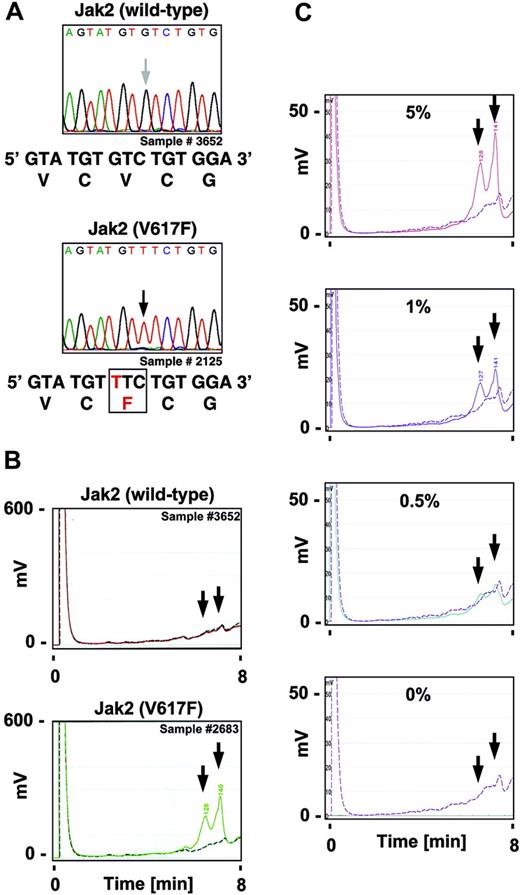
An activating mutation of the Jak2 tyrosine kinase (V617F) is commonly detected in polycythemia vera (65%-97%),1-6 essential thrombocythemia (23%-57%),1,2,4-6 and idiopathic myelofibrosis (35%-57%)1,2,4-6 as well as at low percentages in other myeloproliferative disorders and myelodysplastic syndromes.1,7 Inhibition of Jak2 kinase activity with a small-molecule drug is sufficient to inhibit cell growth of Jak2V617F transformed cells in vitro, hinting at the potential of targeting Jak2 for therapeutic use.2 As Jak2 kinase inhibitors are brought into clinical use, it will be important to screen patients for the presence of the mutation. We developed a simple and sensitive high-throughput denaturing high-performance liquid chromatography (dHPLC) assay that would enable a fast and reliable detection of small amounts of mutant Jak2V617F in peripheral blood. Twenty-five cDNA samples from peripheral-blood leukocytes of patients with a diagnosis of polycythemia vera were used and their mutational status identified by sequencing using a standard fluorescent dye method (Figure 1A). The cDNA was used to amplify a 185–base pair (bp) region proximal to V617 in Jak2 by polymerase chain reaction (PCR) under standard conditions with forward primer (also used for sequencing) 5′-GATGAGCAAGCTTTCTCACAAGC-3′ and reverse primer 5′-GCATGGCCCATGCCAACTGTTT-3′. Twenty samples showed the presence of both wild-type and V617F-mutated Jak2 (not shown) and 2 samples only mutated Jak2, similar to previously reported data.1-6 For the development of a dHPLC assay, a 269-bp region proximal to Jak2V617 was amplified by PCR with forward primer 5′-ACGGTCAACTGCATGAAACA3-′ and reverse primer 5′-CCATGCCAACTGTTTAGCAA-3′ during 45 cycles at 95°C (20 seconds), 54°C (20 seconds), and 72°C (40 seconds). We spiked all amplified patient samples with amplicons from K562 cells (Jak2 wild-type) to enable mismatch hybridization for the endonuclease digest in Jak2V617F homozygous samples. PCR product containing wild-type Jak2 from K562 cells was mixed with patient samples at a ratio of 1:3, denatured at 95°C, and slowly renatured at a rate of 0.5°C decrease/15 seconds. Samples were processed with the Surveyor Nuclease Mutation Detection Kit (Transgenomic, Omaha, NE), labeled with a fluorescent DNA intercalating dye, and analyzed on a WAVE HS system (Transgenomic). Preparation from whole blood to nuclease-treated PCR products can be routinely achieved in fewer than 5 hours and individual samples are analyzed by HPLC in 14-minute cycles. In the presence of Jak2V617F, we detected 2 distinct fragments (129 and 140 bp), visualized as peaks on the chromatogram (Figure 1B bottom panel). In the absence of a mutation, there was no mismatch and thus no peak was detected (Figure 1B top panel). To determine the threshold of sensitivity of this method, we prepared a dilution of HEL (Jak2V617F-expressing) and K562 cell samples and analyzed the peak height in relation to the relative percentage of cells in the mixture. Our control experiments indicate that we can detect the Jak2V617F mutation reliably in at least 1% of a total cell sample preparation under these conditions (Figure 1C). The dHPLC data were consistent with the direct DNA sequencing analysis and confirmed the described frequency of 88% Jak2V617F mutations in the polycythemia vera samples (Table 1). Overall, Surveyor/dHPLC analysis is a fast, reliable, and sensitive method to analyze Jak2V617F mutations in peripheral blood of patients with myeloproliferative disorders.
Evaluation of Jak2V617F expression in patients with polycythemia vera by DNA sequencing and WAVE dHPLC
. | Jak2 (V617F) . | . | |
|---|---|---|---|
| Sample . | DNA sequencing . | WAVE dHPLC . | |
| 0158 | + | + | |
| 0264 | + | + | |
| 0361 | + | + | |
| 0739 | + | + | |
| 0751 | + | + | |
| 0904 | + | + | |
| 0923 | + | + | |
| 0964 | + | + | |
| 1235 | + | + | |
| 1316 | + | + | |
| 1845 | + | + | |
| 1877 | + | + | |
| 1881 | – | – | |
| 2023 | + | + | |
| 2125 | + | + | |
| 2647 | + | + | |
| 2673 | + | + | |
| 2683 | + | + | |
| 2790 | – | – | |
| 3116 | + | + | |
| 3550 | + | + | |
| 3586 | + | + | |
| 3652 | – | – | |
| 3701 | + | + | |
| 3714 | + | + | |
. | Jak2 (V617F) . | . | |
|---|---|---|---|
| Sample . | DNA sequencing . | WAVE dHPLC . | |
| 0158 | + | + | |
| 0264 | + | + | |
| 0361 | + | + | |
| 0739 | + | + | |
| 0751 | + | + | |
| 0904 | + | + | |
| 0923 | + | + | |
| 0964 | + | + | |
| 1235 | + | + | |
| 1316 | + | + | |
| 1845 | + | + | |
| 1877 | + | + | |
| 1881 | – | – | |
| 2023 | + | + | |
| 2125 | + | + | |
| 2647 | + | + | |
| 2673 | + | + | |
| 2683 | + | + | |
| 2790 | – | – | |
| 3116 | + | + | |
| 3550 | + | + | |
| 3586 | + | + | |
| 3652 | – | – | |
| 3701 | + | + | |
| 3714 | + | + | |
Identification of the Jak2V617F mutation by direct DNA sequencing and dHPLC analysis in polycythemia vera. RNA was isolated and cDNA was prepared from the chronic myelogenous leukemia (CML) cell line K562, the erythroleukemia cell line HEL, and peripheral blood from patients with polycythemia vera, obtained with informed consent and according to institutional protocol approved by the Department of Clinical Medicine at Mannheim University of Heidelberg, per the Declaration of Helsinki. (A) Chromatograms of peripheral-blood samples from patients with polycythemia vera after direct sequencing of the forward strand of a PCR-amplified V617 proximal region in Jak2 is shown. The arrows indicate the position of the base implicated in the V617F substitution. The bottom panels show the expected mRNA and protein sequence in Jak2 (wild-type) and Jak2 with the V617F substitution. (B-C) A V617 proximal region within Jak2 was amplified by PCR, digested by Surveyor enzyme, and subjected to dHPLC analysis. Test samples (solid line) were compared to a control sample (dashed line). The arrows indicate the expected retention of the fragments in the presence of the V617F mutation. Chromatograms of peripheral-blood samples from patients with polycythemia vera (B) or HEL-cell samples indicated as a relative percentage of cells in a mixture with K562-cell samples (C) are shown.
Identification of the Jak2V617F mutation by direct DNA sequencing and dHPLC analysis in polycythemia vera. RNA was isolated and cDNA was prepared from the chronic myelogenous leukemia (CML) cell line K562, the erythroleukemia cell line HEL, and peripheral blood from patients with polycythemia vera, obtained with informed consent and according to institutional protocol approved by the Department of Clinical Medicine at Mannheim University of Heidelberg, per the Declaration of Helsinki. (A) Chromatograms of peripheral-blood samples from patients with polycythemia vera after direct sequencing of the forward strand of a PCR-amplified V617 proximal region in Jak2 is shown. The arrows indicate the position of the base implicated in the V617F substitution. The bottom panels show the expected mRNA and protein sequence in Jak2 (wild-type) and Jak2 with the V617F substitution. (B-C) A V617 proximal region within Jak2 was amplified by PCR, digested by Surveyor enzyme, and subjected to dHPLC analysis. Test samples (solid line) were compared to a control sample (dashed line). The arrows indicate the expected retention of the fragments in the presence of the V617F mutation. Chromatograms of peripheral-blood samples from patients with polycythemia vera (B) or HEL-cell samples indicated as a relative percentage of cells in a mixture with K562-cell samples (C) are shown.
Supported in part by National Institutes of Health grant DK66996, Leukemia and Lymphoma Society Specialized Center of Research (SCOR) grant (J.D.G.), and American Cancer Society Research Scholar grant (M.S.).
M.S., C.W., B.J.C., A.M.R., and Y.K. performed research; M.S. and J.D.G wrote the letter; M.S., C.W., P.A.J., Y.K., R.J.D., and J.D.G. designed research; E.L. and A.R. contributed vital new reagents; and M.S., Y.K., and R.J.D. analyzed data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal