Abstract
Chemokines are key regulators of hematopoiesis and host defense. We report here that functional expression of the chemokine receptor CXCR4 on human immature CD34+ hematopoietic progenitors was increased as a result of sustained elevation in cellular cAMP by dbcAMP and prostaglandin E2. This effect of cAMP was specifically mediated by PKCζ activity. CXCR4 expression and PKCζ activation by cAMP were decreased after the inhibition of cAMP effector-Rap1 by Spa1 overexpression. Interference with the activation of Rac1, a downstream target of Rap1, prevented the cAMP-induced increase in PKCζ activity and CXCR4 levels. Functional manifestation of the effects of cAMP-elevating agents revealed an increased ability of human CD34+ cells to transmigrate the bone marrow (BM) endothelial layer and adhere to BM stroma in vitro, and it augmented the homing potential to the BM and spleens of immunodeficient mice in a Rac1- and a PKCζ-dependent manner. cAMP- and TNFα-stimulated pathways converged in PKCζ-activated CXCR4 expression and MMP-2/MMP-9 secretion. cAMP treatment had a beneficial effect on CD34+ cell survival in a PKCζ-mediated fashion. Taken together, our data reveal major roles for cAMP-induced PKCζ activation in signaling governing the motility and development of CD34+ cells.
Introduction
Considerable effort has been invested in recent years toward understanding the mechanisms that govern hematopoietic stem/progenitor cell trafficking and development. Chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) is the only known powerful chemoattractant for defined populations of primitive human (CD34+CD38-/low) and murine (Sca-1+Thy-1lowc-kit+Lin-) hematopoietic progenitor cells.1-3 The primary receptor for SDF-1, CXCR4, is expressed on a variety of immature and mature hematopoietic cells and on neuronal, endothelial, and epithelial cells.4-6 Multiple studies have demonstrated major roles for SDF-1/CXCR4 interaction in human stem cell migration in vivo. Mice lacking either SDF-1 or CXCR4 exhibit several lethal defects, including impairment in hematopoiesis and stem cell seeding of the fetal BM.7-9 We have previously shown that homing and engraftment of human CD34+CD38-/low hematopoietic progenitors transplanted into immunodeficient mice are regulated by cell surface human CXCR4 expression and BM-produced murine SDF-1.2,10-13 The in vitro migratory capacity of enriched CD34+ progenitors, particularly the expression and functionality of CXCR4 on these cells, directly correlates with clinical hematopoietic recovery after autologous stem cell transplantation.14-16 Thus, understanding the mechanisms and molecular pathways that affect CXCR4 expression and cellular signaling might have important implications for clinical stem/progenitor cell transplantation.
Recently, we identified a specific isoform of the PKC family, atypical PKCζ, as a key regulator of SDF-1/CXCR4-activated signaling in human hematopoietic progenitors, demonstrating that ectopic PKCζ expression increases SDF-1-induced motility, whereas the inhibition of PKCζ activity impairs survival, proliferation, adhesion and, engraftment of immature CD34+ progenitors.17 Unlike conventional (α, β, γ) and novel (δ, ϵ, η, θ) PKC isoenzymes, atypical PKCs ζ and λ/ι are not activated by calcium and diacylglycerol.18,19 Atypical PKCs are key regulators of cell polarity, motility, and gene expression.20-22 Others' and our findings17,23-25 have positioned PKCζ in the center of chemoattractant- and immunoregulator-induced responses, yet the molecular events regulating PKCζ activation and signaling in hematopoietic cells have not been completely elucidated.
In general, cAMP has been well established as a secondary messenger regulating a wide variety of cell functions, such as motility, growth, and metabolism. The effects of cAMP are primarily mediated by PKA- and Rap1-activated signaling pathways (for recent reviews, see Bos et al26 and Kopperud et al27 ). Multiple stimuli, including immunomodulators such as prostaglandins, cause increases in cellular cAMP production.28 The fact that changes in cellular cAMP level have been implicated in the activation of CXCR4 expression in T cells,29-32 maturing dendritic cells,33,34 endothelium,35 and astroglia36 calls attention to its importance in the regulation of hematopoietic progenitor cell biology.
In the present work, we have examined the effect of cAMP-activated signaling on primary normal human CD34-enriched (CD34+) hematopoietic progenitor cells that are commonly used for clinical stem cell transplantation, obtained from both cord blood (CB) and adult mobilized peripheral blood (MPBL). We found that a sustained elevation in cellular cAMP level significantly increased CXCR4 expression, adhesion, survival, and homing of CD34+ progenitors, and we describe a novel role for PKCζ in this pathway.
Materials and methods
Human cells and reagents
Human cord blood (CB) and adult G-CSF MPBL mononuclear cells were obtained after informed consent in accordance with procedures approved by the human ethics committee of The Weizmann Institute of Science. CD34+ cell enrichment was performed using magnetic bead separation, as previously described.12 Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, antibiotics, and glutamine. The pre-B-cell acute lymphocyte leukemia (ALL) cell lines G2 and B1 (kindly provided by M. Freedman, Hospital for Sick Children, Toronto, Canada) were cultured in 10% FCS-supplemented IMDM. For the stimulation of cAMP-mediated signaling, cells were treated with 500 μM N,6 2′-O-dibutyryl adenosine 3′,5′-cyclic monophosphate (dbcAMP), 10 nM prostaglandin E2 (PGE2), or 2 μM cholesterol sulfate, all purchased from Sigma-Aldrich (Rehovot, Israel). Cell-permeable myristolated pseudosubstrate (PS) inhibitor peptides for PKCζ, PKCα/β, and PKCϵ were purchased from BioMol Research Laboratories (Plymouth Meeting, PA), and Rac1 inhibitor (NSC23766) was purchased from Calbiochem, Merck Biosciences GmbH (Darmstadt, Germany).
Flow cytometry analysis of membranal and intracellular CXCR4 expression
Membranal CXCR4 expression was determined by flow cytometry (FACS-calibur; Becton Dickinson, San Jose, CA) using purified anti-human CXCR4 mAb, clone 12G5 (R&D Systems, Minneapolis, MN), followed by secondary Alexa 488-conjugated donkey anti-mouse IgG (Molecular Probes, Eugene, OR). Isotype-matched IgG2a was used as control, showing background labeling with no differences between the treatments. Ratios of mean values of Ab-labeled cells to secondary IgG-only control samples were calculated using CellQuest software (Becton Dickinson), and the results were expressed in arbitrary units (AU). Flow cytometry analysis with additional anti-CXCR4 mAbs, clones 6H8 and 1D9, demonstrated increases similar to that of the 12G5 increase after dbcAMP treatment. For the analysis of intracellular CXCR4 levels, after blocking of membranal receptor by 1-hour incubation with 10 μg/mL nonconjugated 12G5 mAb, G2 cells were fixed, permeabilized, and immunolabeled with anti-human CXCR4-PE Ab (12G5; R&D Systems), as previously described.37 Membranal CXCR4 internalization was assayed as described38 : G2 cells were preincubated for 30 minutes at 4°C with 10 μg/mL anti-human CXCR4-PE Ab, incubated at 37°C for the indicated times, and washed for 2 minutes on ice at normal (pH 7.4) or acidic (pH 2.2) buffer to remove the surface-bound Ab. The relative proportion of internalized receptor (labeling after acid wash) out of total fluorescence intensity was determined by flow cytometry. For the analysis of CXCR4 membranal re-expression after SDF-1-induced internalization, G2 cells were incubated for 2 hours at 37°C with 250 nM SDF-1 (PeproTech, Rocky Hill, NJ), washed in excess growth medium, and further incubated at 37°C for the indicated times. CXCR4 expression was monitored by flow cytometry, as described.
Activated Rap1 pull-down assay
GTP-Rap1 pull-down was performed using Ral GDS-RBD agarose slurry (Upstate Cell Signaling Solutions, Lake Placid, NY), according to the manufacturer's instruction, and was detected, after 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by anti-Rap1 mAb (BD Biosciences, Bedford, MA).
Spa1 overexpression
MPBL CD34+ cells were transiently transfected with Spa1-GFP or GFP-only expressing vector,39 kindly provided by M. Hattori and N. Minato (Kyoto University, Kyoto, Japan), using the Amaxa Nucleofector kit (Amaxa, Berlin, Germany) according to the manufacturer's protocol.
Immunoprecipitation and immunoblot
Immunoprecipitation of PKCζ was performed as previously described17 by 2-hour incubation at 4°C of G2 cell lysates with protein A-coated beads (Bio-Rad Laboratories, Hercules, CA) preconjugated to polyclonal anti-PKCζ Ab (Santa Cruz Biotechnology, Santa Cruz, CA). Samples were resolved by 7.5% SDS-PAGE and immunoblotted with anti-phospho-PKCζ or monoclonal anti-total neutrophil PKCζ (both purchased from Santa Cruz). For the detection of ERK1/2 phosphorylation level, 20 μg G2 cell lysate was subjected to 10% SDS-PAGE followed by immunoblot with either anti-phospho-ERK1/2 Ab or anti-total-ERK1/2 Ab (both obtained from Sigma-Aldrich).
Membranal PKCζ translocation
MPBL CD34+ cells were transiently transfected as described, fixed, permeabilized, and indirectly immunolabeled with anti-PKCζ Ab (C-20; Santa Cruz Biotechnology), as described.40 CXCR4 was labeled with 10 μg/mL anti-human CXCR4 mAb (12G5; R&D Systems). Cells were mounted on poly-L-lysine (Sigma-Aldrich)-coated glass coverslips. Images were acquired by the DeltaVision system using Resolve 3D software (Applied Precision, Issaquah, WA) with an Axiovert 100TV inverted microscope (Zeiss, Oberkochen, Germany) and a 100×/1.40 Plan-Apochromat objective lens, and images were processed in Adobe Photoshop 7.0 ME software (Adobe Systems, Mountain View, CA).
Real-time RT-PCR (reverse transcriptase polymerase chain reaction)
Total RNA was isolated from G2 cells (5 × 106 cells per sample) using Tri-reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol and was subjected to cDNA synthesis as previously described.13 CXCR4 expression was analyzed by real-time quantitative PCR using a LightCycler instrument and LightCycler-Fast Start DNA Master SyBr Green I reagents (Roche Molecular Biochemicals, Indianapolis, IN). The following primers were used: CXCR4 sense, 5′-TCTAGGCAGGACCTGT-3′; CXCR4 antisense, 5′-CACTTTGGGCTTTGGTT-3′; β-actin sense, 5′-GCTCAGGAGGAGCAAT-3′; β-actin antisense, 5′-GGCATCCACGAAACTAC-3′. Standard curve was calculated using 5 different cDNA concentrations. Reactions were performed in triplicate at 95°C for 10 seconds, followed by 45 cycles of 95°C for 15 seconds, 60°C for 10 seconds, 72°C for 20 seconds, 65°C for 40 seconds, and a melt curve analysis to confirm the purity of the reaction products. Raw data from reactions using CXCR4 primers were normalized to the corresponding data from reactions using β-actin primers.
Analysis of CREB phosphorylation
For flow cytometry analysis of phosphorylated CREB content (P-CREB), cells were stimulated for 1 hour with 500 μM dbcAMP, washed in PBS, and fixed in 70% ice-cold ethanol. After overnight incubation at 4°C, samples were washed in PBS, permeabilized with 0.25% Triton X100 in PBS for 10 minutes on ice, washed, and indirectly immunolabeled with anti-P-CREB Ab (Upstate Cell Signaling Solutions, Waltham, MA), followed by Alexa 488-goat anti-rabbit IgG (Molecular Probes).
Adhesion and transmigration assays
Adhesion to MS-5 mouse stroma cells (kindly provided by J.C. Gutierrez-Ramos, Millennium Pharmaceuticals, Cambridge, MA) was assayed as previously described.17 Briefly, CB CD34+ cells were preincubated for 24 hours with or without 500 μM dbcAMP, labeled with CFSE (Molecular Probes), and allowed to adhere for 30 to 40 minutes to a confluent MS-5 cell layer. After extensive washings, cells were collected in 5 mM EDTA containing buffer, and the number of CFSE+ cells in each sample was determined by flow cytometry. Where indicated, before the assay, cells were pretreated for 20 minutes at room temperature with 10 μg/mL anti-CD49d (VLA-4; Serotec, Oxford, United Kingdom) mAb or IgG1 control. For transendothelial migration, cAMP-treated CFSE-labeled CD34+ cells, treated as above, were allowed to migrate for 6 hours through human bone marrow SV-transformed endothelial cells (BMECs, a kind gift from S. Rafii, Weill Medical College of Cornell University, New York, NY) that were plated (2 × 105 cells/well) 24 hours in advance on Costar transwells precoated for 1 hour at 37°C with 25 μg/cm2 fibronectin (Sigma-Aldrich). The integrity of the BMEC layer was verified microscopically. The number of cells transmigrated spontaneously or toward 15 nM SDF-1 was quantified by flow cytometry.
Homing experiments
β2-microglobulin knockout NOD/LtSz-PrKdcscid (NOD/SCIDB2mnull) mice were bred and housed as previously described.12 All experiments were approved by the animal care committee of The Weizmann Institute of Science. Enriched human CD34+ cells were treated for 24 hours with 500 μM dbcAMP with or without subsequent washing (leading to identical results) and were injected (0.5 × 106 cells/mouse) into the tail veins of mice that were sublethally irradiated (350 cGy) 24 hours before transplantation. Four to 6 hours after transplantation, cells were recovered from the BM and spleens of the recipient mice and were analyzed by flow cytometry using human-specific CD45-FITC mAb (IQ Products, Groningen, The Netherlands) acquiring 1.5 × 106 cells/sample. Cells obtained from mice that did not undergo transplantation, or cells labeled with mouse IgG1-FITC only, were used as negative controls.
Gelatin zymography
Zymography of supernatants obtained from CD34+ cells cultured in serum-free RPMI 1640 for 40 hours untreated or in the presence of 500 μM dbcAMP, 1.15 pM TNFα (PeproTech), or both was performed as previously described.41
Cell survival and cell cycle analysis
CD34+ cells were cultured for the indicated lengths of time in 10% FCS-supplemented RPMI for cell proliferation or serum-free IMDM supplemented with 2% BSA, 25 U/mL insulin, 0.1 mM 2-mercaptoethanol, 10 mM HEPES, 2 mM L-glutamine, and antibiotics for cell survival. For determination of DNA content, cells were fixed in 70% ice-cold ethanol and incubated for 30 minutes at 37°C with 100 μg/mL RNase A and 50 μg/mL propidium iodide (PI) (both from Sigma-Aldrich), and PI intensity was determined by flow cytometry. In some assays, cell viability was determined by PI exclusion after brief incubation with nonfixed cells. For Ki-67 staining, G2 cells were cultured in serum-supplemented IMDM, washed in excess PBS, fixed as described, and indirectly immunolabeled with mouse anti-human Ki-67 mAb (DakoCytomation, Glostrup, Denmark) followed by Alexa 488-conjugated donkey anti-mouse IgG (Molecular Probes).
Statistical analysis
Significance levels of data were determined by Student t test for the differences in mean values.
Results
Cellular cAMP elevation increases CXCR4 expression and membranal presentation on human hematopoietic progenitors
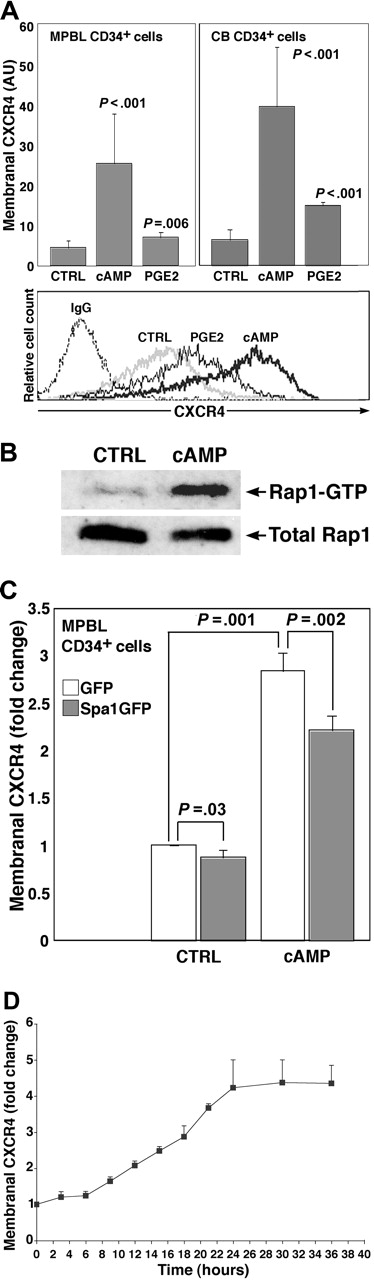
To increase the cellular cAMP level, we applied a cell-permeable cAMP analog, dbcAMP. We found that 24-hour treatment with dbcAMP increased more than 5-fold the membranal CXCR4 expression on MPBL and CB CD34+ cells (Figure 1A). Of interest, CB-derived mononuclear cells of different lineages and primary cells from patients with acute myeloid leukemia and leukemia-derived cell lines displayed heterogeneous responses to the dbcAMP treatment in terms of CXCR4 expression (P.G., unpublished observation, May 2005), suggesting that although the increase in CXCR4 in response to cAMP was not unique to immature hematopoietic progenitors, it was not necessarily a general phenomenon for all the hematopoietic cell types. Given that CD34+ hematopoietic progenitors were shown to respond to prostaglandin E2 (PGE2) stimulation42 and based on the fact that PGE2 is a potent activator of cellular cAMP synthesis,28 we attempted to increase the cellular cAMP level by PGE2 treatment to study early processes in the activation of CD34+ cells. Accordingly, stimulation by PGE2 had also elevated significantly the CXCR4 level expressed on CD34+ progenitors (Figure 1A).
To further elucidate this effect, we used the G2 pre-B ALL cell line that was previously shown to recapitulate several features of normal hematopoietic progenitors.17 Treatment of G2 cells with dbcAMP resulted in Rap1 activation, as assayed by the GTP-bound Rap1 content (Figure 1B). Rap1 is a member of the Ras subgroup of small GTP-binding proteins. To interfere with the Rap1 activity, we transiently overexpressed Spa1, the hematopoietic cell-specific Rap1 GTPase activating protein.43 As depicted in Figure 1C, the excess of Rap1 inhibitor decreased dbcAMP-induced and, to a lesser extent, basal level CXCR4 expression in MPBL CD34+ progenitors, indicating that Rap1 activity was required for the cAMP-mediated effect on CXCR4 expression. Interestingly, membranal CXCR4 elevation stimulated by dbcAMP was not prevented when we inhibited PKA activity by H89, KT5720, or several other serine/threonine kinases—including phosphatidyl inositol 3-kinase (PI3K)—by wortmannin and LY294002 and Rho-kinase by Y27632 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
CXCR4 expression induced by cAMP is PKCζ dependent
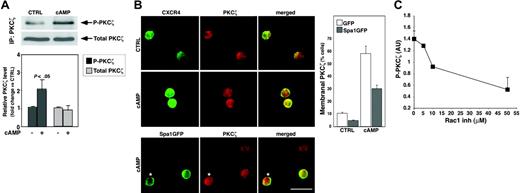
To investigate the mechanisms underlying the cAMP effect on CXCR4 expression, we examined downstream constituents of the cAMP-activated pathway in hematopoietic progenitors. We thus focused on PKCζ, which has been recently implicated in cAMP-induced signaling.44,45 We found that an increase in cellular cAMP level had a major effect on PKCζ activity. As shown in Figure 2A, 24-hour treatment of G2 cells with dbcAMP increased by 2-fold the phosphorylation of PKCζ, a hallmark of its activation. In MPBL CD34+ progenitors, interference with the cAMP signaling by the overexpression of Spa1 resulted in the inhibition of dbcAMP-induced PKCζ membranal translocation (Figure 2B), which is implicated in PKC molecule activation. Surprisingly, despite the profound decrease in the total number of cells with membranal PKCζ in Spa1-overexpressing samples (Figure 2B, histogram), we still detected Spa1GFP-positive cells displaying membranal PKCζ (Figure 2B, lower row), suggesting that Rap1 inhibition might affect signaling upstream to PKCζ translocation. Members of the Rac family of small GTPases have been implicated downstream of cAMP-activated Rap1 in the signaling pathway, regulating the expression of amyloid precursor protein,46 whereas functional Rap1 was found to recruit Rac-activating guanine nucleotide exchange factors.47 It is suggested that in epithelial cells, Rac mediates the localization and activation of atypical PKCs.48 Therefore, we examined the involvement of RacGTPases in the cAMP-induced PKCζ activation in hematopoietic progenitors. Our data showed that blocking Rac1 activation by NSC23766, a pharmacologic compound preventing Rac1 interactions with guanine nucleotide exchange factors Trio and Tiam1,49 resulted in concentration-dependent inhibition of the cAMP-induced PKCζ phosphorylation in G2 cells (Figure 2C), implying that functional Rac1 is required for PKCζ activation by cAMP.
cAMP-stimulated CXCR4 expression in CD34+ hematopoietic progenitors is Rap1 dependent. (A) Membranal CXCR4 expression on human MPBL or CB CD34+ cells treated for 24 hours with 500 μM dbcAMP (cAMP) and 10 nM PGE2, untreated, or vehicle EtOH-treated as control (CTRL), indirectly immunolabeled with anti-human CXCR4 mAb. Results are shown as mean ± SD. For each cell source, 20 cAMP and 5 PGE2 independent experiments were conducted. P values indicate statistically significant differences compared with CTRL. Representative flow cytometry analysis is shown at the bottom. IgG indicates secondary Ab-only labeled samples. (B) Representative analysis of activated Rap1 (Rap1-GTP) status in G2 cells stimulated for 24 hours with 500 μM cAMP or left untreated (CTRL). (C) Effect of Spa1GFP overexpression on membranal CXCR4 level in MPBL CD34+ cells incubated 18 hours after transfection and left untreated (CTRL) or treated with 500 μM cAMP. Results are shown as fold change compared with the CXCR4 intensity in GFP-only transfected samples taken as 1 (mean ± SD of 4 independent experiments). (D) Time-lapse analysis of membranal CXCR4 expression in G2 cells treated with 500 μM cAMP for the indicated time periods. Results (mean ± SD of 3 independent experiments) are expressed as fold change compared with CXCR4 intensity of untreated counterparts.
cAMP-stimulated CXCR4 expression in CD34+ hematopoietic progenitors is Rap1 dependent. (A) Membranal CXCR4 expression on human MPBL or CB CD34+ cells treated for 24 hours with 500 μM dbcAMP (cAMP) and 10 nM PGE2, untreated, or vehicle EtOH-treated as control (CTRL), indirectly immunolabeled with anti-human CXCR4 mAb. Results are shown as mean ± SD. For each cell source, 20 cAMP and 5 PGE2 independent experiments were conducted. P values indicate statistically significant differences compared with CTRL. Representative flow cytometry analysis is shown at the bottom. IgG indicates secondary Ab-only labeled samples. (B) Representative analysis of activated Rap1 (Rap1-GTP) status in G2 cells stimulated for 24 hours with 500 μM cAMP or left untreated (CTRL). (C) Effect of Spa1GFP overexpression on membranal CXCR4 level in MPBL CD34+ cells incubated 18 hours after transfection and left untreated (CTRL) or treated with 500 μM cAMP. Results are shown as fold change compared with the CXCR4 intensity in GFP-only transfected samples taken as 1 (mean ± SD of 4 independent experiments). (D) Time-lapse analysis of membranal CXCR4 expression in G2 cells treated with 500 μM cAMP for the indicated time periods. Results (mean ± SD of 3 independent experiments) are expressed as fold change compared with CXCR4 intensity of untreated counterparts.
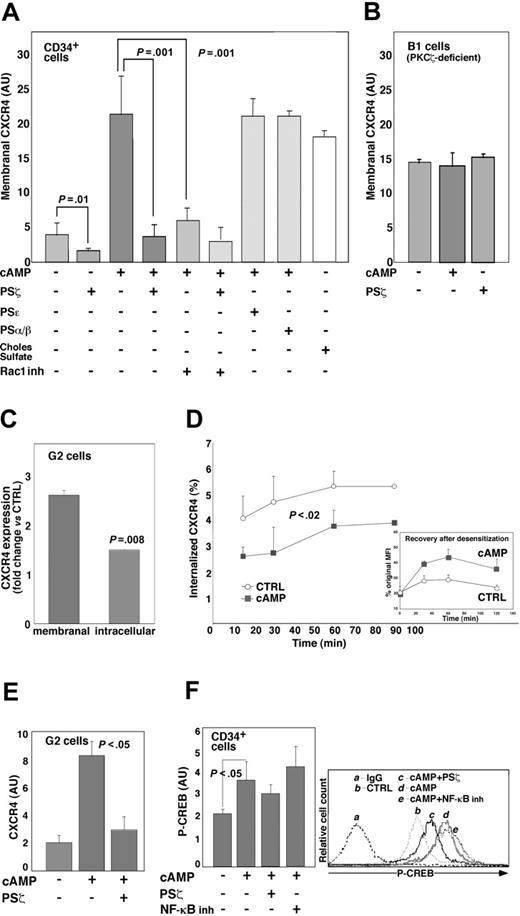
We next examined PKCζ involvement in cAMP-activated CXCR4 expression. Adding the PKCζ inhibitory pseudosubstrate (PS) substantially affected basal and completely abrogated cAMP-induced CXCR4 expression in CD34+ cells. Blocking PKCζ activity by a similar approach also inhibited the CXCR4 expression in dbcAMP-treated human peripheral blood CD3+ T cells (data not shown). In contrast, inhibiting the other PKC isoenzymes, namely PKCϵ and PKCα/β, had no effect on CXCR4 expression (Figure 3A). In addition, cholesterol sulfate, a chemical activator of PKCζ,50 increased the membranal CXCR4 level to one similar to that for dbcAMP-treated cells (Figure 3A). To further confirm these findings, we used a PKCζ-deficient B1 cell line.17 As shown in Figure 3B, neither dbcAMP nor PKCζ PS affected CXCR4 expression in these cells. Importantly, blocking Rac1 activation in CD34+ cells caused a reduction comparable to that for PKCζ PS in membranal CXCR4 expression (Figure 3A) in a manner that was neither synergistic nor additive in the presence of both inhibitors, supporting our suggestion that Rac1 and PKCζ constitute the same pathway in cAMP-stimulated CXCR4 elevation.
The changes in membranal and intracellular CXCR4 levels were then examined comparatively in G2 cells incubated for 24 hours with dbcAMP. Results revealed that whereas cell surface (membranal) expression of the receptor was up-regulated 2.5 times, the intracellular molecule content was increased by just 1.4-fold (Figure 3C). Furthermore, time-course analysis of surface-bound anti-CXCR4 mAb internalization showed that in cAMP-stimulated cells, CXCR4 receptor endocytosis was reduced 2 times compared with untreated counterparts (Figure 3D), suggesting that in the absence of a ligand, cAMP stimulation prolonged the time of CXCR4 presence on the cell membrane. In addition, cAMP-treated cells displayed a more efficient recovery from SDF-1-induced CXCR4 desensitization (Figure 3D, inset). These results are in line with previous findings demonstrating that cAMP treatment reduces the CXCR4 internalization rate and antagonizes SDF-1-induced receptor endocytosis in peripheral blood mononuclear cells.29
In view of reports that cAMP-induced CXCR4 expression in T cells relies on de novo protein synthesis,31,32 we examined whether this was also the case in hematopoietic cells. Time-lapse analysis of changes in membranal CXCR4 expression in dbcAMP-stimulated G2 cells revealed that the increase in CXCR4 took place after at least 6 hours of incubation, reaching maximal level at approximately 24 hours of treatment (Figure 1D). Accordingly, we found that in G2 cells cycloheximide treatment antagonized the CXCR4 elevation by cAMP (data not shown). Real-time RT-PCR analysis of G2 cells stimulated with dbcAMP for 6 hours showed a 4-fold increase in CXCR4 mRNA level that was significantly attenuated in the presence of the PKCζ PS (Figure 3E). The transcription factor CREB is a major cAMP target in the regulation of gene expression.51 CREB-mediated transcription was implicated in the cAMP-induced CXCR4 expression.31 We found that in CD34+ progenitors treated for 1 hour with dbcAMP (the time period of maximal CREB binding to the CXCR4 promoter in peripheral blood T cells),31 the phosphorylated (activated) CREB content was increased by almost 2-fold, whereas pretreatment with PKCζ PS attenuated the cAMP-induced CREB phosphorylation (Figure 3F). Interestingly, cAMP-activated CREB phosphorylation and membranal CXCR4 expression in these cells were not affected by the inhibition of NF-κB activity when we applied a cell-permeable inhibitory peptide NF-κBSN50 (Figures 3F and S1, respectively), implying that NF-κB is not involved in cAMP-induced CXCR4 transcription.
CXCR4 induced by cAMP is functional and depends on PKCζ for its activity
The following experiments were designed to investigate the ability of cAMP-treated cells to respond to SDF-1, the CXCR4 ligand. The first set of experiments was based on the in vitro assays. As depicted in Figure 4A, cAMP-stimulated progenitors migrated more efficiently than controls through BMEC-coated transwell filters. The results were further substantiated by the observation that cAMP treatment increased by 40% the ability of CD34+ cells to adhere to the SDF-1-expressing BM stroma cell line MS-552 (Figure 4B). In accordance with our previous findings showing that CD34+ cell adhesion to MS-5 is CXCR4 and PKCζ dependent,17 we found that pretreatment with PKCζ PS abolished the cAMP-induced adhesion (Figure 4B). SDF-1-activated adhesion of CB CD34+ cells to MS-5 is partially dependent on VLA-4/VCAM-1 interactions.17 We observed that blocking VLA-4 activity by neutralizing Abs had an effect similar to that of PKCζ PS on the cAMP-induced adhesion (Figure 4B), further supporting our suggestion that cAMP-stimulated cells adhere to the SDF-1-expressing stroma by the CXCR4/PKCζ-mediated pathway.
The function of the cAMP-induced CXCR4 receptor was then tested in vivo with the homing assay. We found that human CD34+ cells exposed for 24 hours to dbcAMP home more efficiently to the BM and spleens of recipient NOD/SCIDB2mnull mice, as measured 4 to 6 hours after transplantation (Figure 4C). Similar results were obtained with PGE2-treated progenitors (data not shown), yet there was no increase in the long-term repopulation capacity of dbcAMP-treated cells, and the positive effect of cAMP on homing declined with time. In fact, it was already less significant 24 hours after transplantation (Figure S2A) because cAMP-induced effects, including CXCR4 elevation (Figure S2B), are rapidly reversed once dbcAMP is removed from the immediate cell environment, as in intravenous injection. Increases in short-term progenitor cell homing were abrogated in cAMP-stimulated cells pretreated with the PKCζ inhibitor just before transplantation (Figure 4C, cAMP+PSζ). In agreement with our findings implicating Rac1 activity in cAMP-induced PKCζ activation (Figures 2C, 3A), we found that Rac1 inhibition opposed the effect of cAMP on BM homing of CD34+ progenitors (Figure 4C, cAMP+Rac1 inh).
cAMP activates PKCζ in hematopoietic progenitor cells. (A) PKCζ phosphorylation and expression levels in G2 cells cultured for 24 hours either untreated (CTRL) or in the presence of 500 μM dbcAMP (cAMP), as assayed by immunoblot. PKCζ-phosphorylated (P-PKCζ) level was determined by densitometry, normalized to the PKCζ protein level (Total), and expressed as fold change versus CTRL cells. Results are mean ± SD of 5 independent experiments. (B) PKCζ membranal translocation in cAMP-treated cells. MPBL CD34+ cells were transfected with GFP (green fluorescent protein) only (top and middle rows) or with Spa1GFP (bottom row) expressing vector and further incubated for 18 hours either untreated (CTRL) or in the presence of 500 μM cAMP and indirectly immunolabeled for PKCζ (red). GFP vector transfected cells were colabeled for CXCR4 (green) because no GFP signal was retained after the permeabilization procedure. Note the increased CXCR4 expression and colocalization with PKCζ at the membrane of cAMP-stimulated cells (middle row). Spa1GFP overexpression decreased the proportion of cells displaying membranal PKCζ labeling (histogram on the right, representative of 2 independent experiments; 250 cells in each sample), yet PKCζ translocation to the membrane could be detected in Spa1GFP-transfected cells (bottom row, asterisks). Bar represents 10 μm. (C) Inhibition of the cAMP-induced PKCζ phosphorylation (P-PKCζ) by the NSC23766 (Rac1 inh) in G2 cells treated and assayed as in panel A. Results of the densitometry analysis, expressed in arbitrary units (AU), are represented as mean ± SD of 3 independent experiments.
cAMP activates PKCζ in hematopoietic progenitor cells. (A) PKCζ phosphorylation and expression levels in G2 cells cultured for 24 hours either untreated (CTRL) or in the presence of 500 μM dbcAMP (cAMP), as assayed by immunoblot. PKCζ-phosphorylated (P-PKCζ) level was determined by densitometry, normalized to the PKCζ protein level (Total), and expressed as fold change versus CTRL cells. Results are mean ± SD of 5 independent experiments. (B) PKCζ membranal translocation in cAMP-treated cells. MPBL CD34+ cells were transfected with GFP (green fluorescent protein) only (top and middle rows) or with Spa1GFP (bottom row) expressing vector and further incubated for 18 hours either untreated (CTRL) or in the presence of 500 μM cAMP and indirectly immunolabeled for PKCζ (red). GFP vector transfected cells were colabeled for CXCR4 (green) because no GFP signal was retained after the permeabilization procedure. Note the increased CXCR4 expression and colocalization with PKCζ at the membrane of cAMP-stimulated cells (middle row). Spa1GFP overexpression decreased the proportion of cells displaying membranal PKCζ labeling (histogram on the right, representative of 2 independent experiments; 250 cells in each sample), yet PKCζ translocation to the membrane could be detected in Spa1GFP-transfected cells (bottom row, asterisks). Bar represents 10 μm. (C) Inhibition of the cAMP-induced PKCζ phosphorylation (P-PKCζ) by the NSC23766 (Rac1 inh) in G2 cells treated and assayed as in panel A. Results of the densitometry analysis, expressed in arbitrary units (AU), are represented as mean ± SD of 3 independent experiments.
To further define the role of PKCζ in cAMP-stimulated processes, we looked into the effects of TNFα because PKCζ has been well established as a major downstream target of TNFα.53 The experiments were based on the reports that TNFα is a potent activator of matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in human CD34+ cells,41 in a PKCζ-dependent manner,54 and that MMP-9 activity is implicated in the regulation of progenitor cell trafficking in vivo.41,55-57 Surprisingly, we found that dbcAMP treatment also increased MMP-2 and MMP-9 expression by CD34+ progenitors, though to a much lower extent than that of TNFα (Figure 5A). Moreover, MMP-2 and MMP-9 secretion was apparently further elevated in cells costimulated with dbcAMP and TNFα (Figure 5A). We have previously shown that MMP-9 expression induced by SDF-1 in CD34+ cells is PKCζ dependent.17 We here found that PKCζ inhibition abrogated dbcAMP- and TNFα-activated MMP-2 and MMP-9 secretion and, significantly, MMP expression in cells costimulated with both agents (Figure 5A, PSζ). Furthermore, we observed that dbcAMP and TNFα had an additive effect on CXCR4 expression in immature MPBL CD34+ progenitors (Figure 5B) but not on CB CD34+ cells (data not shown). These findings indicate that cAMP- and TNFα-activated pathways use PKCζ for MMP-2, MMP-9, and CXCR4 induction in hematopoietic progenitors.
cAMP-induced increase in CXCR4 expression depends on PKCζ activity. Membranal CXCR4 expression in CB and MPBL CD34+ (A) or B1 (B) cells, incubated for 24 hours with 500 μM dbcAMP (cAMP), 2 μM cholesterol sulfate (Choles Sulfate), or left untreated (-). Where indicated, 10 μM PS of PKCζ (PSζ), PKCϵ (PSϵ), PSα/β (PSα/β), or 25 μM NSC23766 (Rac1 inh) were applied. Flow cytometry analysis data are shown in arbitrary units (AU) as mean ± SD; 10 independent experiments were conducted for PSζ, and 6 were conducted for Rac1 inh. (C) Increase in membranal and intracellular CXCR4 labeling in G2 cells incubated for 24 hours in the presence of 500 μM cAMP compared with untreated cells (CTRL) taken as 1. Flow cytometry analysis data (mean ± SD of 3 independent experiments) are shown. (D) Decreased internalization rate of anti-CXCR4-PE mAb in G2 cells treated for 24 hours with cAMP compared with CTRL. Results are mean ± SD of 3 independent experiments. P < .02; statistically significant differences compared with CTRL at each time point. Increased recovery after SDF-1-induced receptor internalization is shown in the inset. Mean fluorescence intensity (MFI) is calculated as percentage of CXCR4 expression in CTRL or cAMP-treated cells before the application of SDF-1 (original MFI). (E) Relative CXCR4 expression in G2 cells either untreated (-) or stimulated for 6 hours with 500 μM cAMP in the absence or presence of 10 μM PSζ, as analyzed by real-time RT-PCR. Data are expressed in AU as a ratio of CXCR4 and β-actin mRNA level (mean ± SD of 3 independent experiments). (F) Changes in CREB phosphorylation (P-CREB) in MPBL CD34+ progenitors stimulated for 1 hour with 500 μM cAMP (mean ± SD of 3 independent experiments). Where specified, cells were pretreated with either 10 μM PSζ or 18 μM NF-κBSN50 (NF-κB inh). Representative flow cytometry analysis is shown. IgG indicates secondary Ab-labeled cells; CTRL, untreated samples.
cAMP-induced increase in CXCR4 expression depends on PKCζ activity. Membranal CXCR4 expression in CB and MPBL CD34+ (A) or B1 (B) cells, incubated for 24 hours with 500 μM dbcAMP (cAMP), 2 μM cholesterol sulfate (Choles Sulfate), or left untreated (-). Where indicated, 10 μM PS of PKCζ (PSζ), PKCϵ (PSϵ), PSα/β (PSα/β), or 25 μM NSC23766 (Rac1 inh) were applied. Flow cytometry analysis data are shown in arbitrary units (AU) as mean ± SD; 10 independent experiments were conducted for PSζ, and 6 were conducted for Rac1 inh. (C) Increase in membranal and intracellular CXCR4 labeling in G2 cells incubated for 24 hours in the presence of 500 μM cAMP compared with untreated cells (CTRL) taken as 1. Flow cytometry analysis data (mean ± SD of 3 independent experiments) are shown. (D) Decreased internalization rate of anti-CXCR4-PE mAb in G2 cells treated for 24 hours with cAMP compared with CTRL. Results are mean ± SD of 3 independent experiments. P < .02; statistically significant differences compared with CTRL at each time point. Increased recovery after SDF-1-induced receptor internalization is shown in the inset. Mean fluorescence intensity (MFI) is calculated as percentage of CXCR4 expression in CTRL or cAMP-treated cells before the application of SDF-1 (original MFI). (E) Relative CXCR4 expression in G2 cells either untreated (-) or stimulated for 6 hours with 500 μM cAMP in the absence or presence of 10 μM PSζ, as analyzed by real-time RT-PCR. Data are expressed in AU as a ratio of CXCR4 and β-actin mRNA level (mean ± SD of 3 independent experiments). (F) Changes in CREB phosphorylation (P-CREB) in MPBL CD34+ progenitors stimulated for 1 hour with 500 μM cAMP (mean ± SD of 3 independent experiments). Where specified, cells were pretreated with either 10 μM PSζ or 18 μM NF-κBSN50 (NF-κB inh). Representative flow cytometry analysis is shown. IgG indicates secondary Ab-labeled cells; CTRL, untreated samples.
Cellular cAMP elevation has opposing effects on progenitor cell survival and proliferation
Activation of cAMP signaling has been well documented as a way to promote the survival of a variety of cell types, including hematopoietic cells.58,59 In accordance with this, we found that dbcAMP stimulation substantially increased the survival of CD34+ progenitors under cytokine-deprived conditions, as determined through monitoring the viable cell count by flow cytometry and the number of dead cells with fragmented DNA (Figure 6A). This effect of cAMP was abrogated in the presence of PKCζ PS (Figure 6A, inset). Strikingly, DNA content analysis revealed that dbcAMP treatment also resulted in a 2-fold decrease in the percentage of dividing CD34+ cells (Figure 6B). We found that the expression of proliferation-associated antigen Ki-67 was reduced by 5-fold in cAMP-treated G2 cells (Figure 6C). To address this issue, we examined the activity of extracellular signal-regulated protein kinase (ERK1/2), a major regulator of cell-cycle progression. The outcome of the cAMP elevation on ERK activity and proliferation is cell type and signal specific.60 We found that 24-hour exposure to dbcAMP almost completely decreased the ERK1/2 phosphorylation in G2 cells (Figure 6D), suggesting that the negative effect of cAMP on cell proliferation might be exerted through the inhibition of ERK1/2 activity.
CXCR4 overexpressed on cAMP-stimulated CD34+ progenitors is functional. (A) Transendothelial migration of dbcAMP (cAMP)-treated cells. CD34+ progenitors were incubated for 24 hours with 500 μM cAMP or left untreated (-), labeled with CFSE, and allowed to migrate either spontaneously (spont) or toward SDF-1 (directional) through BMEC-coated transwells. Data represent the percentages of migrated cells (mean ± SD of 2 independent experiments). (B) Adhesion to BM cells after cAMP treatment. Cells treated for 24 hours with 500 μM cAMP or left untreated (CTRL) were labeled with CFSE and allowed to adhere for 40 minutes to MS-5 cell layer (mean ± SD of at least 2 independent experiments, each in triplicate samples). Where indicated, cAMP-treated cells were preincubated with 10 μM PKCζ PS (PSζ), 10 μg/mL VLA-4 blocking mAb (αVLA-4), or IgG1 control. (C) Homing efficiency of cAMP-treated cells. CB or MPBL CD34+ cells were treated for 24 hours with 500 μM cAMP or left untreated (CTRL) and injected intravenously into the NOD/SCIDB2mnull mice. Where indicated, cAMP-treated cells were incubated for 30 minutes before injection with 10 μM PSζ or cultured in the presence of 25 μM NSC23766 (Rac1 inh). BM and spleen of recipient mice were analyzed for the presence of human cells (mean ± SD of at least 3 independent experiments, 2 to 3 mice per treatment in each experiment; *P < .05 relative to other treatments). Representative flow cytometry analyses are shown on the right. IgG indicates samples labeled with the isotype control Ab. Number of CD45+ human cells per 106 acquired is indicated in each case.
CXCR4 overexpressed on cAMP-stimulated CD34+ progenitors is functional. (A) Transendothelial migration of dbcAMP (cAMP)-treated cells. CD34+ progenitors were incubated for 24 hours with 500 μM cAMP or left untreated (-), labeled with CFSE, and allowed to migrate either spontaneously (spont) or toward SDF-1 (directional) through BMEC-coated transwells. Data represent the percentages of migrated cells (mean ± SD of 2 independent experiments). (B) Adhesion to BM cells after cAMP treatment. Cells treated for 24 hours with 500 μM cAMP or left untreated (CTRL) were labeled with CFSE and allowed to adhere for 40 minutes to MS-5 cell layer (mean ± SD of at least 2 independent experiments, each in triplicate samples). Where indicated, cAMP-treated cells were preincubated with 10 μM PKCζ PS (PSζ), 10 μg/mL VLA-4 blocking mAb (αVLA-4), or IgG1 control. (C) Homing efficiency of cAMP-treated cells. CB or MPBL CD34+ cells were treated for 24 hours with 500 μM cAMP or left untreated (CTRL) and injected intravenously into the NOD/SCIDB2mnull mice. Where indicated, cAMP-treated cells were incubated for 30 minutes before injection with 10 μM PSζ or cultured in the presence of 25 μM NSC23766 (Rac1 inh). BM and spleen of recipient mice were analyzed for the presence of human cells (mean ± SD of at least 3 independent experiments, 2 to 3 mice per treatment in each experiment; *P < .05 relative to other treatments). Representative flow cytometry analyses are shown on the right. IgG indicates samples labeled with the isotype control Ab. Number of CD45+ human cells per 106 acquired is indicated in each case.
cAMP and TNFα signaling converge in MMP-2/MMP-9 secretion and induction of CXCR4 expression by CD34+ progenitors. (A) MMP-2 and MMP-9 levels in the conditioned media of CB and MPBL CD34+ cells cultured for 24 hours with the indicated agents, as determined by gelatin zymography. Positive control (contr) is the conditioned medium of HT1080 cells. Representative gel and densitometry analysis data are shown as mean ± SD of 3 independent experiments expressed in arbitrary units (AU). Note that the presence of PKCζ PS (PSζ) abrogates MMP-2/MMP-9 secretion by cAMP and TNFα costimulated cells. (B) Membranal CXCR4 expression in MPBL CD34+ cells treated as in panel A. Data are shown as mean ± SD of 6 independent experiments. Representative flow cytometry analysis is presented at the bottom. IgG indicates secondary IgG only; CTRL, untreated cells.
cAMP and TNFα signaling converge in MMP-2/MMP-9 secretion and induction of CXCR4 expression by CD34+ progenitors. (A) MMP-2 and MMP-9 levels in the conditioned media of CB and MPBL CD34+ cells cultured for 24 hours with the indicated agents, as determined by gelatin zymography. Positive control (contr) is the conditioned medium of HT1080 cells. Representative gel and densitometry analysis data are shown as mean ± SD of 3 independent experiments expressed in arbitrary units (AU). Note that the presence of PKCζ PS (PSζ) abrogates MMP-2/MMP-9 secretion by cAMP and TNFα costimulated cells. (B) Membranal CXCR4 expression in MPBL CD34+ cells treated as in panel A. Data are shown as mean ± SD of 6 independent experiments. Representative flow cytometry analysis is presented at the bottom. IgG indicates secondary IgG only; CTRL, untreated cells.
Discussion
The present study addressed molecular aspects of the regulation of CXCR4 in human hematopoietic progenitors, namely primary normal CD34+ cells enriched by MPBL and CB and pre-B ALL G2 cells. Our results showed that CXCR4 expression was increased after elevation of the cellular cAMP level and implicated PKCζ activity in the cAMP-mediated effects. We showed that in progenitor cells, PKCζ was directly stimulated by cAMP-activated signaling. We further found that cAMP-induced and, to a lesser extent, basal CXCR4 expression are PKCζ dependent because, in the PKCζ-deficient cell line B1, the CXCR4 level was not affected by cAMP stimulation or PKCζ inhibition, whereas the blocking of PKCζ activity in CD34+ cells completely abrogated membranal CXCR4 expression. Importantly, we found that the inhibition of several other PKC isoenzymes had no effect on CXCR4 expression in hematopoietic progenitors (present work) or on their adhesion, polarization, and chemotaxis.17
cAMP increases CD34+ progenitor cell survival by PKCζ-activated pathway while inhibiting their proliferation. (A) Increased survival of CB CD34+ cells cultured for 3 days under cytokine-deprived conditions in the presence of 500 μM dbcAMP (cAMP) as analyzed by flow cytometry. Data represented as fold change (mean ± SD of 6 independent experiments each in triplicate) in the number of PI-positive (Dead cells) in cAMP-treated compared with untreated (CTRL) samples. Inset shows representative changes in the proportion of CD34+ cells with fragmented DNA (dead cells [%]) cultured for the indicated time periods untreated (CTRL), treated with cAMP, or treated with cAMP and 10 μM PKCζ PS (cAMP+PSζ). (B) Comparison of the percentage of dividing CD34+ cells (mean ± SD of 4 independent experiments) cultured for 3 days in serum-supplemented medium with 500 μM cAMP or without (CTRL), fixed, and PI labeled for cell cycle analysis. (C) Ki-67 expression in G2 cells cultured in full growth medium for 3 days either untreated (CTRL) or with 500 μM cAMP. Data are shown as mean ± SD of 3 independent experiments. Representative flow cytometry analyses are shown on the right; numbers indicate percentages of Ki-67-positive cells. (D) Immunoblot analysis of ERK1/2 phosphorylation (P-ERK1/2) status compared with the total ERK1/2 level in G2 cells incubated for 24 hours with 500 μM cAMP or left untreated (CTRL). Representative of 1 of 3 independent experiments with similar results.
cAMP increases CD34+ progenitor cell survival by PKCζ-activated pathway while inhibiting their proliferation. (A) Increased survival of CB CD34+ cells cultured for 3 days under cytokine-deprived conditions in the presence of 500 μM dbcAMP (cAMP) as analyzed by flow cytometry. Data represented as fold change (mean ± SD of 6 independent experiments each in triplicate) in the number of PI-positive (Dead cells) in cAMP-treated compared with untreated (CTRL) samples. Inset shows representative changes in the proportion of CD34+ cells with fragmented DNA (dead cells [%]) cultured for the indicated time periods untreated (CTRL), treated with cAMP, or treated with cAMP and 10 μM PKCζ PS (cAMP+PSζ). (B) Comparison of the percentage of dividing CD34+ cells (mean ± SD of 4 independent experiments) cultured for 3 days in serum-supplemented medium with 500 μM cAMP or without (CTRL), fixed, and PI labeled for cell cycle analysis. (C) Ki-67 expression in G2 cells cultured in full growth medium for 3 days either untreated (CTRL) or with 500 μM cAMP. Data are shown as mean ± SD of 3 independent experiments. Representative flow cytometry analyses are shown on the right; numbers indicate percentages of Ki-67-positive cells. (D) Immunoblot analysis of ERK1/2 phosphorylation (P-ERK1/2) status compared with the total ERK1/2 level in G2 cells incubated for 24 hours with 500 μM cAMP or left untreated (CTRL). Representative of 1 of 3 independent experiments with similar results.
Our attempts to elucidate the molecular mechanisms underlying the processes of cAMP-induced CXCR4 elevation led us to Rap1, a member of the Ras family of small GTPases, which is a key regulator of cell polarity, motility, adhesion, and proliferation.26,61,62 Our data indicate that Rap1 activity is required for cAMP-induced CXCR4 up-regulation because the overexpression of Spa1, a principal Rap1GAP in hematopoietic cells, attenuated CXCR4 expression by cAMP. We have also observed reduced membranal PKCζ translocation in Spa1-overexpressing CD34+ progenitors, indicating that interference with Rap1 signaling affects PKCζ activity. During the establishment of cell polarity and intercellular junction formation, atypical PKCs are activated by binding to the Par6/Par3/Cdc42 molecular complex.20,63 Rap1 was positioned upstream of Cdc42 in determining neuronal cell asymmetry mediated by atypical PKC family members.64 It has been shown that Rac1 binds Par6 and potentially activates PKCζ.65 Accordingly, we found that Rac1 inhibition decreased cAMP-induced PKCζ phosphorylation and had an inhibitory effect on CXCR4 expression identical to that of PKCζ PS. Therefore, as summarized in the model depicted in Figure 7, our data suggested that Rap1, Rac1, and PKCζ constitute a signaling pathway of cAMP-induced CXCR4 expression. Our current and previous17 studies also point to a positive feedback loop between PKCζ activation by cAMP, which results in elevated CXCR4 expression and thus might further enhance PKCζ activity.
We found that the increase in CXCR4 expression in cAMP-stimulated cells resulted from enhanced transcription and, primarily, prolonged time of membranal receptor representation. Previous studies29 and our present findings on anti-CXCR4 Ab internalization suggest that cAMP treatment leads to a decrease in CXCR4 endocytosis. Our preliminary results indicated that PKCζ inhibition significantly increases membranal CXCR4 turnover; this is under further investigation. Consistent with previous reports, we also observed cAMP-activated CXCR4 mRNA expression attenuated in the presence of PKCζ inhibitor. We show that CREB phosphorylation was induced by cAMP treatment of CD34+ progenitors, whereas PKCζ inhibition reduced this effect. Given that in T cells cAMP-mediated CXCR4 expression is partially regulated by CREB,31 we suggest that PKCζ activation increases the CXCR4 mRNA level by CREB-mediated transcription. In several cellular systems, CXCR4 expression required NF-κB activity.66-68 Interestingly, however, we found no significant effect of NF-κB inhibition on CREB phosphorylation or cAMP-induced membranal CXCR4 elevation. Similarly, NF-κB-independent PKCζ-mediated gene transcription in human epithelial and monocytic cells was demonstrated in response to Toll-like receptor 2 activation.69 Our data also suggest that cAMP-stimulated CXCR4 expression by PKCζ probably did not require PI3K (Figure S1). Accordingly, it was recently reported that PKCζ activation by TC10 (a member of the Rho small GTPase family), which mediates insulin-stimulated PKCζ activation in adipocytes, is PI3K-independent and is, rather, regulated by the guanyl nucleotide exchange factor C3G.70 C3G is also a Rap1-activating protein.71 These findings imply that members of the small GTP-binding protein superfamily might compose a PI3K-independent pathway for PKCζ activation (Figure 7).
Schematic representation of a model of the PKCζ regulation by cAMP and SDF-1/CXCR4. Cellular cAMP accumulation is induced by the ligands (eg, PGE2) that activate Gαs-coupled receptors. Persistent (“prolonged” hours) stimulation with the cAMP-elevating agents results in PKCζ activation in a Rap1/Rac1-dependent manner and a subsequent increase in membranal CXCR4 expression. CXCR4 binding to SDF-1 further activates PKCζ, resulting in a positive feedback loop. On the other hand, SDF-1/CXCR4 interactions might reduce the cellular cAMP level as a result of Gαi activity, establishing a negative feedback loop on the cAMP-induced PKCζ activation. SDF-1 activates PKCζ by a distinct from the cAMP, PI3K-dependent pathway.17 PKCζ activity is required for multiple cellular functions, including MMP-2/MMP-9 secretion, motility, adhesion, and survival. cAMP reduces cell proliferation in a PKCζ-independent manner because of a negative effect on ERK1/2 activity. In contrast to “prolonged” cAMP activation, “short” (minutes) priming with the cAMP-elevating agents antagonizes PKCζ-mediated SDF-1/CXCR4-induced responses.
Schematic representation of a model of the PKCζ regulation by cAMP and SDF-1/CXCR4. Cellular cAMP accumulation is induced by the ligands (eg, PGE2) that activate Gαs-coupled receptors. Persistent (“prolonged” hours) stimulation with the cAMP-elevating agents results in PKCζ activation in a Rap1/Rac1-dependent manner and a subsequent increase in membranal CXCR4 expression. CXCR4 binding to SDF-1 further activates PKCζ, resulting in a positive feedback loop. On the other hand, SDF-1/CXCR4 interactions might reduce the cellular cAMP level as a result of Gαi activity, establishing a negative feedback loop on the cAMP-induced PKCζ activation. SDF-1 activates PKCζ by a distinct from the cAMP, PI3K-dependent pathway.17 PKCζ activity is required for multiple cellular functions, including MMP-2/MMP-9 secretion, motility, adhesion, and survival. cAMP reduces cell proliferation in a PKCζ-independent manner because of a negative effect on ERK1/2 activity. In contrast to “prolonged” cAMP activation, “short” (minutes) priming with the cAMP-elevating agents antagonizes PKCζ-mediated SDF-1/CXCR4-induced responses.
Our data suggest that PKCζ stimulation and CXCR4 expression in cAMP-treated hematopoietic progenitors increased their adhesion and migration in vitro. Indeed, Rap family proteins, effectors of cAMP signaling, are critical regulators of chemokine-induced integrin activation and motility in lymphocytes,61,72 including SDF-1/CXCR4-mediated cell polarization, adhesion, and migration.73,74 It was also shown that along with RhoA, PKCζ stimulates CCL21-induced adhesion and in vivo homing of lymphocytes.75 Human hematopoietic progenitor cell homing in immunodeficient mice and adhesion to BM endothelial and stromal cells are CXCR4-dependent processes (for a review, see Lapidot and Kollet5 ). In accordance, we observed facilitated short-term homing of cAMP-treated CD34+ cells. However, because of the transient nature of the stimulation by cAMP, reversed within a few hours, we did not detect improved repopulation capacity by these cells. The positive effect of cAMP treatment on homing was abrogated on the inhibition of Rac1 or PKCζ activity. Our findings imply that functional Rac1 is required for cAMP-induced PKCζ activation. In murine hematopoietic progenitors, lack of Rac1 results in significant impairment in hematopoietic reconstitution, and the combined Rac1/Rac2 deficiency causes progenitor mobilization.76 Recent reports indicate that a defect in progenitor retention in the stem cell niche at the endosteum occurs in the absence of Rac1 and is more severe in cells lacking both Rac1 and Rac2.77 Reminiscent of our findings on PKCζ inhibition,17 the injection of the Rac1 inhibitory compound NSC23766 increases the number of circulating murine hematopoietic progenitors,77 pointing to the direct role of PKCζ and RacGTPases in progenitor adhesion and retention. At present, the hematopoietic progenitors of PKCζ knockout mice have not been characterized. However, PKCζ-deficient mice display profound defects in B-cell development and function,25,78 the processes that are dependent on SDF-1/CXCR4 signaling. Data from the present study and our previous work17 indicate that a variety of clinical conditions might benefit from approaches targeting PKCζ,79 a key signaling molecule in cAMP- and SDF-1-activated pathways.
Numerous studies imply that an increase in cellular cAMP level inhibits immune cell responses to chemotactic stimuli.80,81 In that respect, we found that whereas sustained cAMP elevation activated PKCζ, resulting in increased CXCR4-mediated responses, short (30-minute) priming with dbcAMP antagonized PKCζ activity and largely inhibited SDF-1-induced PKCζ phosphorylation, cell polarization, and spreading, with no apparent effect on CXCR4 expression (Figure S3). The duality of cell responses to the changes in cellular cAMP might reflect physiologic situations such as inflammation, in which chemokines and cytokines act in concert with noncytokine mediators, among them PGE2 and neuromediators, in modulating the immune response.28,82 These ligand-induced changes in cAMP concentration, which vary in duration and amplitude, are integrated and converged on the signaling pathways activated by cytokines, regulating cell trafficking. In accordance, we found that sustained cAMP elevation in CD34+ progenitors had additive and synergistic effects on PKCζ activation by TNFα, manifested by the increased CXCR4 expression and MMP-2 and MMP-9 production.
We further show that cAMP treatment affected progenitor cell survival and proliferation. Cellular cAMP elevation was demonstrated to have an antiapoptotic/survival effect on various experimental settings.83-86 Interestingly, enhanced CREB phosphorylation was recently correlated with improved SDF-1-induced survival in the MO7e megakaryocytic cell line.87 In that respect, we observed a significantly better survival of cAMP-stimulated CD34+ cells under the cytokine-deprived conditions, and this effect of cAMP was abrogated in the presence of PKCζ PS or NF-κB inhibitor (data not shown). These findings, in line with our previous results,17 indicate that cAMP increases hematopoietic progenitor cell survival by activating the PKCζ pathway. PKCζ activity is controlled by several adaptor proteins, including the negative regulator Par-4,53 which is shown to antagonize the positive effect of PKCζ on cell survival.23,88-90 Thus, one may speculate that by decreasing Par-4 expression/function, cAMP stimulates PKCζ activity and cell survival. In addition, we have found that sustained stimulation with cAMP significantly decreased CD34+ progenitor cell proliferation, pointing to a variety of mechanisms affecting the ultimate size of progenitor cell population. It was previously shown that cAMP-elevating agents reduce the proliferation of murine hematopoietic precursors.91 In contrast, deregulated Rap1 activity in Spa-1-deficient mice causes enhanced expansion of the BM hematopoietic progenitors.62 Our data suggest that the effect of cAMP on the cell cycle is mediated by the inhibition of ERK1/2 activity. Several studies have demonstrated the negative role of Rap1 on cell division and ERK activation,92,93 suggesting that cAMP-induced inhibition of CD34+ cell proliferation is Rap1 dependent. Finally, given that we have previously shown that a positive effect of SDF-1 on cell proliferation and ERK activation was mediated by PKCζ,17 we propose that in progenitor cells the cAMP-induced inhibition of cell division and ERK phosphorylation is PKCζ independent (Figure 7).
In conclusion, we present novel evidence of the role and molecular mechanisms of cAMP-induced PKCζ activation in human immature hematopoietic progenitor cells that result in increased CXCR4 expression and signaling and that affect cell motility and growth.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-03-0941.
Supported by grants from the Ares-Serono Group, the Israel Science Foundation, and the Charles and David Wolfson Charitable Trust, through the Stem Cell Center in Establishment at the Weitzmann Institute of Science.
P.G. designed and performed the experiments, analyzed the data, and wrote the manuscript. A.K. and N.B. performed the experiments. M.T. performed real-time polymerase chain reaction analysis. I.P. assisted in designing the experiments. A.N. and I.H. provided cord blood and mobilized peripheral blood. T.L. supervised the work and prepared the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank LoyaAbel and Yaron Vagima for their excellent technical help and Amiela Globerson for her valuable contribution to manuscript preparation. We also thank Masakazu Hattori and Nagahiro Minato for providing us with the Spa1-GFP-expressing vector. Tsvee Lapidot holds the professional Edith Stein Immunology Chair.



![Figure 6. cAMP increases CD34+ progenitor cell survival by PKCζ-activated pathway while inhibiting their proliferation. (A) Increased survival of CB CD34+ cells cultured for 3 days under cytokine-deprived conditions in the presence of 500 μM dbcAMP (cAMP) as analyzed by flow cytometry. Data represented as fold change (mean ± SD of 6 independent experiments each in triplicate) in the number of PI-positive (Dead cells) in cAMP-treated compared with untreated (CTRL) samples. Inset shows representative changes in the proportion of CD34+ cells with fragmented DNA (dead cells [%]) cultured for the indicated time periods untreated (CTRL), treated with cAMP, or treated with cAMP and 10 μM PKCζ PS (cAMP+PSζ). (B) Comparison of the percentage of dividing CD34+ cells (mean ± SD of 4 independent experiments) cultured for 3 days in serum-supplemented medium with 500 μM cAMP or without (CTRL), fixed, and PI labeled for cell cycle analysis. (C) Ki-67 expression in G2 cells cultured in full growth medium for 3 days either untreated (CTRL) or with 500 μM cAMP. Data are shown as mean ± SD of 3 independent experiments. Representative flow cytometry analyses are shown on the right; numbers indicate percentages of Ki-67-positive cells. (D) Immunoblot analysis of ERK1/2 phosphorylation (P-ERK1/2) status compared with the total ERK1/2 level in G2 cells incubated for 24 hours with 500 μM cAMP or left untreated (CTRL). Representative of 1 of 3 independent experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-03-0941/4/m_zh80030690380006.jpeg?Expires=1769153680&Signature=3R16aPdvoM0gkiBlqA8DXtP8LjhU55OI58A-4wkTvOQj0UXBq2bmE-WGNpoeeRLOXjz9Q8-s5g9KIUJqpwb2R5~GnbjM8ET7tM935e-US80HOzLjBK9cqDBaZEZxMLapg0qNSuZO-kW9gQm1~lh0rl1sJub5FMErsUvhQZBP~2Maq3f1A01uLRj-eygal~FTUQ8SAuAE5VOh-rQ85mfkC5QWp5nOhOYS9otKhxsfY9nFfkC6ZY0v6G8HmWcczZm572OQ4N652zea16LqJZ730GJc7itc7ku2-A0iirrO1XHjlu-790rQDhlnI7HckTARL0gwYMqg4GGl4XVaOfJv2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal