mM HMBA after 96 hours using anti-GATA1 (pS310), anti-GATA1 (N6), or anti-topoisomerase I (Topo I) antibodies (i). Benzidine staining of hemoglobin (ii) confirmed the undifferentiated and differentiated status of MEL cells used (i). (B-C) The Epo-dependent erythroleukemic HCD57 cells were Epo-starved overnight, and 5 × 107 cells were stimulated with Epo (2 U/mL) for the indicated time points, in the presence or absence of inhibitors of PI3-kinase LY294002 (LY; 10 μM), Wortmannin (WO; 100 nM), inhibitor of ERK/MAP kinase PD98059 (PD; 50 μM), or inhibitor of p38 MAP kinase SB203580 (SB; 10 μM). Western blot analysis of nuclear extracts with the indicated antibodies. (D) Cells (from B-C) were stimulated with increasing doses of Epo for 1 hour, and nuclear extracts were subjected to Western blot analysis as in panel B. Results shown are representative of at least 3 independent experiments. (E) Fetal liver cells enriched for erythroid progenitors (TER 119- cells) were starved overnight in vitro in serum-free media (SFEM; StemCell Technologies) in the presence of SF (50 ng/mL) and IL-6 (10 ng/mL) and stimulated or not with Epo (2 U/mL) for 2 hours. Nuclear extracts were subjected to Western blot analysis as in panel B.
GATA-1S310 is phosphorylated in response to Epo stimulation of primary fetal liver and cultured erythroid cells. (A) Western blot analysis of nuclear extracts of MEL cells induced to differentiate in the presence or absence of 5 mM HMBA after 96 hours using anti-GATA1 (pS310), anti-GATA1 (N6), or anti-topoisomerase I (Topo I) antibodies (i). Benzidine staining of hemoglobin (ii) confirmed the undifferentiated and differentiated status of MEL cells used (i). (B-C) The Epo-dependent erythroleukemic HCD57 cells were Epo-starved overnight, and 5 × 107 cells were stimulated with Epo (2 U/mL) for the indicated time points, in the presence or absence of inhibitors of PI3-kinase LY294002 (LY; 10 μM), Wortmannin (WO; 100 nM), inhibitor of ERK/MAP kinase PD98059 (PD; 50 μM), or inhibitor of p38 MAP kinase SB203580 (SB; 10 μM). Western blot analysis of nuclear extracts with the indicated antibodies. (D) Cells (from B-C) were stimulated with increasing doses of Epo for 1 hour, and nuclear extracts were subjected to Western blot analysis as in panel B. Results shown are representative of at least 3 independent experiments. (E) Fetal liver cells enriched for erythroid progenitors (TER 119- cells) were starved overnight in vitro in serum-free media (SFEM; StemCell Technologies) in the presence of SF (50 ng/mL) and IL-6 (10 ng/mL) and stimulated or not with Epo (2 U/mL) for 2 hours. Nuclear extracts were subjected to Western blot analysis as in panel B.
Immunocomplexes of constitutively active AKT phosphorylated wild-type GATA-1 (Figure 4B top, lane 3), and to a much lesser extent a GATA-1 mutant (Figure 1C) having an alanine residue in place of the serine at position 310 (S310A) (Figure 4B top, lane 6) in vitro. Constitutively active AKT did not phosphorylate a GATA-1 mutant (Figure 1C) in which 7 serine residues were mutated to alanine (GATA-1 Dephos) (Figure 4B top, lane 4). However, when a serine was added back to residue 310 of the GATA-1 Dephos mutant (Dephos A310S) (Figure 1C), activated AKT induced its phosphorylation almost to the same extent as GATA-1 wild type (Figure 4B top, lane 5). In addition, following incubation with activated AKT, wild-type GATA-1 and the GATA-1 mutant Dephos A310S, but not GATA-1S310A or GATA-1 Dephos (Figure 4B, lanes 4 and 6), were recognized by the anti-GATA-1pS310 antibody (Figure 4B, lanes 3 and 5; GATA-1pS310).
Activated AKT phosphorylates GATA-1S310. Purified recombinant constitutively active AKT (act), kinase inactive AKT (inact), or constitutively active SGK protein (act) (A) or immunocomplexes of Flag-tagged constitutively active AKT (AKT*) or HA-tagged kinase-inactive mutant of AKT (AKT K-D) (B) were used in an in vitro kinase assay and incubated in the presence of [γ-32P]ATP with GST-GATA-1 WT or mutants as substrates. Samples were resolved on SDS-PAGE, and 32P-labeled proteins were detected by autoradiography (A-B). Half of the reaction was subjected to Western blot analysis using the indicated antibodies (B). (C) 293T cells were cotransfected with GST alone or GST-GATA-1 wild type or mutants and a Flag-tagged constitutively active AKT (AKT*) or a dominant-negative AKT (HA-AKT DN), lysates were prepared 48 hours later and subjected to Western blot analysis by using the indicated antibodies.
Activated AKT phosphorylates GATA-1S310. Purified recombinant constitutively active AKT (act), kinase inactive AKT (inact), or constitutively active SGK protein (act) (A) or immunocomplexes of Flag-tagged constitutively active AKT (AKT*) or HA-tagged kinase-inactive mutant of AKT (AKT K-D) (B) were used in an in vitro kinase assay and incubated in the presence of [γ-32P]ATP with GST-GATA-1 WT or mutants as substrates. Samples were resolved on SDS-PAGE, and 32P-labeled proteins were detected by autoradiography (A-B). Half of the reaction was subjected to Western blot analysis using the indicated antibodies (B). (C) 293T cells were cotransfected with GST alone or GST-GATA-1 wild type or mutants and a Flag-tagged constitutively active AKT (AKT*) or a dominant-negative AKT (HA-AKT DN), lysates were prepared 48 hours later and subjected to Western blot analysis by using the indicated antibodies.
In addition, overexpressed (Figure 4C, lanes 5 and 7) or endogenous constitutively active AKT (Figure 4C, lane 9) but not kinase-dead AKT (Figure 4C, lane 2) phosphorylated GATA-1S310 in 293T cells.
These combined data demonstrate that GATA-1 is specifically phosphorylated in vitro and in heterologous cells by a constitutively active AKT. S310 is the principal serine residue phosphorylated by AKT, but other serine or threonine residues are also phosphorylated.
Phosphoacceptor sites are required for GATA-1 induction of erythroid maturation in GATA-1-deficient cells
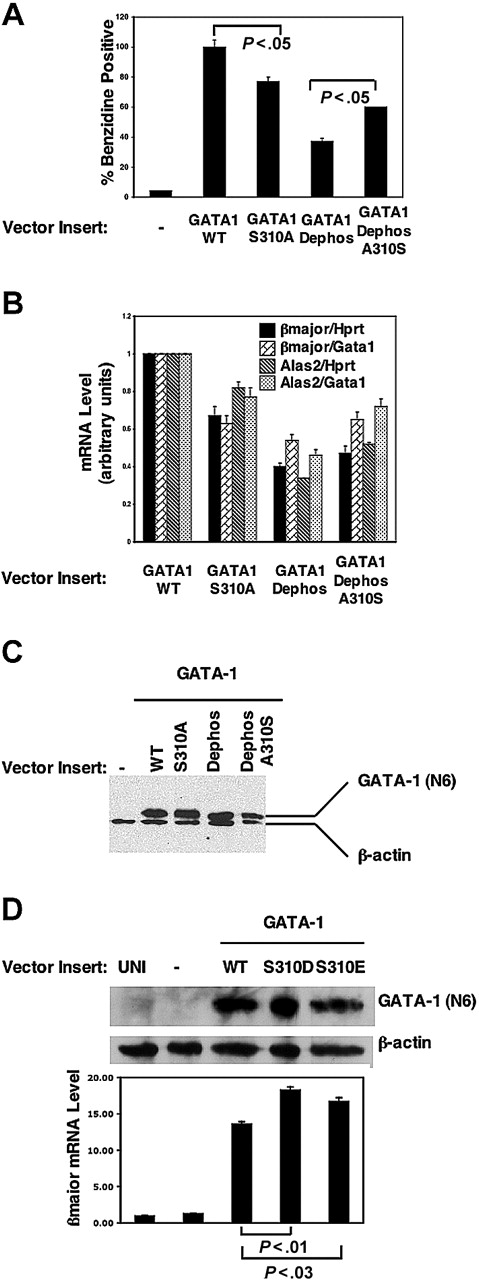
To evaluate the effect of phosphoacceptor sites on GATA-1 transactivation activity in erythroid cells, the differentiation induced by GATA-1 mutants in GATA-1- G1E cells was examined. G1E cells are derived from GATA-1- ES cells and are developmentally arrested at the precursor stage of CFU-E/proerythroblasts.29,39 G1E cells proliferate continuously in the absence of GATA-1 and are induced to undergo erythroid differentiation when GATA-1 is introduced, making G1E cells a stringent system for evaluating GATA-1 transactivation activity.39 Introduction of GATA-1 with a mutation of S310A reduced the differentiation of G1E cells, as measured by diaminobenzidine staining of hemoglobin, by 25% as compared with differentiation induced by WT GATA-1 (Figure 5A) and inhibited the expression of β-globin by almost 40% (Figure 5B). The levels of GATA-1S310A expression, as determined by GFP expression, correlated inversely with the degree of maturity of G1E cells (data not shown), further suggesting that phosphorylation of S310 modulates transactivation activity of GATA-1. Erythroid differentiation and expression of erythroid specific genes, β-globin and δ-aminolevulinate synthase-2 (Alas2) of G1E cells overexpressing GATA-1 Dephos, was reduced by greater than 60% as compared with wild-type GATA-1 (Figure 5A-B). The lack of differentiation of GATA-1 Dephos-expressing cells was accompanied by an increased DNA synthesis (data not shown). Readdition of only serine 310 on the GATA-1 Dephos backbone enhanced significantly erythroid differentiation (Figure 5A) and expression of erythroid specific genes (Figure 5B) as compared with cells transduced with GATA-1 Dephos. The differences in erythroid maturation induced by mutants could not be attributed to variations in GATA-1 mRNA or protein expression (Figure 5B-C). Similarly, introduction of GATA-1 bearing phosphomimetic mutations at S310 (GATA-1S310D or GATA-1 S310E) induced higher levels of β-globin expression than GATA-1 wild type in G1E cells cultured in a suboptimum dose of Epo (Figure 5D). These results suggest that (1) phosphorylation of GATA-1 on multiple residues is required for its transactivation activity in inducing erythroid differentiation and maturation; (2) S310 is required for optimum activity of GATA-1 and supports significant erythroid maturation in the absence of the other 6 phosphoacceptor sites.
AKT phosphorylates GATA-1 and enhances its activity in erythroid cells
Next, the potential of AKT to phosphorylate GATA-1S310 in erythroid cells was investigated. G1E-R2H cells are a subclone of GATA-1- G1E cells engineered to stably express GATA-1 protein fused to the ligand binding domain of the estrogen receptor. G1E-R2H cells proliferate without differentiation in the absence of induced nuclear translocation of GATA-1. Addition of β-estradiol induces the transactivation activity of GATA-1 in the nucleus which triggers differentiation of G1E-R2H cells to mature erythroblasts.27
Phosphorylation enhances GATA-1 transactivation activity. (A) GATA-1-deficient G1E cells were transduced with an empty retroviral vector (MIG) alone or vectors containing the indicated inserts, and 48 hours later the differentiation was analyzed by diaminobenzidine staining of hemoglobin in total cells in the absence of cell sorting. Results are expressed as percentages of positive control GATA-1 WT-transduced cells. P values determined by Student t test (GATA-1S310A versus wild type, n = 6; GATA-1 A310S versus Dephos, n = 3). (B) Real-time PCR analysis of gene expression in total cells from panel A. Results shown are normalized to both Gata1 and Hprt mRNA expression. (C) Western blot analysis of GATA-1 protein expression in G1E cells after 48 hours of retroviral transduction. (D) Experiments performed as in panel A, using GATA-1 phosphomimetic mutants to transduce G1E cells cultured in 0.1 U/mL Epo. Real-time PCR analysis, representative Western blot analysis of GATA-1 mutant protein expression in transduced G1E cells. UNT indicates untransduced. In panels A, B, and D, values shown are mean ± SEM.
Phosphorylation enhances GATA-1 transactivation activity. (A) GATA-1-deficient G1E cells were transduced with an empty retroviral vector (MIG) alone or vectors containing the indicated inserts, and 48 hours later the differentiation was analyzed by diaminobenzidine staining of hemoglobin in total cells in the absence of cell sorting. Results are expressed as percentages of positive control GATA-1 WT-transduced cells. P values determined by Student t test (GATA-1S310A versus wild type, n = 6; GATA-1 A310S versus Dephos, n = 3). (B) Real-time PCR analysis of gene expression in total cells from panel A. Results shown are normalized to both Gata1 and Hprt mRNA expression. (C) Western blot analysis of GATA-1 protein expression in G1E cells after 48 hours of retroviral transduction. (D) Experiments performed as in panel A, using GATA-1 phosphomimetic mutants to transduce G1E cells cultured in 0.1 U/mL Epo. Real-time PCR analysis, representative Western blot analysis of GATA-1 mutant protein expression in transduced G1E cells. UNT indicates untransduced. In panels A, B, and D, values shown are mean ± SEM.
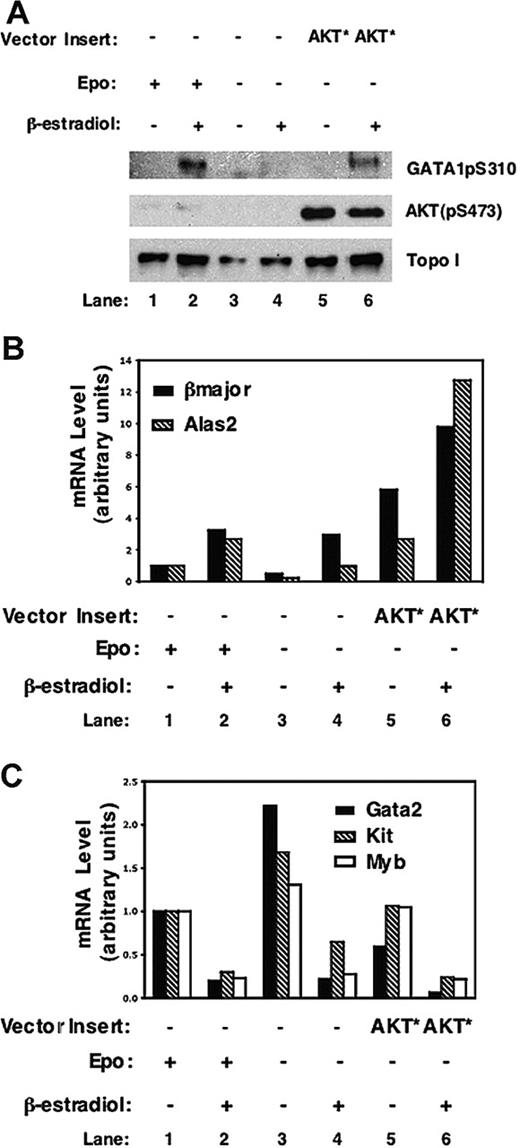
GATA-1pS310 was readily detected in the nuclear extracts of G1E-R2H cells only when these cells were stimulated with β-estradiol and either incubated with Epo or transduced with activated AKT (AKT*) (Figure 6A, lanes 2 and 6). These findings further confirmed the ability of activated AKT* to phosphorylate GATA-1S310 in cells (Figure 4C). As anticipated, phosphorylated AKT was also found in the nucleus (Figure 6, lanes 5 and 6) where it may phosphorylate its targets.40,41 The phosphorylation of GATA-1S310 was concomitant with transactivation of GATA-1 responsive genes by active AKT* in G1E-R2H cells (Figure 6B). Activated AKT and GATA-1 had a synergistic effect on the expression of Alas2 because AKT* enhanced the expression of Alas2 by 13-fold as compared with the effect of GATA-1 translocation alone (Figure 6B, lanes 4 and 6). The cooperation of GATA-1 and activated AKT on the expression of β-globin seemed to be additive in G1E-R2H cells (Figure 6B, lanes 4-6). During the erythroid differentiation program, GATA-1 positively up-regulates red cell gene expression, whereas it represses the expression of Gata2 and mitogenic genes such as Kit and Myb.28,30 Thus, as predicted by a model in which AKT induction of erythroid cell differentiation and maturation is mediated by enhancing GATA-1 activity, the induction of erythroid genes in AKT*-transduced cells was concomitant with its inhibition of expression of Gata2, Myb, and Kit (Figure 6C). In repressing the expression of these genes, activated AKT mimicked the effect of Epo whether GATA-1 was nuclear or not (Figure 6C, lanes 1 and 5; and lanes 2 and 6). When GATA-1 was cytoplasmic (ie, in the absence of β-estradiol), significantly higher levels of expression of erythroid genes were observed in cells with ectopic expression of activated AKT as compared with the Epo-stimulated cells (Figure 6B, lanes 1 and 5). Overexpression of activated AKT may enhance, more efficiently than Epo, GATA-1-independent mechanisms25 that operate in GATA-1-deficient cells to induce red cell genes at very low levels (Figure 6B, lanes 1 and 5, compare also effect of Epo alone lanes 1 and 3). This is likely because ectopic expression of activated AKT in GATA-1-deficient G1E cells has some minimal effect above the background on the expression of erythroid specific genes (Figure 7). Because AKT does not regulate the ligand binding moiety within the chimeric protein,42 it cannot translocate GATA-1. Alternatively, AKT* may directly phosphorylate and activate GATA-1 in the absence of β-estradiol, because relative activation of red cell genes induced by constitutively active AKT* (Figure 6B, lanes 3 and 5) concurred with repression of Gata2, Kit, and Myb (Figure 6C, lanes 3 and 5).
Activated AKT phosphorylates and enhances GATA-1 transactivation activity in erythroid cells. (A) G1E-R2H cells were retrovirally transduced with an empty vector (MIG) or MIG-AKT* and cultured in the presence or absence of Epo for 16 hours before adding β-estradiol (10-7 M). Nuclear extracts were prepared 7 hours later and subjected to Western blot analysis using the indicated antibodies. Total RNA was prepared from an aliquot of cells from panel A and subjected to real-time PCR for analysis of genes whose expression is up-regulated (B) or repressed (C) by nuclear GATA-1. Representative graphs from 3 independent experiments are shown.
Activated AKT phosphorylates and enhances GATA-1 transactivation activity in erythroid cells. (A) G1E-R2H cells were retrovirally transduced with an empty vector (MIG) or MIG-AKT* and cultured in the presence or absence of Epo for 16 hours before adding β-estradiol (10-7 M). Nuclear extracts were prepared 7 hours later and subjected to Western blot analysis using the indicated antibodies. Total RNA was prepared from an aliquot of cells from panel A and subjected to real-time PCR for analysis of genes whose expression is up-regulated (B) or repressed (C) by nuclear GATA-1. Representative graphs from 3 independent experiments are shown.
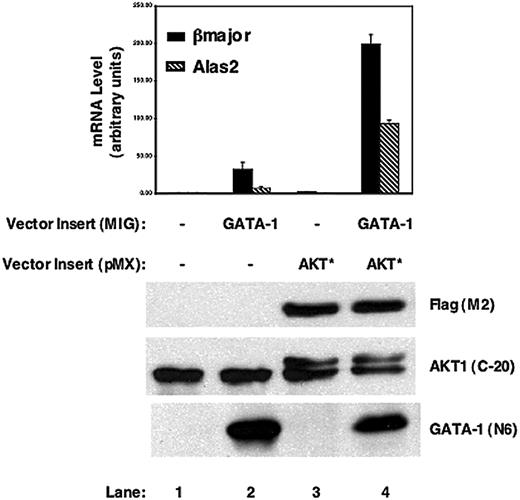
To conclusively determine whether AKT cooperates with GATA-1 in regulating erythroid gene expression, AKT and GATA-1 were introduced from 2 distinct retroviral vectors in GATA-1-deficient G1E cells, and the levels of expression of erythroid genes were measured (Figure 7). Overexpression of active AKT* up-regulated above the background by 2- to 3-fold the levels of β-globin and Alas2 expression (Figure 7, lane 3) in G1E cells significantly below the levels induced by GATA-1 alone (Figure 7, lane 2) in these cells, suggesting that AKT may indeed enhance mildly GATA-1-independent pathways to regulate red cell genes in G1E cells. Interestingly, introduction of AKT and GATA-1 together in G1E cells had a synergistic effect on the levels of β-globin and Alas2 expression, suggesting that AKT enhances GATA-1 transactivation activity.
In addition to GATA-1 phosphorylation, activated AKT may enhance the transactivation activity of GATA-1 in complex with a coactivator such as the CREB-binding protein (CBP), because it has been shown for AKT regulation of the neuronal bHLH protein complex formation with CBP.43 Given the multifaceted functions of AKT, its interactions with many regulatory proteins and the potency of AKT effect on inducing red cell differentiation, it would not be surprising if the observed effects were the result of the combination of more than one regulatory mechanism by which AKT exerts its effects in erythroid cells.
AKT and GATA-1 synergize in up-regulating red cell gene expression in G1E cells. GATA-1 and constitutively active AKT* were retrovirally transduced in G1E cells in the absence of Epo; 24 hours later, cells were FACS sorted and cultured for another 24 hours before preparing RNA for real-time PCR analysis (top) and protein for Western blot analysis (bottom) using the indicated antibodies. One of 2 independent experiments is shown.
AKT and GATA-1 synergize in up-regulating red cell gene expression in G1E cells. GATA-1 and constitutively active AKT* were retrovirally transduced in G1E cells in the absence of Epo; 24 hours later, cells were FACS sorted and cultured for another 24 hours before preparing RNA for real-time PCR analysis (top) and protein for Western blot analysis (bottom) using the indicated antibodies. One of 2 independent experiments is shown.
Together these results demonstrate that activated AKT phosphorylates GATA-1 and enhances the expression of erythroid-specific genes by modulating GATA-1-dependent and -independent mechanisms in erythroid cells.
Discussion
Our key finding is that erythropoietin stimulation of erythroid cells results in phosphorylation and activation of GATA-1 transcription factor. The data presented here demonstrate that phosphorylation of GATA-1 is required for erythroid differentiation of fetal liver progenitor cells. In addition, phosphorylation of S310 is essential for optimum GATA-1 activity in erythroid cells (Figure 5). We have identified the PI3-kinase/AKT pathway as a mediator of EpoR-stimulation of phosphorylation and regulation of GATA-1 function. AKT phosphorylates GATA-1S310 in vitro and in vivo and enhances GATA-1 activity in erythroid cells.
Crossley and Orkin36 have previously shown that S310 is constitutively phosphorylated in GATA-1 extracted from MEL cells, and phosphorylated GATA-1S310 increases during DMSO-induced MEL cell differentiation. However, phosphorylation of S310 was not found to have any effect on DNA binding, transactivation activity, or DNA bending of GATA-1.36 These results may be due to the cellular context in which GATA-1 is synthesized,44,45 because increased phosphorylation of GATA-1 enhances its binding affinity for DNA during erythroid differentiation of K562 but not MEL cells.45
At the late CFU-E/proerythroblast stage of erythroid differentiation, during a temporal window in the sequence of maturation and completion of the erythroid program, GATA-1 and EpoR signaling act in concert. The transition to subsequent stages of differentiation requires both EpoR and GATA-1 function (Figure 6) and their coordinated activity.28,30 It is becoming increasingly clear that during this sequence of events, EpoR signaling is required for the optimum activity of at least some of GATA-1 functions in orchestrating the full erythroid maturation such as in the induction of β-globin, BclXL, and EpoR genes (Figure 6).27,31 On the basis of our findings we propose a model of erythroid differentiation program during which the activity of EpoR signaling and GATA-1 transcription factor is coordinated, at this transition stage, by Epo-induced phosphorylation of GATA-1 that is mediated by the PI3-kinase/AKT signaling pathway.
Serine 310 is one GATA-1 residue targeted by EpoR signaling that accounts for a fraction of GATA-1 ability to support normal erythroid differentiation. Because levels of GATA-1 expression and activity are directly correlated with the physiologic erythropoietic rate,46 a moderate modulation of GATA-1 activity may be of major significance during situations of acute demands such as blood loss or hemolysis.47 On the basis of our findings we predict that regulation of the phosphorylation status of GATA-1S310 alone, albeit subtle, may participate in modulating the GATA-1 activity in determining the erythropoietic rate in response to available Epo and the amplitude of EpoR signaling. Such a model is in agreement with the notion that cytokine dosage modulates the fate of hematopoietic cells.48,49 It is not clear how Epo-induced phosphorylation may modulate GATA-1 activity. The nuclear magnetic resonance (NMR) analysis of the structure of the carboxy-terminal region of chicken GATA-1 bound to DNA encompasses GATA-1S310 and its flanking sequences.50 According to an analysis of the NMR structure of GATA-1 in solution (Steve Johnston, Biocomputing, Whitehead Institute for Biomedical Research, Cambridge, MA), GATA-1S310 is free of contact from the sugar-phosphate backbone in the minor groove of DNA which suggests that phosphorylation of GATA-1 at this site may regulate its interaction with other protein(s). Phosphorylation of GATA-1 may therefore be involved in distinct biologic functions such as the stability of GATA-1 protein, its subcellular localization ([S310] is within a nuclear localization sequence),36,51 or its acetylation by CBP/p300 transcriptional coactivators.52-54 In support of this latter hypothesis, note that S310 falls within the C-terminal acetylation motif of GATA-1 that is required for CBP to bind GATA-1 and stimulates its transcriptional activity in support of erythroid differentiation.52-54 Many proteins, including transcription factors such as neuronal bHLH, p53, and CREB, recruit and interact with CBP/p300 through their phosphorylated residues.43,55,56 It is therefore tempting to speculate that phosphorylation of S310 may facilitate the recruitment of CBP by GATA-1. Alternatively, phosphorylation of GATA-1 may modulate its affinity for regulatory domains of certain genes as has been previously suggested.45
Abnormal expression or mutations of GATA-1 are associated with leukemias in children with Down syndrome, familial dyserythropoietic anemia, thrombocytopenia, and myelodysplastic syndromes (reviewed in Gurbuxani et al57 ). Whether mutations of GATA-1 phosphoacceptor sites have any pathophysiologic consequences is not known, but our data are consistent with a potential role for these sites in oncogenic transformation or in anemias.
Given the importance of AKT in coordinating signaling pathways to balance cellular outcome, and the essential role of GATA-1 in coordinating cell proliferation, differentiation, and survival, it is perhaps not surprising for AKT to mediate Epo regulation of GATA-1 activity. However, the effect of AKT during the program of erythroid maturation does not appear to be limited to phosphorylation of GATA-1S310 (compare Figures 5, 6B, and 7). In particular, AKT or other PI3-kinase-dependent kinases may phosphorylate additional serine or threonine residues in GATA-1.58,59 These additional sites may also be targets of other EpoR-JAK2-dependent signaling pathways such as the MAPK pathway.60
Abnormalities of the PI3-kinase/AKT signaling pathway may be particularly relevant to patients with polycythemia vera (PV). PV is a myeloproliferative and clonal disease of multipotential hematopoietic progenitor cells characterized by an enhanced erythropoiesis and increased red cell mass.61 It was recently uncovered that a single dominant-acting mutation in JAK2 tyrosine kinase leads to constitutive signaling in the majority of patients with polycythemia vera.62-66 Interestingly, the Epo-independent terminal differentiation of PV erythroid progenitors was found to involve the PI3-kinase but not the MAP-kinase signaling pathway by at least one of these groups,67 suggesting that enhanced AKT phosphorylation,66 and activation of its downstream signaling,68 may have a dominant role in erythropoiesis of patients with polycythemia vera. In this context, note that PV progenitors are hypersensitive to insulin-like growth factor-1 (IGF-1), a major stimulator of AKT, that is increased in patients' peripheral blood.69,70 However, it is not clear whether IGF-1 stimulation of normal or PV human erythroid progenitors activates AKT.71 Whether abnormal GATA-1 expression or function is associated with PV is not known.
In summary, we have uncovered an Epo-generated signal that targets GATA-1. AKT is a mediator of Epo modulation of GATA-1 and may coordinate EpoR signaling and GATA-1 activities during hematopoietic cell differentiation. Given the major functions of GATA transcription factors we believe these findings may have important implications for the cytokine control of transcription factors in normal and leukemic hematopoiesis.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-06-2516.
Supported by a National Cancer Institute Clinician Scientist Career Award (NIH5K08 CA77675) (S.G.), Mount Sinai School of Medicine Foundation fund (S.G.), and the National Institutes of Health (NIH) (grant NIH/NHLBI P01 no. HL32262) (H.F.L.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Zhou Songyang (Baylor College of Medicine) for AKT constructs, Dr Stuart Orkin (Harvard Medical School) for pXM-GATA-1 WT and pXM-GATA-1 Dephos constructs and G1E/G1E-R2H cells, and Dr James Bieker (Mount Sinai) for MEL cells. We also thank Dr Steve Johnston (Bioinformatic Group, Whitehead Institute) for help with Bioinformatics; Glenn Paradis (MIT, Cancer Center) and Italas George (Mount Sinai) for flow cytometry; and Drs Stefan Constantinescu, Stuart Orkin, James Bieker, Gerd Blobel, Mitchell Weiss and Heather Rooke for helpful discussions. We thank Drs Gordon Keller, Bill Schiemann, and Nai-Wen Chi for critically reading the manuscript.

![Figure 4. Activated AKT phosphorylates GATA-1S310. Purified recombinant constitutively active AKT (act), kinase inactive AKT (inact), or constitutively active SGK protein (act) (A) or immunocomplexes of Flag-tagged constitutively active AKT (AKT*) or HA-tagged kinase-inactive mutant of AKT (AKT K-D) (B) were used in an in vitro kinase assay and incubated in the presence of [γ-32P]ATP with GST-GATA-1 WT or mutants as substrates. Samples were resolved on SDS-PAGE, and 32P-labeled proteins were detected by autoradiography (A-B). Half of the reaction was subjected to Western blot analysis using the indicated antibodies (B). (C) 293T cells were cotransfected with GST alone or GST-GATA-1 wild type or mutants and a Flag-tagged constitutively active AKT (AKT*) or a dominant-negative AKT (HA-AKT DN), lysates were prepared 48 hours later and subjected to Western blot analysis by using the indicated antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-06-2516/4/m_zh80030690270004.jpeg?Expires=1767725940&Signature=n-HED7tD11HrROpsAycgo5T1g0rxFZrGgR-ElMOUlZykSDAyy8ER1rJ3SorJ1EU3JsKZc5Z~LHSouSX4Fn5WXokg~WalDByeYzODJIlMeSyJ9-pBtQZ~40JwoB0VUOSq6LSgGtdc7ye9Qwp5Ft3v1Tw9qjjroOYyFQo5LFxLg1gqzzlqz0IulXDgRFQqde4jGFwGcDKk4rT75C73aD7O6-qZKP8oQXsTwq7ReJaflI-uP7z9KF2TVdXBIeQX2fupCup17DFWSjWiVHL5Dlo6JJMAYD-cR-WMxH-zypjQOVpaZbP3uNzqw88wuYbdo1cNqs96AmlrTK5UlCQhmt2Jfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal