Abstract
Gene silencing by CpG island promoter hypermethylation has awakened the interest for DNA demethylating agents as chemotherapy drugs. Zebularine (1-[β-D-ribofuranosil]-1,2-dihydropyrimidin-2-1) has been recently described as a new DNA methylation inhibitor. Here we have studied its effects in a mouse model of radiation-induced lymphomagenesis using nuclear magnetic resonance (NMR) and positron emission tomography (PET). All control animals presented large thymic T lymphomas and died between 4 and 5.5 months. In contrast, 40% (12 of 30) of zebularine-treated animals were still alive after 1 year (Kaplan-Meier P < .001). NMR and PET imaging showed that surviving animals presented a thymus structure/volume similar to normal mice of the same age. Most important, zebularine demonstrated a complete lack of toxicity in nonirradiated control mice. DNA hypomethylation induced by zebularine occurred in association with depletion in extractable DNA methyltransferase 1 protein. Thus, our data support the role of zebularine as a DNA demethylating agent with antitumor activity and little toxicity.
Introduction
Hypermethylation-associated silencing of tumor suppressor genes has been shown to occur in lymphoid/hematopoietic malignancies, disrupting many cellular pathways.1-3 Unlike genetic alterations in cancer, hypermethylation changes are potentially reversible by pharmacologic inhibition of DNA methylation.4 The cytidine analogs 5-aza-2′-deoxycytidine and 5-azacytidine are capable of reactivating tumor suppressor genes that were silenced by hypermethylation.4 These compounds act through DNA methyltransferase (DNMT) inhibition.4 Thus far, it is in hematologic malignancies where DNA demethylating agents have had their greatest success.4-6 For high-risk myelodysplastic syndrome (MDS) using 5-aza-2-deoxycytidine, it has been reported overall response rates of 49% to 54%, with 23% to 50% complete responses7-9 with a lasting effect on the platelet count.10 In myelogenous leukemia (CML), 5-aza-2-deoxycytidine achieved objective responses in 55% of patients,11 including imatinib-refractory patients.12 For 5-azacytidine, the studies have demonstrated significant complete and partial remissions in MDS patients13,14 and improved quality of life.15 In 2004, the Food and Drug Administration (FDA) approved 5-azacytidine for the treatment of all MDS subtypes.
Zebularine (1-[β-D-ribofuranosyl]-1,2-dihydropyrimidin-2-1) is a new cytidine analog that forms a covalent complex with DNA methyltransferases,16 depletes human DNA methyltransferase 1 (DNMT1),17,18 reactivates hypermethylated genes in yeast and solid tumor cells,19 has antitumor effects in mouse xenografts,19 and shows a preferential effect on cancer cells.18 To gain insight into the effects of zebularine, we used a mouse model of radiation-induced T-cell lymphoma.20 Here, we show that zebularine is effective against the development of thymic lymphoma, inducing longer overall survival. Nuclear magnetic resonance-positron emission tomography (NMR-PET) images and a pathologic histology show that zebularine-treated lymphomas closely resemble the normal thymus. These therapeutic changes occur in a context of lack of toxicity, global genomic hypomethylation, and a depletion of the Dnmt1.
Study design
Murine models and drug treatments
C57BL/6J 4-week-old mice were irradiated by gamma rays (1.75 γ) each week during 4 consecutive weeks.20 About 90% of these mice developed thymic T-cell lymphomas, with an average latency of 4.5 months.20 These thymus-dependent T-cell malignancies are formed by blasts that are positive for CD7, CD2, IgHG, and T-cell receptor βR (TCRβR) and negative for myeloperoxidase (MPO) and VpreB.20 Two groups of 30 mice (each 1 with 15 males and 15 females) were generated: the drug group receiving zebularine dissolved in phosphate-buffered saline (PBS), and the control group receiving only PBS. Intraperitoneal inoculation was used. For toxicity determination, 2 groups of 20 nonirradiated mice were also intraperitoneally inoculated with zebularine or PBS. The histopathology analysis used tissue samples stained with hematoxylin and eosin (H&E). For the mouse xenograft model of T-cell acute lymphoblastic leukemia, 106 cells of the cell line MOLT-4 were injected subcutaneously into 10 6-week-old female athymic nude mice nu/nu (Harlam Sprague Dawley, Indianapolis, IN).
Nuclear magnetic resonance and positron emission tomography
Images were acquired on a Bruker Biospec spectrometer (Bruker, Ettlingen, Germany) 4.7 T 40-cm bore horizontal-magnet (Magnex, Oxford, United Kingdom) magnetic resonance imaging (MRI) system. Images of the mouse thymus taken from a 3-dimensional data set were acquired with standard gradient coil capable of a 200-microsecond rise time and 300 milliteslas/meter (mT/m) maximum gradient strength. We analyzed the mice using PET F-18 FDG whole-body scans. The F-18 FDG intravenous dose was 0.3626 MBq (9.8 μCi). In all mice, torso PET acquisition was performed using the UGM PENN PET 240H camera (HGM, Madrid, Spain). A transmission scan was acquired after injection of F-18 FDG, with cesium 137.
Determination of 5-methylcytosine (5mC) DNA content, CpG island methylation status, and Western blot of Dnmt1
The 5-methylcytosine DNA content was determined by high-performance capillary electrophoresis (HPCE)21,22 using a P/ACE MDQ system (Beckman-Coulter España, Madrid, Spain). Three replicate analyses were performed. Western blot analysis was developed using the Dnmt1 antibody (Abcam, Cambridge, United Kingdom), as previously described.22 The cytosine-phosphate-guanosine (CpG) island methylation status of the p16INK4a, p15INK4b, MGMT, MLT1, RASSF1A, and E-cadherin genes was analyzed by bisulfite genomic sequencing and methylation-specific polymerase chain reaction (PCR), as previously described.22
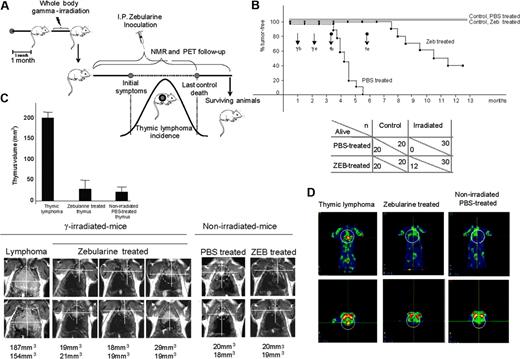
Effect of zebularine on a mouse model of gamma-induced thymic lymphoma determined by overall survival, NMR, and PET. (A) Schematic diagram of the gamma-induced mouse model of lymphomagenesis, zebularine treatment, and NMR and PET imaging follow-up. (B) Up indicates Kaplan-Meier survival curve; γ, gamma irradiation; Tb, start of zebularine treatment; and Te, end of zebularine treatment. Black and gray straight lines show nonirradiated control mice treated with PBS and zebularine (Zeb), respectively; ♦ line shows irradiated and PBS treated animals; and ▪ line shows irradiated and zebularine treated mice. Below, summary of total number of animals used in the study (n) and the surviving animals (alive). (C) Follow-up of thymus and thymic lymphoma sizes by NMR using a Bruker Biospec spectrometer/Magnex 4.7 Teslas 40-cm bore horizontal magnet MRI system. Image analysis and tumor volume measurements were performed with the public domain software ImageJ (NIH, http://rsb.info.nih.gov/ij). A marked volume reduction in the thymic mass is observed in zebularine-treated mice. (D) Metabolic activity in FDG-PET imaging; PET image shows a significant “hot spot” (yellow) confined in the thymic lymphoma that is lost in the zebularine-treated mice.
Effect of zebularine on a mouse model of gamma-induced thymic lymphoma determined by overall survival, NMR, and PET. (A) Schematic diagram of the gamma-induced mouse model of lymphomagenesis, zebularine treatment, and NMR and PET imaging follow-up. (B) Up indicates Kaplan-Meier survival curve; γ, gamma irradiation; Tb, start of zebularine treatment; and Te, end of zebularine treatment. Black and gray straight lines show nonirradiated control mice treated with PBS and zebularine (Zeb), respectively; ♦ line shows irradiated and PBS treated animals; and ▪ line shows irradiated and zebularine treated mice. Below, summary of total number of animals used in the study (n) and the surviving animals (alive). (C) Follow-up of thymus and thymic lymphoma sizes by NMR using a Bruker Biospec spectrometer/Magnex 4.7 Teslas 40-cm bore horizontal magnet MRI system. Image analysis and tumor volume measurements were performed with the public domain software ImageJ (NIH, http://rsb.info.nih.gov/ij). A marked volume reduction in the thymic mass is observed in zebularine-treated mice. (D) Metabolic activity in FDG-PET imaging; PET image shows a significant “hot spot” (yellow) confined in the thymic lymphoma that is lost in the zebularine-treated mice.
Results and discussion
We used a mouse model of radiation-induced lymphomagenesis (Figure 1A) in which thymic T-cell lymphomas kill all mice in a period of 4 to 5.5 months.20 We performed a complete and exhaustive noninvasive imaging follow-up by NMR and PET of zebularine-treated mice compared with a control group treated with PBS. An 8 mg/mouse dose (400 mg/kg), pH 7, of zebularine dissolved in PBS was given with the following experimental procedure: 200 μL/mouse once a day, every day, for 78 consecutive days.
All the PBS-treated control animals presented large thymic lymphomas and died, as expected, 4 to 5.5 months after irradiation (Figure 1B). However, 40% (12 of 30) of zebularine-treated irradiated animals were still alive after 1 year (Kaplan-Meier P < .001) (Figure 1B). The lymphoma relapse in the remaining mice always occurred after completion of the treatment, and in these cases the contribution of methylation-independent events to our lymphomagenesis model, such as homozygous deletions at the p16INK4a and p15INK4b loci, could also be relevant.23 NMR and PET imaging results confirmed that surviving animals presented a thymus similar in structure and size to normal mice of the same age, with a 5- to 8-fold lower volume than thymic lymphomas arising in the irradiated PBS-treated animals (Figure 1C-D). Furthermore, a high rate of metabolic activity was observed in the thymic lymphomas, while the thymus of zebularine-treated animals demonstrated a low metabolic activity, as in the thymus of normal mice (Figure 1D). Most important, zebularine demonstrated a complete lack of toxicity in nonirradiated control mice, showing the same thymic features by NMR and PET and overall survival time than those nonirradiated control mice that received PBS (Figure 1B-C).
Effect of zebularine on histology, lymphoma xenografts, and DNA methylation parameters. (A) Hematoxylin and eosin (H&E)-stained 4-μm section thymus samples. The pathological findings in the thymus of the zebularine-treated mice resemble the structure of the normal thymus. Images were captured with an Olympus Vanox microscope (Olympus, Melville, NY) using a 10 ×/0.35 NA objective. (B) Analysis of global DNA methylation by high-performance capillary electrophoresis (HPCE). The irradiated thymus of the zebularine-treated mice shows DNA hypomethylation compared with the lymphomas appearing in the irradiated mice that only receive PBS. (C) Western blot analysis of DNMT1 protein levels in normal thymus and lymphomas appearing in the irradiated mice that only receive PBS and in irradiated thymus of the zebularine-treated mice. (D) Top panels show female athymic nude mice 16 days after injection of 106 MOLT-4 cells. Note the large tumor on the left, corresponding to a PBS-treated mouse, and the small tumor on the opposite figure, corresponding to zebularine-treated mouse. Tumor detail is shown in millimeters, and weight is shown in milligrams. Bottom panels show effect of zebularine treatment on the in vivo growth of MOLT-4 cells. Tumor size was monitored over time, and size is shown in cubic millimeters. Tumoral weight data at 16 days with PBS- and zebularine-treated MOLT-4 xenografts presented as means ± SD.
Effect of zebularine on histology, lymphoma xenografts, and DNA methylation parameters. (A) Hematoxylin and eosin (H&E)-stained 4-μm section thymus samples. The pathological findings in the thymus of the zebularine-treated mice resemble the structure of the normal thymus. Images were captured with an Olympus Vanox microscope (Olympus, Melville, NY) using a 10 ×/0.35 NA objective. (B) Analysis of global DNA methylation by high-performance capillary electrophoresis (HPCE). The irradiated thymus of the zebularine-treated mice shows DNA hypomethylation compared with the lymphomas appearing in the irradiated mice that only receive PBS. (C) Western blot analysis of DNMT1 protein levels in normal thymus and lymphomas appearing in the irradiated mice that only receive PBS and in irradiated thymus of the zebularine-treated mice. (D) Top panels show female athymic nude mice 16 days after injection of 106 MOLT-4 cells. Note the large tumor on the left, corresponding to a PBS-treated mouse, and the small tumor on the opposite figure, corresponding to zebularine-treated mouse. Tumor detail is shown in millimeters, and weight is shown in milligrams. Bottom panels show effect of zebularine treatment on the in vivo growth of MOLT-4 cells. Tumor size was monitored over time, and size is shown in cubic millimeters. Tumoral weight data at 16 days with PBS- and zebularine-treated MOLT-4 xenografts presented as means ± SD.
All mice receiving zebularine, from the control group and the 1-year survival animals from the irradiated-lymphoma group, were killed, and careful pathologic studies were developed. We failed to identify any sign of toxicity in both groups for any organ or tissue. We did not observe any presence of chromosomal abnormalities after zebularine treatment using a standard G-band karyotype and a spectral karyotype analysis (SKY) (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). In the case of the irradiated animals, the thymus had a phenotype similar to that observed in mice of similar age without any evidence of T-cell lymphoma (Figure 2A). We found abnormal lymphocyte hyperplasia in the thymus in only 2 (16%) of 12 cases.
Our model of lymphomagenesis also demonstrated that the growth inhibitory effects of zebularine occurred in parallel with its DNA demethylating action. The thymus of 1-year survival irradiated-mice receiving zebularine had a 30% reduction of 5-methylcytosine genomic content in comparison with the radiation-induced thymic lymphomas receiving only PBS: 2.25% ± 0.02% versus 3.25% ± 0.1% (Figure 2B). Moreover, we observed demethylation of the hypermethylated CpG islands of the p16INK4a, MGMT, MLT-1, and E-cadherin genes in association with the restoration of their gene expression (Figures S1-S2). Furthermore, this DNA hypomethylating effect was associated with a depletion of the levels of extractable DNMT1 protein (Figure 2C), suggesting the trapping of this enzyme to zebularine-incorporated DNA.
Finally, we tested the efficacy of zebularine in a human experimental model equivalent to our irradiated mice developing thymic T-cell lymphomas. Nude mice received subcutaneous injections of 106 cells from the T-cell acute lymphoblastic leukemia cell line (MOLT-4) and were treated with zebularine or PBS. All mice were killed 16 days after injection, and the tumors were dissected and weighed. While the buffer-treated MOLT-4 xenografts formed tumors rapidly, the zebularine-treated group demonstrated extremely low tumorigenicity (Figure 2D). At the time of death, the lymphomas were 14 times larger in PBS-treated mice (650 ± 117 mg) than in zebularine-treated mice (47 ± 37.6 mg) (Figure 2D), supporting the efficacy of the drug in a human genetic background of T-cell lymphoma. The growth-inhibitory effects of zebularine occurred in parallel with its DNA demethylating action demonstrated by a reduction in the 5-methylcytosine genomic content, the demethylation of the hypermethylated CpG islands of the RASSF1A and p15INK4b genes, and the depletion of DNMT1 protein levels (Figures S1-S3).
In summary, recent important observations place zebularine as an important candidate agent for the epigenetic therapy of cancer.16-19,24 However, before clinical trials are undertaken, it is necessary to test the lack of toxicity and the antitumor effect of zebularine in a well-defined mouse cancer model. We provide here this missing step demonstrating that a long-term administration schedule of zebularine inhibits the growth of thymic lymphomas in mice, in association with its DNA hypomethylating action without evident side effects for normal cells or the well-being of the animals.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-05-2033.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal