Abstract
Sézary syndrome (SS) is a rare form of cutaneous T-cell lymphoma (CTCL) characterized by a distinct metastatic pattern mainly involving blood and skin. Chemokines and their receptors play a critical role in cellular recruitment and homing to tissues and in the metastatic process of several tumors including non-Hodgkin T-cell lymphomas (NHLs). Here we report that SS cells express a functionally active CXCR4 and that its ligand SDF-1 is abundantly produced in the skin, which represents the main destination of SS cell spreading. SDF-1 is normally inactivated by proteolytic cleavage by the CD26/dipeptidylpeptidase IV (DPPIV). The lack of CD26 from the cell surface is a hallmark of circulating SS cells. We also show that the CD26- phenotype is maintained also in skin-infiltrating neoplastic T lymphocytes and that SS-affected individuals exhibit a reduced activity of plasma soluble CD26. Finally, we observe that the addition of soluble CD26 reduces the migratory response of SS cells to SDF-1 whereas the inhibition of the CD26 peptidase activity in Hut78, a CD26+ CTCL cell line, enhances the SDF-1-induced migration of these cells. Our findings suggest that the SDF-1-CXCR4 axis could play an important role in skin homing of SS through the regulatory activity of CD26.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of non-Hodgkin lymphomas (NHLs) that show a considerable variability in clinical presentation, histology, immunophenotype, and prognosis. They represent a clonal malignancy1 of CD4+ helper T cells2,3 with a memory phenotype4 and tendency to accumulate in the skin.5
Mycosis fungoides (MF) and Sézary syndrome (SS) are the 2 major clinical variants of CTCLs. While MF shows a skin-restricted infiltration of the clonal T-cell population and an indolent course, SS is a leukemic and erythrodermic variant of CTCL characterized by the presence of tumor lymphocytes in the skin, lymph nodes, and peripheral blood. These tumor cells, named SS cells, have major abnormalities like a low mitotic index, aberrant but nonuniform chromosomal alterations,6 monoclonal rearrangement7 with a loss of T-cell receptor repertoire complexity8 and loss of surface molecules such as CD7, CD26, and CD49d.9-11 SS cells mainly localize to the skin and express cutaneous lymphocyte-associated antigen (CLA) that has been implicated in skin-specific homing patterns.4,9 However, the mechanisms underlying the accumulation of these atypical lymphocytes to the skin are poorly defined. Extravasation and active locomotion of malignant lymphocytes could suggest the involvement of secreted factors such as chemokines. Chemokines regulate multiple cell functions, including cell chemotaxis, proliferation, and apoptosis, and are involved in leukocyte transendothelial migration and homing to tissues. These biologic activities are mediated through their interaction with G protein-coupled chemokine receptors expressed by target cells such as leukocytes, hematopoietic cells, neuronal cells, glial cells, and cells of the vasculature.12 Recent studies have also shown that chemokines and their receptors are implicated in tumor cell growth, angiogenesis, and metastasis.13 Investigation of expression of chemokine receptors has been performed in extracutaneous NHLs. Expression of CCR4 and its ligand TARC/CCL17 was found in CTCLs such as SS and MF14 while CCR3 and its ligand eotaxin/CCL11 were detected in cutaneous CD30+ T-cell lymphoma.15
One of the hallmarks of SS cells is the loss of CD26/dipeptidylpeptidase IV (DPPIV) from the cell surface,9,11 a membrane-bound protease that, as recently demonstrated, is able in vitro to cleave and inactivate SDF-1, the ligand of the chemokine receptor CXCR4.16,17
Thus, we have hypothesized that a chemokine-mediated mechanism and, particularly, the CXCR4-SDF-1 axis could play a role in SS cells' skin homing. Here we report that CXCR4 is expressed and functionally active in SS cells and that the CXCR4 ligand SDF-1 is abundantly expressed in the skin of affected patients. Moreover, we confirm18 that neoplastic skin-infiltrating lymphoid cells are CD26-, similar to circulating SS cells, and that SS patients have a reduced activity of the soluble CD26/DPPIV enzyme. The importance of the CD26 role is further demonstrated by either the reduced SDF-1-induced chemotaxis of SS cells following the addition of soluble CD26 or the increased migratory response of Hut78, a CD26+ CTCL cell line, after the CD26 activity inhibition.
Materials and methods
Blood samples
We analyzed blood samples from 12 patients affected by SS recruited from Istituto Dermopatico dell'Immacolata (IDI) after obtaining their informed consent. Diagnosis of SS was based on clinical criteria (erythroderma, pruritus, palmoplantar keratoderma, diffuse adenopathies), histologic data (cutaneous epidermotropic T-cell lymphoma), and biologic criteria represented by CD4/CD8 ratio greater than 10, percentage of T cells with a CD4+/CD8-CD7- phenotype greater than 40%, and detection of circulating T-cell clones identified by FACS-Vβ repertoire analysis. Only one patient had been treated by chemotherapy at the time of analysis. All patients included in this study were enrolled in clinical protocols approved by the Instituto Dermopatico dell'Immacolata (IDI-IRCCS) Investigational Review Board. The characteristics of the patients are listed in Table 1.
Patient characteristics
Patient . | Sex/age, y . | Vβ family . | Vβ+ clonal, % . | Vβ+ normal, range (%*) . | Absolute no. CD3+CD4+ Vβ+ . | CD4/CD8 ratio . | T/N/M . | Disease duration, mo . | Current disease . | Current therapy . | Previous therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/61 | Failed | – | – | – | 96 | T4/N0/M0 | 38 | Progress | 30 ECP + steroids syst | ECP, IFN, steroids syst |
| 2 | M/49 | Failed | – | – | – | 7 | T4/N1/M0 | 8 | Steady | 5 ECP + IFN + steroids syst | Chemotherapy |
| 3 | F/61 | 13.6 | 98 | 0.1-3.6 (1.5) | 1 348 | 16 | T4/N0/M0 | 46 | Steady | 30 ECP + IFN + steroids syst | ECP, IFN, steroids syst |
| 4 | M/73 | 2 | 83 | 0.7-20.5 (5.6) | 3 763 | 29 | T4/N0/M0 | 46 | Steady | 1 ECP + steroids syst | None |
| 5 | M/56 | 2 | 92 | 0.7-20.5 (5.6) | 3 162 | 96 | T4/N0/M0 | 10 | Steady | 1 ECP + steroids syst | Chemotherapy + steroids syst |
| 6 | F/58 | 7.2 | 95 | 0.1-5.7 (0.9) | 13 534 | 95 | T4/N1/M0 | 12 | Progress | 1 ECP + steroids syst | IFN + steroids syst + retinoid |
| 7 | M/45 | 5.1 | 68.5 | 1.2-6.1 (2.7) | 2 748 | 17 | T4/N0/M0 | 3 | Progress | 5 ECP + IFN | None |
| 8 | M/66 | 1 | 80 | 2.5-7.9 (3.8) | 2 590 | 15 | T4/N0/M0 | 13 | Progress | 1 ECP | IFN + steroids syst + PUVA |
| 9 | M/45 | 17 | 91 | 1.7-23.2 (4.7) | 2 915 | 24 | T4/N2/M0 | 0 | Beginning | 1 ECP + IFN | Chemotherapy + PUVA |
| 10 | M/60 | 2 | 54 | 0.7-20.5 (5.6) | 1 128 | 8 | T4/N0/M0 | 0 | Beginning | IFN | None |
| 11 | M/62 | Failed | – | – | – | 48 | T4/N1/M1 | 6 | Steady | 1 ECP + IFN | Chemotherapy |
| 12 | M/70 | 12 | 88 | 0.3-4.5 (1.3) | 9 685 | 18 | T4/N0/M0 | 0 | Beginning | 1 ECP + IFN | None |
Patient . | Sex/age, y . | Vβ family . | Vβ+ clonal, % . | Vβ+ normal, range (%*) . | Absolute no. CD3+CD4+ Vβ+ . | CD4/CD8 ratio . | T/N/M . | Disease duration, mo . | Current disease . | Current therapy . | Previous therapy . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/61 | Failed | – | – | – | 96 | T4/N0/M0 | 38 | Progress | 30 ECP + steroids syst | ECP, IFN, steroids syst |
| 2 | M/49 | Failed | – | – | – | 7 | T4/N1/M0 | 8 | Steady | 5 ECP + IFN + steroids syst | Chemotherapy |
| 3 | F/61 | 13.6 | 98 | 0.1-3.6 (1.5) | 1 348 | 16 | T4/N0/M0 | 46 | Steady | 30 ECP + IFN + steroids syst | ECP, IFN, steroids syst |
| 4 | M/73 | 2 | 83 | 0.7-20.5 (5.6) | 3 763 | 29 | T4/N0/M0 | 46 | Steady | 1 ECP + steroids syst | None |
| 5 | M/56 | 2 | 92 | 0.7-20.5 (5.6) | 3 162 | 96 | T4/N0/M0 | 10 | Steady | 1 ECP + steroids syst | Chemotherapy + steroids syst |
| 6 | F/58 | 7.2 | 95 | 0.1-5.7 (0.9) | 13 534 | 95 | T4/N1/M0 | 12 | Progress | 1 ECP + steroids syst | IFN + steroids syst + retinoid |
| 7 | M/45 | 5.1 | 68.5 | 1.2-6.1 (2.7) | 2 748 | 17 | T4/N0/M0 | 3 | Progress | 5 ECP + IFN | None |
| 8 | M/66 | 1 | 80 | 2.5-7.9 (3.8) | 2 590 | 15 | T4/N0/M0 | 13 | Progress | 1 ECP | IFN + steroids syst + PUVA |
| 9 | M/45 | 17 | 91 | 1.7-23.2 (4.7) | 2 915 | 24 | T4/N2/M0 | 0 | Beginning | 1 ECP + IFN | Chemotherapy + PUVA |
| 10 | M/60 | 2 | 54 | 0.7-20.5 (5.6) | 1 128 | 8 | T4/N0/M0 | 0 | Beginning | IFN | None |
| 11 | M/62 | Failed | – | – | – | 48 | T4/N1/M1 | 6 | Steady | 1 ECP + IFN | Chemotherapy |
| 12 | M/70 | 12 | 88 | 0.3-4.5 (1.3) | 9 685 | 18 | T4/N0/M0 | 0 | Beginning | 1 ECP + IFN | None |
According to the EORTC classification, all patients had SS.
ECP indicates extracorporeal photopheresis; IFN, interferon α; PUVA, psoralens ultraviolet A; syst, systemic; and progress, that disease is progressing.
The percentage (range + median) of polyclonal TCR Vβ+ T cells expressing the indicated TCR Vβ gene observed in healthy controls
Cell line
Hut78 from American Type Culture Collection (ATCC no. TIB161; Manassas, VA), a human cell line established from peripheral blood of CTCL patients, was grown in complete RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% of fetal bovine serum (Gibco, Grand Island, NY).
Flow cytometry of Sézary cells
Peripheral blood mononuclear cells (PBMCs) from blood of 12 SS patients and 10 healthy donors used as controls were studied in detail for T-cell clonality by the detection of a dominant TCR-Vβ rearrangement using a panel of 23 Vβ monoclonal antibodies (MoAbs) (Immunotech, Marseilles, France) as previously described.9 Polymerase chain reaction (PCR) analysis, direct sequencing, and spectratyping also confirmed clonality of SS cells.9 To study chemokine receptors' expression within the neoplastic T-cell clone(s), we then performed a 3-color flow cytometry analysis using TCR-Vβ MoAbs in combination with fluorescein isothiocyanate (FITC)-conjugated anti-CD3; R-phycoerythrin (PE)-conjugated anti-CXCR3, anti-CXCR4, anti-CCR1, anti-CCR2, anti-CCR3, anti-CCR4, anti-CCR5, anti-CCR6, anti-CCR7, and anti-CCR8; and peridin chlorophyll protein (PCP)-conjugated anti-CD4 and anti-CD8 (Becton Dickinson, Heidelberg, Germany).
Quantitative analysis for 3-color flow cytometry was carried out using a FACScan instrument (Becton Dickinson). At least 10 000 events were acquired in list mode, and all data were analyzed by the CellQuest software (Becton Dickinson). Live lymphomonocytes were electronically gated by forward and right angle scatters. Results are listed in Table 2.
Expression of chemokine receptors on circulating Sézary cells
. | Within TCR-Vβ+CD3+CD4+, % . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CXCR3 . | CXCR4 . | CCR1 . | CCR2 . | CCR3 . | CCR4 . | CCR5 . | CCR6 . | CCR7 . | CCR8 . | |||||||||
| Patient no. | |||||||||||||||||||
| 1 | 1.5* | 95* | 0* | 1* | 0* | 97* | 0.9* | 0* | 96* | 0* | |||||||||
| 2 | 8* | 82* | 0* | 0.2* | 0* | 31* | 1* | 0* | 38* | 0.8* | |||||||||
| 3 | 1 | 76 | 0 | 0 | 0 | 78 | 0.7 | 0 | 82 | 2 | |||||||||
| 4 | 7 | 92 | 0 | 0 | 0 | 63 | 3.5 | 0 | 87 | 0 | |||||||||
| 5 | 0 | 65 | 0 | 0 | 0 | 92 | 0.2 | 0 | 97 | 2 | |||||||||
| 6 | 52 | 39 | 0 | 0.4 | 0 | 88 | 0.7 | 1.6 | 24 | 2 | |||||||||
| 7 | 5 | 40 | 0 | 2 | 0 | 100 | 0 | 1 | 78 | 4 | |||||||||
| 8 | 7 | 63 | 0 | 0 | 0 | 95 | 96 | 0 | 53 | 0 | |||||||||
| 9 | 18 | 56 | 0 | 0 | 0 | 34 | 8 | 0.5 | 61 | 4 | |||||||||
| 10 | 2 | 40 | 0 | 0 | 4 | 80 | 0 | 0 | 90 | 0 | |||||||||
| 11 | 50* | 71* | 0* | 0* | 0* | 100* | 0* | 0* | 82* | 1* | |||||||||
| 12 | 1 | 36 | 0 | 0 | 0 | 100 | 0 | 0 | 94 | 1.5 | |||||||||
| Healthy donors | |||||||||||||||||||
| Mean | 35 | 64 | 0.7 | 1 | 0.1 | 27 | 11.2 | 5.4 | 65 | 0.76 | |||||||||
| SD | 9 | 7.7 | 0.85 | 0.9 | 0.3 | 5.1 | 4 | 2.7 | 12.7 | 0.23 | |||||||||
. | Within TCR-Vβ+CD3+CD4+, % . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CXCR3 . | CXCR4 . | CCR1 . | CCR2 . | CCR3 . | CCR4 . | CCR5 . | CCR6 . | CCR7 . | CCR8 . | |||||||||
| Patient no. | |||||||||||||||||||
| 1 | 1.5* | 95* | 0* | 1* | 0* | 97* | 0.9* | 0* | 96* | 0* | |||||||||
| 2 | 8* | 82* | 0* | 0.2* | 0* | 31* | 1* | 0* | 38* | 0.8* | |||||||||
| 3 | 1 | 76 | 0 | 0 | 0 | 78 | 0.7 | 0 | 82 | 2 | |||||||||
| 4 | 7 | 92 | 0 | 0 | 0 | 63 | 3.5 | 0 | 87 | 0 | |||||||||
| 5 | 0 | 65 | 0 | 0 | 0 | 92 | 0.2 | 0 | 97 | 2 | |||||||||
| 6 | 52 | 39 | 0 | 0.4 | 0 | 88 | 0.7 | 1.6 | 24 | 2 | |||||||||
| 7 | 5 | 40 | 0 | 2 | 0 | 100 | 0 | 1 | 78 | 4 | |||||||||
| 8 | 7 | 63 | 0 | 0 | 0 | 95 | 96 | 0 | 53 | 0 | |||||||||
| 9 | 18 | 56 | 0 | 0 | 0 | 34 | 8 | 0.5 | 61 | 4 | |||||||||
| 10 | 2 | 40 | 0 | 0 | 4 | 80 | 0 | 0 | 90 | 0 | |||||||||
| 11 | 50* | 71* | 0* | 0* | 0* | 100* | 0* | 0* | 82* | 1* | |||||||||
| 12 | 1 | 36 | 0 | 0 | 0 | 100 | 0 | 0 | 94 | 1.5 | |||||||||
| Healthy donors | |||||||||||||||||||
| Mean | 35 | 64 | 0.7 | 1 | 0.1 | 27 | 11.2 | 5.4 | 65 | 0.76 | |||||||||
| SD | 9 | 7.7 | 0.85 | 0.9 | 0.3 | 5.1 | 4 | 2.7 | 12.7 | 0.23 | |||||||||
Calculated within TCR-Vβ unreactive T cells (see text)
Chemotaxis assay
PBMCs isolated from 7 SS patients and 4 healthy donors were allowed to migrate to different concentrations of SDF-1 (R&D Systems, Minneapolis, MN) in a chemotaxis assay as previously described.19 Briefly, chemotaxis was performed using 24-well transwell inserts with 5 μm pore size filters (Corning Costar, Corning, NY). SDF-1 at 0, 5, 100, 300, and 500 ng/mL was added to the lower chamber in 0.6 mL migration medium (MM) composed of Iscove medium, 0.5% FBS, and 25 mM HEPES. Cells from SS patients and healthy controls were washed twice and resuspended at 107/mL in MM; 100 μL of cell suspension was placed in the upper chamber and allowed to migrate for 2 hours at 37°C. CD4+ cells that migrated into the lower chamber were counted with FACScan for 60 seconds at a flow rate of 60 mL/min, as described.20 Results are shown as migration index, which represents the ratio between the number of cells that migrated in the presence of SDF-1 and the number of cells that migrated in response to MM alone.
PBMCs from 3 SS patients and controls were evaluated for their ability to migrate in response to SDF-1 in the presence or absence of soluble CD26 (sCD26) (Alexis Biochemicals, San Diego, CA). SDF-1 alone or SDF-1 mixed with sCD26 at a molecular ratio of 200:1 and 20:1, respectively, was resuspended in 100 mM Tris-HCl (pH 8) in a final volume reaction of 15 μL. After 1 hour of incubation at 37°C, the mixtures were diluted in MM and added to the lower chamber in a final volume of 600 μL. Chemotaxis assay was performed at 37 °C for 2 hours.
Three independent experiments were performed in duplicate, and migrated cells were counted as described above. The results are shown as migration index, and statistical analysis was made using the Student t test.
Hut78 chemotaxis was performed using a 24-well transwell insert with 8-μm pore size filters. Inhibition of endogenous CD26 activity on Hut78 cells was accomplished by pretreating cells with 5 mM of specific inhibitor Diprotin A (Sigma-Aldrich) for 15 minutes at 37°C in 5% of CO2. Diprotin A was kept throughout the assay. Chemotaxis was performed in the presence or absence of Diprotin A and SDF-1 at 50 ng/mL. Three independent experiments were performed, and migrated cells were counted by a hemocytometer. The results are shown as migration index, and statistical analysis was made using the Student t test.
Immunohistochemistry and immunofluorescence
Biopsies of SS patients were selected from the files of the Service of Pathology of the IDI. Pathological specimens were classified according to the European Organization for Research and Treatment of Cancer (EORTC) classification.5
According to standard protocols for immunohistochemical studies, the paraffin sections were heated for 1 hour at 55°C. After dewaxing, the slides were rehydrated through a graded ethanol series and distilled water, immersed in PBS (pH 7.4), and then boiled for 10 minutes in EDTA buffer (pH 8.0). Endogenous peroxidase was blocked with 0.3% hydrogen peroxide, and nonspecific binding was blocked with 10% normal serum. Immunostaining for SDF-1 was performed on 5 SS and 10 MF samples by incubating with anti-human SDF-1 antibody (R&D Systems) or with an isotype-matched negative control. Using streptoavidin-biotin peroxidase labeling method (DAKO, Fort Collins, CO) we performed SDF-1 immunohistochemical stainings. Sections were counterstained with hematoxylin. For CXCR4 immunofluorescence studies, frozen tissue sections (5 μM) from 5 SS skin biopsies were fixed in acetone at -20°C for 10 minutes. After the preincubation with PBS/BSA plus 10% of horse serum for 1 hour at room temperature (RT) to inhibit unspecific binding, sections were incubated overnight at 4°C with a mouse anti-human CXCR4 Ab (R&D Systems) followed by incubation with a horse biotinylated anti-mouse and avidin D-Texas Red-conjugated Ab (Vector Laboratories, Burlingame, CA). T lymphocytes were immunostained by incubation with the mouse anti-human CD3 (DAKO) for 30 minutes at RT followed by incubation with biotinylated anti-mouse (DAKO) and avidin D-FITC-conjugated Ab (Hoechst; Vector Laboratories, Burlingame, CA). Nuclei were counterstained with Hoechst, and sections were analyzed by fluorescence microscopy (Axioplan 2; Zeiss, Jena, Germany). For CD26 immunofluorescence studies, frozen tissue sections (5 μM) from 5 SS skin biopsies were incubated overnight at +4°C with a mouse anti-human CD26-antibody (Ab)-FITC conjugated (Exalpha Biologicals, Maynard, MA) followed by incubation with a rabbit anti-FITC and a goat anti-rabbit FITC conjugate (Jackson ImmunoResearch Laboratories, Bar Harbor, ME). T lymphocytes were immunostained by incubation with the mouse anti-human CD3 for 30 minutes at RT followed by incubation with a goat anti-mouse Cy5 (Jackson ImmunoResearch Laboratories). Nuclei were counterstained with propidium iodide (PI) (2 μg/mL), and sections were analyzed by confocal microscopy (Zeiss LSM 510 Meta).
In situ hybridization
In situ hybridization was performed on frozen sections from 5 SS skin biopsies according to the method already described.21 FITC-labeled sense and antisense riboprobes were synthesized by in vitro transcription of a 447-bp coding fragment of the human SDF-1 cDNA cloned into TA vector (Invitrogen, Carlsbad, CA). Linearized plasmids were transcribed by SP6 and T7 polymerase (Roche, Milan, Italy). The hybridization signals were detected using a rabbit anti-FITC Ab and FITC-conjugated goat anti-rabbit Ab. Neoplastic T lymphocytes, already evaluated by serial histologic sections of the same specimens, were immunostained by incubating a mouse anti-human CD3 and a Cy3-conjugated anti-mouse Ab (Jackson ImmunoResearch Laboratories). Nuclei were stained by PI (2 μg/mL). Preparations were analyzed using a confocal microscope (Zeiss LSM 510 Meta).
CD26/DPPIV activity
CD26/DPPIV activity was assayed on plasma of 11 SS patients, 7 MF patients, and 7 healthy donors using Gly-Pro-p-nitroanilide (Gly-Pro-pNA) (Sigma-Aldrich) as chromogenic substrate. Eight microliters of plasma was incubated at 37°C for 10 to 60 minutes with 4 mM Gly-Pro-pNA in 100 μL PBS buffer (pH 7.4) containing 10 mg/mL BSA into 96-well flat microplates. Proteolytic activity was determined by monitoring pNA release with spectrophotometry at 405 nm. Inhibition of Gly-Pro-pNA cleavage was determined in the presence of 4 mM Diprotin A preincubated for 15 minutes at 37°C prior to the addition of the substrate. Tests were run in triplicate. Absorbance was measured at 405 nm by microplate reader (Bio-Rad, Hercules, CA).
Statistical analysis
Results are expressed as mean plus or minus SD or SEM of 3 or more experiments performed in triplicate. Statistical analysis was performed using the 2-tailed Student t test.
Results
CXCR4, CCR4, and CCR7 receptors are expressed by a high percentage of circulating Sézary cells
We have analyzed 12 patients with SS, diagnosed by both clinical and morphologic criteria (Table 1), and 10 healthy donors as controls for chemokine receptor expression.
T-cell clonality was assessed by flow cytometric analysis of the TCR-Vβ repertoire using a panel of TCR-Vβ antibodies that covers 65% to 70% of all TCR-Vβ domains on CD3+CD4+ peripheral lymphocytes from patients and healthy controls. Nine of the 12 SS patients showed a restricted TCR-Vβ reactivity ranging from 54% to 98% with a Vβ2 expansion observed in 3 cases (Table 1). Clonality was also confirmed through PCR, direct sequencing, and spectratyping analysis (not shown). TCR-Vβ repertoire analysis failed to identify a clonal population in 3 examined patients (case nos. 1, 2, and 11). Thus, in these 3 latter patients, we applied, as previously described,9 an indirect approach probing all the circulating CD3/CD4 cells with a mixture of all commercially available Vβ MoAbs in a single reaction. This let us detect an extremely reduced proportion of stained cells (less than 5% compared with about 70% in healthy donors). This result is compatible with the presence of a large unreactive T-cell population undetectable by the current panel of antibodies.22
To define the phenotype of these expanded tumor cells, we used a 3-color fluorescence-activated cell sorter (FACS) analysis approach, employing, for each patient, the specific reactive Vβ MoAb in combination with CXCR3, CXCR4, and CCR1-CCR8 chemokine receptors.
As shown in Table 2, we found that CCR4, CCR7, and CXCR4 were consistently expressed within the specific TCR-Vβ-positive population detected in SS patients. CCR4 expression ranged from 31% to 100% with an average value of 80% ± 25% (versus 27% ± 5.1% of healthy polyclonal controls), CCR7 from 24% to 97% with an average value of 74% ± 24% (versus 65% ± 12.7% of controls), and CXCR4 from 36% to 95% with an average value of 63% ± 21% (versus 64% ± 7.7% of controls). CCR1, CCR2, CCR3, CCR6, and CCR8 were absent in all SS patients, similarly to controls. Heterogeneity was otherwise observed in the expression of CCR5 and CXCR3. CCR5, in fact, was absent in all patients with the exception of patient no. 8 (with 96% of positive cells), whereas CXCR3 was found in 3 of 12 samples ranging from 18% to 52%.
Similar data, confirming the expression of all chemokine receptors investigated, were supported at the RNA level by the use of Affymetrix U133A Gene Chips (Affymetrix, Santa Clara, CA) on a group of 12 SS patients (data not shown).
CXCR4 receptor is expressed by skin-infiltrating neoplastic T lymphocytes of SS patients
Because roles of CCR4 and CCR7 have been partially disclosed by Ferenczi et al,14 Kallinich et al,23 and Sokolowska-Wojdylo,24 we focused our attention on possible function of CXCR4 and its ligand SDF-1 in SS. Therefore, we first investigated whether CXCR4 was also expressed by skin-infiltrating neoplastic T lymphocytes by performing CXCR4 immunofluorescence on 5 frozen SS skin biopsies.
The morphologic features of cell malignancy were determined by histologic analysis performed with hematoxylin-eosin staining shown in Figure 1A-B. The immunofluorescence staining clearly demonstrated the CXCR4 expression in dermal/epidermal-infiltrating neoplastic T lymphocytes (Figure 1C), which were recognized also by a CD3 staining (Figure 1D). CD26 was absent on these atypical T lymphocytes while it was abundantly expressed in CD3 cells. (Figure 1E). In situ hybridization of SDF-1 mRNA also showed no expression in SS skin-infiltrating neoplastic T lymphocytes (Figure 1F).
SDF-1 is a chemotactic factor for circulating SS cells
To assess whether CXCR4 expressed by SS cells was functionally active, we analyzed circulating T lymphocytes in a chemotaxis assay in response to SDF-1.
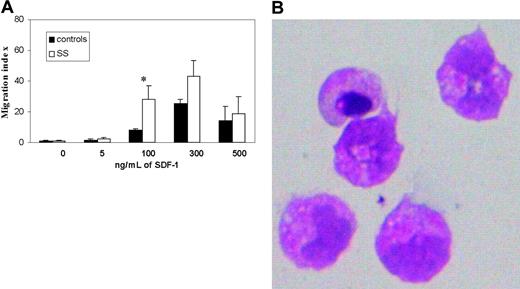
A total of 7 SS cases and 4 healthy controls were studied. Results shown in Figure 2A revealed the ability of PBMCs from all SS-affected individuals (7 of 7) to migrate toward SDF-1 with a typical bell-shaped dose-response curve peaking at 300 ng/mL. As expected, PBMCs from healthy donors also efficiently migrated to SDF-1 although migration indexes were lower than in SS PBMCs. (Figure 2A). Cytologic evaluation of SS cells that migrated into the lower chambers confirmed their morphologic neoplastic features (Figure 2B).
In addition to chemoattraction, SDF-1 might also contribute to the metastasis process acting either as a survival factor or by affecting tumor cell apoptosis.25,26 To evaluate such a possibility, we cultured PBMCs from 6 SS patients and 6 healthy donors in serum-free conditions and in media supplemented with 10% FBS in the presence of 100 and 300 ng/mL SDF-1.After 3 and 6 days of culture, the cells were stained with a PE conjugate anti-CD4, annexin V-FITC, and PI and were evaluated for apoptosis by FACS analysis. No significant differences in cells undergoing apoptosis were observed between SDF-1-stimulated and unstimulated CD4+ neoplastic cells at all doses of SDF-1 tested (not shown).
SDF-1 is abundantly expressed in the skin of SS patients but is not constitutively produced by neoplastic T lymphocytes colonizing the skin
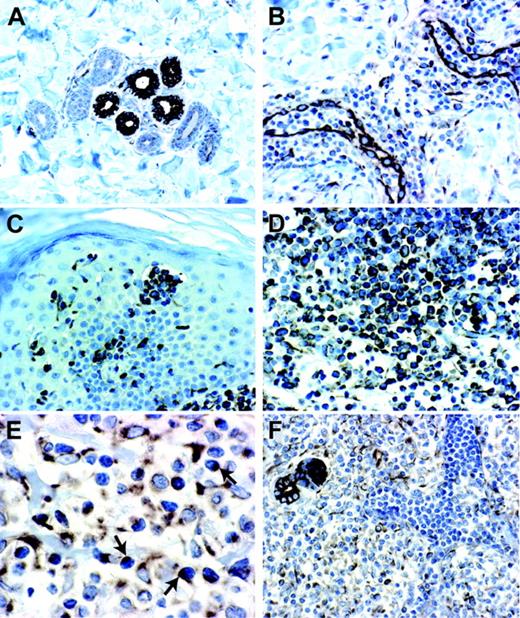
Because SS cells can migrate in vitro in response to SDF-1, we determined whether this chemokine was expressed in the skin of SS patients where it could possibly be involved in the recruitment of neoplastic cells. To this aim, we used immunohistochemistry (IHC) analysis to determine the expression levels of SDF-1 in lesional skin biopsies of CTCLs as well as in normal and inflamed skin control samples. The histopathological sections examined were from 5 patients with SS and 10 patients with MF, because they represent 2 commonly associated diseases sharing many morphologic and biologic abnormalities.27 In substantial agreement with previously described data,28 we found that SDF-1 is normally expressed in epithelial, dendritic, endothelial, and small perivascular lymphocyte cells of both healthy and inflamed skin (Figure 3A-B); SDF-1 immunoreactivity, in all cases of SS and MF, was strongly associated with the dermal/epidermal malignant lymphocyte infiltrate as shown in Figure 3C-F. Differently from normal lymphocytes, which show a uniform SDF-1 localization both into the cytoplasm and on the cell surface (Figure 3B), neoplastic cells in some cases exhibited a dotlike immunostaining (Figure 3E), possibly representing the consequence of a CXCR4 receptor polarization to the leading edge in response to the chemotactic SDF-1 stimulus.29,30
To establish whether SDF-1 immunoreactivity shown by skin neoplastic T lymphocytes reflected the chemokine uptake from the extracellular environment31 or was the consequence of autocrine synthesis, we performed an in situ hybridization on 5 frozen samples obtained from the same patients already evaluated by SDF-1 IHC. Confocal microscopy analysis failed to reveal any signal corresponding to a specific SDF-1 mRNA hybridization associated with the neoplastic T lymphocytes (Figure 1F). In keeping with this result, microarray analysis showed comparable very low levels of SDF-1 mRNA in circulating SS cells and healthy CD4+ lymphocytes (data not shown)
CXCR4 and CD26 immunoreactivity and in situ hybridization analysis of SDF-1 expression in an SS skin lesion. (A) Hematoxylin-eosin (H&E) staining of serial sections from a frozen SS skin biopsy reveals the morphologic features of neoplastic T lymphocytes that infiltrate the dermis (magnification × 10/0.30 NA) and (B) (magnification × 40/0.75 NA) where eccrine glands are indicated by white arrows. (C-D) CXCR4-CD3 immunofluorescence shows that the totality of neoplastic CD3-expressing T lymphocytes (green [D]) also express CXCR4 (red [C]) (magnification × 40/0.75 NA). (E) CD26-CD3 costaining analyzed by confocal microscopy shows that the totality of CD3+ neoplastic T lymphocytes (red) do not express CD26 on their surface (green) while few CD3-CD26+ cells are present. (F) In situ hybridization analysis did not reveal any signal corresponding to specific SDF-1 mRNA expression in neoplastic T lymphocytes. SDF-1 mRNA is detected in epithelial cells of eccrine glands (arrows). Magnification for panels E and F, × 40/1.13 NA.
CXCR4 and CD26 immunoreactivity and in situ hybridization analysis of SDF-1 expression in an SS skin lesion. (A) Hematoxylin-eosin (H&E) staining of serial sections from a frozen SS skin biopsy reveals the morphologic features of neoplastic T lymphocytes that infiltrate the dermis (magnification × 10/0.30 NA) and (B) (magnification × 40/0.75 NA) where eccrine glands are indicated by white arrows. (C-D) CXCR4-CD3 immunofluorescence shows that the totality of neoplastic CD3-expressing T lymphocytes (green [D]) also express CXCR4 (red [C]) (magnification × 40/0.75 NA). (E) CD26-CD3 costaining analyzed by confocal microscopy shows that the totality of CD3+ neoplastic T lymphocytes (red) do not express CD26 on their surface (green) while few CD3-CD26+ cells are present. (F) In situ hybridization analysis did not reveal any signal corresponding to specific SDF-1 mRNA expression in neoplastic T lymphocytes. SDF-1 mRNA is detected in epithelial cells of eccrine glands (arrows). Magnification for panels E and F, × 40/1.13 NA.
CD26/DPPIV is not detectable in SS skin lesions, and its soluble activity is reduced in SS/MF patients' plasma
It has previously been described that the lack of CD26 is a distinguishing feature of circulating SS cells,9,11 and several studies have highlighted how CD26 is able to cleave SDF-1, generating an inactive chemokine both in vitro and under physiological conditions.16,17 We therefore investigated whether SS-infiltrating neoplastic T lymphocytes also exhibited lack of CD26 expression. Thus, frozen tissue sections were subjected to CD26 immunofluorescence and analyzed by confocal microscopy. Results shown in Figure 1E revealed that CD26 is undetectable in all SS patients' skin biopsies.
Chemotactic response to SDF-1 of circulating T lymphocytes isolated from SS patients and healthy donors. (A) Chemotactic response of CD4+ SS cells and CD4+ control cells following exposure to increasing concentrations of SDF-1 (5 to 500 ng/mL) with a typical bell-shaped response curve. Results (mean ± SEM from 3 separate experiments) are shown as migration indexes representing the ratio between the number of cells that migrated in the presence of SDF-1 and the number of cells that migrated in response to medium alone. *Significant differences between controls (▪) and SS (□) by the Student t test (controls, n = 4; SS, n = 7; P < .05). (B) Cells that migrated in the lower chambers in response to SDF-1 were collected and stained with hematoxylin-eosin to evaluate their morphologic features. Original magnification × 40.
Chemotactic response to SDF-1 of circulating T lymphocytes isolated from SS patients and healthy donors. (A) Chemotactic response of CD4+ SS cells and CD4+ control cells following exposure to increasing concentrations of SDF-1 (5 to 500 ng/mL) with a typical bell-shaped response curve. Results (mean ± SEM from 3 separate experiments) are shown as migration indexes representing the ratio between the number of cells that migrated in the presence of SDF-1 and the number of cells that migrated in response to medium alone. *Significant differences between controls (▪) and SS (□) by the Student t test (controls, n = 4; SS, n = 7; P < .05). (B) Cells that migrated in the lower chambers in response to SDF-1 were collected and stained with hematoxylin-eosin to evaluate their morphologic features. Original magnification × 40.
Immunohistochemistry for SDF-1 in normal, inflamed, and neoplastic CTCL skin biopsies. (A) Normal skin showing epithelial cells of eccrine glands with a strong SDF-1 immunoreactivity associated with membrane and cytoplasm (magnification 20×/0.40 NA). (B) Chronic dermatitis showing endothelial cells of dermal capillary vessels and scant perivascular lymphoid infiltrate with a strong SDF-1 immunoreactivity (magnification 20×/0.40 NA). (C) SS skin lesion showing a strong SDF-1 immunoreactivity associated with neoplastic T lymphocytes of pathognomonic Pautrier microabscess and neoplastic T lymphocytes that infiltrate dermis and epidermis (magnification 20×/0.40 NA). (D) MF skin lesion showing an SDF-1 immunoreactivity associated with neoplastic T lymphocytes that infiltrate dermis and epidermis (magnification × 40/0.65 NA). (E) SS skin lesion showing neoplastic T lymphocytes with an SDF-1 dotlike immunoreactivity (magnification × 60/0.80 NA). (F) SS skin lesion showing SDF-1 positivity associated with neoplastic T lymphocytes infiltrating the dermis, whereas the intravascular neoplastic T lymphocytes appear SDF-1 negative (magnification 20×/0.40 NA).
Immunohistochemistry for SDF-1 in normal, inflamed, and neoplastic CTCL skin biopsies. (A) Normal skin showing epithelial cells of eccrine glands with a strong SDF-1 immunoreactivity associated with membrane and cytoplasm (magnification 20×/0.40 NA). (B) Chronic dermatitis showing endothelial cells of dermal capillary vessels and scant perivascular lymphoid infiltrate with a strong SDF-1 immunoreactivity (magnification 20×/0.40 NA). (C) SS skin lesion showing a strong SDF-1 immunoreactivity associated with neoplastic T lymphocytes of pathognomonic Pautrier microabscess and neoplastic T lymphocytes that infiltrate dermis and epidermis (magnification 20×/0.40 NA). (D) MF skin lesion showing an SDF-1 immunoreactivity associated with neoplastic T lymphocytes that infiltrate dermis and epidermis (magnification × 40/0.65 NA). (E) SS skin lesion showing neoplastic T lymphocytes with an SDF-1 dotlike immunoreactivity (magnification × 60/0.80 NA). (F) SS skin lesion showing SDF-1 positivity associated with neoplastic T lymphocytes infiltrating the dermis, whereas the intravascular neoplastic T lymphocytes appear SDF-1 negative (magnification 20×/0.40 NA).
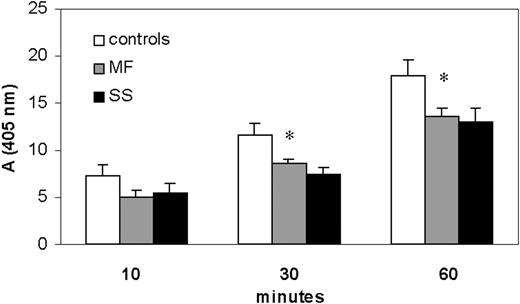
Plasma CD26/DPPIV activity in SS and MF patients and healthy donors. CD26 activity measured in the plasma of SS and MF patients and compared with that of healthy donors. Using the chromogenic substrate Gly-Pro-pNA, the production of pNA by CD26 activity was monitored at 405 nm. After 30 minutes of reaction, adsorbance values (mean ± SEM from 3 separate experiments) indicated that MF and SS patients displayed, respectively, a significant reduction of CD26 activity of 26% (8.6% ± 0.43%) and 33% (7.4% ± 0.74%) when compared with controls (116% ± 1.3%). After 60 minutes the reduction was about 26% in both MF and SS. *Significant differences between MF/SS and controls by Student t test (MF, n = 7; SS, n = 11; P < .05).
Plasma CD26/DPPIV activity in SS and MF patients and healthy donors. CD26 activity measured in the plasma of SS and MF patients and compared with that of healthy donors. Using the chromogenic substrate Gly-Pro-pNA, the production of pNA by CD26 activity was monitored at 405 nm. After 30 minutes of reaction, adsorbance values (mean ± SEM from 3 separate experiments) indicated that MF and SS patients displayed, respectively, a significant reduction of CD26 activity of 26% (8.6% ± 0.43%) and 33% (7.4% ± 0.74%) when compared with controls (116% ± 1.3%). After 60 minutes the reduction was about 26% in both MF and SS. *Significant differences between MF/SS and controls by Student t test (MF, n = 7; SS, n = 11; P < .05).
The precise source for plasma CD26 is unclear; it may derive from all CD26-expressing cells that come into contact with blood. Nevertheless, circulating neoplastic T lymphocytes in SS do not express CD26, an immunologic feature that has been described also in MF disease11,32 ; hence, we assessed whether soluble CD26/DPPIV activity was detectable in the plasma of SS/MF patients. Using the chromogenic substrate Gly-Pro-pNA, we monitored the production of pNA determined by CD26/DPPIV cleavage and found that SS/MF patients' plasma displayed a lower CD26/DPPIV activity than healthy controls (Figure 4). In particular, after 30 minutes of reaction, absorbance values (mean ± SEM of 3 independent experiments) indicated a statistically significant enzymatic activity reduction of a mean of 26% (8.6% ± 0.43%) and 33% (7.4% ± 0.74%,) respectively, in MF and SS patients when compared with controls (11.6% ± 1.3%). After 60 minutes we observed a reduction of about 26% in both MF and SS. The addition of CD26 antagonist, Diprotin A, to plasma reduced the level of pNA over 95%, demonstrating the specificity of the assay (not shown).
Lack of CD26 from the cell surface enhances the in vitro migratory response of SS cells induced by SDF-1
Recently it has been demonstrated that N-terminal-truncated SDF-1 fails to induce in vitro migration of CD34+ hematopoietic progenitor cells and that the inhibition (or loss) of CD26 activity on these cells enhances their migratory response to SDF-1.33,34
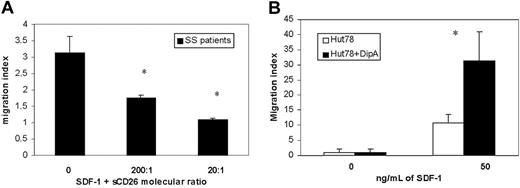
To evaluate if exogenous CD26 activity could reduce the migratory response of CD26-circulating SS cells induced by SDF-1, we performed a chemotaxis assay using PBMCs from 3 SS patients in response to SDF-1 in the absence and presence of soluble CD26. As shown in Figure 5A, SDF-1 supplemented with sCD26 at molecular ratios of 200:1 and 20:1 reduced the SDF-1 chemotactic activity, respectively, 44% and 65% if compared with the response induced by SDF-1 alone. Similar results were observed with PBMCs from healthy controls (not shown). Furthermore, to verify that the inhibition of CD26 activity could instead improve the responsiveness to SDF-1 in SS cells, we performed a chemotaxis assay using Hut78, a human CTCL cell line.
The immunophenotype characterization performed by a FACS analysis revealed that, although Hut78 cells possess a typical CD4+, CD7-, CXCR4+ SS cell phenotype, they express CD26 on their cell surface, and they were therefore used for CD26 inhibition studies.
Although we assessed the chemotatic response of Hut78 to different concentrations of SDF-1 (50 to 300 ng/mL), we observed that Hut78 cells show the higher chemotactic activity in response to 50 ng/mL SDF-1. Treatment with 5 mM Diprotin A demonstrated up to 3.1-fold of SDF-1-directed chemotactic activity (Figure 5B).
Discussion
Chemokine receptors have been involved in the regulation of tissue homing, metastasis, proliferation, and apoptosis. In our attempt to identify crucial molecules involved in the regulation of SS cell functions, we analyzed the expression of several chemokine receptors and found that CXCR4 was abundantly expressed by circulating SS cells and skin-infiltrating neoplastic T lymphocytes. CXCR4 activity is regulated by its interaction with the ligand SDF-1, and this SDF-1-CXCR4 axis is essential for leukocytes trafficking19 and can mediate both proliferative35 and apoptotic stimuli25,26 in hematopoietic cells.
Furthermore, the CXCR4-SDF-1 axis is involved in the metastasis of breast,36 lung,37 ovarian,38 pancreatic,39 neuroblastoma,40 renal,41 thyroid,42 rhabdomyosarcoma,43 prostate,44 and colorectal45 cancers as well as several types of lymphomas.46-49
In this study we have demonstrated that the expression of CXCR4 might have a functional relevance in both circulating and skin-infiltrating SS cells. Its ligand, SDF-1, that is expressed in skin lesions of these patients but not in the circulating SS cells is a potent inducer of chemotaxis of SS cells. Moreover, the absence of CD26 peptidase, the lack of which is known to be a hallmark of circulating SS cells, is potentially involved in SDF-1 processing. Together, these findings suggest that CXCR4-mediated signals may represent a homing mechanism of SS cells to the skin, their preferred metastatic site.
The effects of CD26 activity on chemotaxis of SS cells induced by SDF-1. (A) Chemotactic response of CD4+ SS cells following exposure of SDF-1 (300 ng/mL) in the absence or presence of soluble CD26 (sCD26), respectively, at a molecular ratio of SDF-1 and sCD26 of 200:1 and 20:1. Results (mean ± SEM from 3 separate experiments) are shown as migration indexes representing the ratio between the number of cells that migrated in the presence of SDF-1 alone or supplemented with sCD26 and the number of cells that migrated in response to medium alone. *Significant differences between SDF-1 supplemented with both sCD26 concentrations and SDF-1 alone calculated by the Student t test (SS, n = 3; P < .05). (B) Chemotaxis assay performed comparing Diprotin A-treated (▪) and untreated (□) Hut78 cell line. Treatment with 5 mM Diprotin A enhances up to 3.1-fold of SDF-1-directed chemotactic activity. *Significant differences between untreated and treated Hut78 cells by the Student t test (n = 6; P < .05).
The effects of CD26 activity on chemotaxis of SS cells induced by SDF-1. (A) Chemotactic response of CD4+ SS cells following exposure of SDF-1 (300 ng/mL) in the absence or presence of soluble CD26 (sCD26), respectively, at a molecular ratio of SDF-1 and sCD26 of 200:1 and 20:1. Results (mean ± SEM from 3 separate experiments) are shown as migration indexes representing the ratio between the number of cells that migrated in the presence of SDF-1 alone or supplemented with sCD26 and the number of cells that migrated in response to medium alone. *Significant differences between SDF-1 supplemented with both sCD26 concentrations and SDF-1 alone calculated by the Student t test (SS, n = 3; P < .05). (B) Chemotaxis assay performed comparing Diprotin A-treated (▪) and untreated (□) Hut78 cell line. Treatment with 5 mM Diprotin A enhances up to 3.1-fold of SDF-1-directed chemotactic activity. *Significant differences between untreated and treated Hut78 cells by the Student t test (n = 6; P < .05).
One might argue that SDF-1 is constitutively expressed in a number of tissues, including bone marrow and lung, yet Sézary cells rarely enter these tissues. This conundrum might be explained, for example, by the coexistence of specific skin-homing receptors such as CLA and CCR4, and of CXCR4, in SS cells. On the contrary, the lack of SS spreading into other tissues may be dictated by the absence of additional signals. In this regard, recently Xu et al50 demonstrated that lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is dependent on CD49d-VCAM1 interaction. Interestingly, CD49d is not present on the SS cell surface.9
In this study we also investigated several functional aspects of CXCR4-SDF-1 interaction. One aspect, in our opinion, is given by the mechanisms that inactivate SDF-1. In vitro, SDF-1 can be enzymatically cleaved by a variety of proteases51 and, among these, matrix metalloproteinases and CD26/DPPIV are well characterized.17,52 CD26 is absent on both circulating and skin-infiltrating SS tumor cells. A soluble form of CD26 lacking the transmembrane domain is also found in plasma and other biologic fluids. The origin of this soluble form is not clear, although it could be generated by the cleavage of CD26 expressed by cells in contact with the blood.53 Therefore, the reduced level of CD26 activity that we found in the plasma of both SS and MF patients could be secondary to the diminished number of circulating CD26+ cells present in these variants of CTCLs.
The proteolytic cleavage of endogenous SDF-1 in the bone marrow has been identified as a key modulator of hematopoietic progenitor cell mobilization to peripheral blood.54-56 Further, the increase in SDF-1 expression in ischemia has a critical role in mobilization of endothelial progenitor cells (EPCs), recruitment to the injury site, and differentiation into vascular cells.57-59 Recently, functional studies have shown that the inhibition of CD26 enhances the migratory response to SDF-1 in hematopoietic progenitor cells.34 Interestingly, we have shown that the addition of soluble CD26 reduces the SDF-1-induced chemotaxis of SS cells whereas the inhibition of CD26 activity on Hut78, a CD26+ CTCL cell line, by a specific antagonist increases the migration response of these cells to SDF-1 when compared with healthy CD4+ cells. Thus, these results support the hypothesis that CXCR4-mediated signals may regulate CTCL skin accumulation and that the CD26 molecule may play a role in this process. Furthermore, these data suggest that the lack of CD26 from SS tumor cell surface might act as a reinforcing mechanism of SDF-1-directed chemoattraction in these patients and could also explain the abundance of this chemokine detected at the skin level in SS patients. These results also might assume relevance for MF, which, differently from SS, has an indolent clinical course with slow progression, over years or sometimes decades, from patches to more infiltrated skin plaques and eventually tumor.5 Because high SDF-1 levels have been detected in MF skin and a reduced soluble CD26 activity was measured in MF patients' plasma, the CXCR4-dependent skin-homing mechanism hypothesized above for the SS could also apply to this indolent variant of CTCL.
Cells that express both a growth factor and its receptor have the potential for self-stimulatory or autocrine growth in normal or malignant hematopoietic cells.60,61 In CTCL, for example, IL-15 has been shown to exert autocrine growth.62 Circulating SS cells are usually in resting phase and unable to properly respond to T-cell mitogen stimulations.63,64 On the contrary, neoplastic lymphocyte proliferation is observable in skin infiltrates.65 We therefore investigated whether SDF-1 may be produced by skin-infiltrating tumor cells and found that, while neoplastic T lymphocytes filling the capillary vessels do not express SDF-1, those infiltrating the skin nearby are moderately positive for SDF-1, possibly suggesting a chemokine uptake from the surrounding microenvironment as described for other citokines/chemokines.31 It is in fact unlikely that SS cells could acquire the ability to express SDF-1 once they have infiltrated the skin, because no SDF-1 mRNA expression was found in infiltrating neoplastic cells by in situ hybridization. Our data suggest that no SDF-1-mediated autocrine regulation of SS cell functions is likely to occur in the skin.
Unlike their normal CD4+ counterparts, SS cells have prolonged life spans and are resistant to chemotherapeutic agent-induced cell death.66 We have therefore investigated whether SDF-1 could be involved in the regulation of apoptosis of SS cells and found no significant differences between SS and healthy CD4+ cells following in vitro SDF-1 stimulation.
In conclusion, these findings suggest that the SDF-1-CXCR4 axis, through the regulatory activity of CD26, may contribute to CTCL cell skin recruitment and accumulation. Because SS cells preferentially home and proliferate into skin, CXCR4 signaling may contribute to CTCL disease progression. It can therefore be hypothesized that blocking the CXCR4-SDF-1 axis, by means of specific CXCR4 antagonists, could represent a useful tool to interfere with tumor progression in CTCL patients.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-04-1492.
Supported by Associazione Italiana Ricerca sul Cancro (M.G.N.) and Ministero della Salute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Tullio Faraggiana and Dr Marie Perez of the Pathology Service of the IDI; Dr Fabio Facchetti and Ms Silvana Festa of Università degli studi di Brescia, respectively, for scientific expertise and technical assistance to arrange the SDF-1 IHC analysis; Dr Antonella Mangoni at IDI, Laboratorio di Patologia Vascolare, for technical assistance; Dr Fausto Titti at Istituto Superiore di Sanità of Rome for providing cell lines; Dr Antonio Facchiano at IDI, Laboratorio di Patologia Vascolare, and Dr Monica Pascucci at IDI, Laboratorio di Biologia Molecolare, for helpful discussions during the preparation of this work; and Dr Gioia Di Luigi at IDI for expert secretarial assistance.

![Figure 1. CXCR4 and CD26 immunoreactivity and in situ hybridization analysis of SDF-1 expression in an SS skin lesion. (A) Hematoxylin-eosin (H&E) staining of serial sections from a frozen SS skin biopsy reveals the morphologic features of neoplastic T lymphocytes that infiltrate the dermis (magnification × 10/0.30 NA) and (B) (magnification × 40/0.75 NA) where eccrine glands are indicated by white arrows. (C-D) CXCR4-CD3 immunofluorescence shows that the totality of neoplastic CD3-expressing T lymphocytes (green [D]) also express CXCR4 (red [C]) (magnification × 40/0.75 NA). (E) CD26-CD3 costaining analyzed by confocal microscopy shows that the totality of CD3+ neoplastic T lymphocytes (red) do not express CD26 on their surface (green) while few CD3-CD26+ cells are present. (F) In situ hybridization analysis did not reveal any signal corresponding to specific SDF-1 mRNA expression in neoplastic T lymphocytes. SDF-1 mRNA is detected in epithelial cells of eccrine glands (arrows). Magnification for panels E and F, × 40/1.13 NA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/3/10.1182_blood-2005-04-1492/4/m_zh80030690390001.jpeg?Expires=1767732462&Signature=xkwX6~WFiofiLuo0wyv7N01gDCXAfAd1wTx8AvdhSW8I662JE8Q~cL0zrL0iWUXryHbjHPX~7eoFzrZYFDbygU7x6i0a6W5Kug9Gaf8DOJy74VhUeOECgB6ge~IgjNry9WxEQEqkexMVZ1hhwzEGeskRjxnFgSGLQGA6qCnhq4Bd65byEi4sxQSBsLmEvgzAZIOD~E-Is1u~0H4yX6SaRC9T7JqkY9mliBXsSHCYuXLepcu5kdUpPYd1zvB~SRft2OQZlTfqTz8o0j1Q48ViqRq1gBADwH04yxwnWHDJwRMFpQ1gfQOVtzRkJyDj4q4FLpu7wyUC3PAsnNDRWqojuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal