Abstract
Localization of circulating lymphocytes to a site of inflammation is paramount for the development and maintenance of an immune response. In vitro studies using cell lines have previously demonstrated that rolling and adhesion of lymphocytes on endothelium requires CD44 interactions with hyaluronan (HA). To date, whether CD44 has a role in mediating CD4+-polarized T-helper 1 (Th1) and Th2 lymphocyte interactions with the endothelium in vivo is yet to be determined. In this study we used intravital microscopy to demonstrate that both Th1 and Th2 lymphocytes use CD44 to roll and adhere to tumor necrosis factor-α (TNFα)–activated microvasculature. Furthermore, chimeric studies imply that CD44 expression by both the endothelium and lymphocytes is essential for these interactions to occur. HA was also necessary for T cell–endothelial cell interactions in vivo and Th1 and Th2 cells rolled on immobilized HA in vitro via CD44. In vitro, both Th1 and Th2 lymphocytes have increased expression of CD44 and greater binding of fluorescent HA than naive cells. The interactions of Th1 and Th2 cells were entirely dependent upon both P-selectin and CD44 in vivo, but did not appear to be counter ligands in vitro. Taken together, these results suggest that CD44 and HA are key to both Th1 and Th2 lymphocyte interactions with the TNFα-activated endothelium and raises the possibility of cooperativity between the P-selectin/PSGL-1 and HA/CD44 pathways for Th1 and Th2 rolling in vivo.
Introduction
The CD44 family of transmembrane glycoproteins is present on a wide variety of cell types, including endothelial cells, lymphocytes, neutrophils, fibroblasts, and neurons.1 The family consists of some 20 different isoforms, each generated through differential splicing of 10 variably expressed exons, with CD44H (hematopoietic), also known as CD44s (standard) containing none of the variably expressed exons, being the most abundant form.2 The region encoded by the variant exons is highly hydrophilic and is heavily modified by O-linked glycosylation and in some cases by glycosaminoglycan addition, which can influence ligand binding. Hyaluronan (HA) has been proposed as the principal ligand for CD44,3,4 but CD44 has also been shown to bind to collagens, fibronectin, laminin, chondroitin sulfate, and osteopontin.5-7
The strategic positioning of HA on the endothelium as well as CD44 on both the endothelium and leukocytes suggests that this molecule could be a mechanism by which circulating lymphocytes are recruited to sites of inflammation. Indeed, the seminal study by DeGrendele and colleagues8 demonstrated in vitro with murine cell lines that CD44 and HA mediate lymphocyte rolling on endothelium under physiologic flow. This same group later showed that CD44 was required for staphylococcal enterotoxin B (SEB)–activated Vβ8+ T-cell extravasation into the inflammatory sites.9 In addition, Estess et al10 observed that circulating T cells bearing increased levels of activated CD44 are elevated in chronic inflammatory diseases and provide a reliable marker for disease activity. In vivo administration of anti-CD44 monoclonal antibodies in hapten-sensitized mice was found to inhibit their ability to mount a cutaneous delayed-type hypersensitivity response within the first 24 hours after hapten challenge.11 Similarly, studies using anti-CD44 antibodies have demonstrated significant suppression of chronic inflammation in animal models of experimental allergic encephalomyelitis,12 diabetes in the nonobese diabetic (NOD) mouse,13 and a murine model of rheumatoid arthritis.14
An immune response is largely regulated by cytokines differentially produced by 2 distinct CD4+ T-helper (Th) lymphocytes, Th1 and Th2 cells. Moreover, the unregulated accumulation of either Th1 or Th2 lymphocytes has been well described as critical for the development of various diseases.15,16 Th1 effector lymphocytes promote a Th1 cytokine milieu (eg, tumor necrosis factor-α [TNFα], interferon-γ [IFNγ], and interleukin-1β [IL-1β]), are involved in cellular immunity and are usually observed in clinical cases of chronic inflammation. They have also been implicated in the development of Crohn disease, multiple sclerosis and arthritis.17-20 By contrast, Th2 effector lymphocytes initiate a Th2 cytokine milieu (eg, IL-4, IL-5, and IL-10), promote humoral immunity, and are associated with allergic reactions to environmental antigens such as asthma, ulcerative colitis, and other diseases.16,17 One well-known consequence of T-cell activation is increased surface expression of CD44.21 As cited, evidence has accumulated that T-cell receptor (TCR) stimulation of circulating T lymphocytes in vitro and in vivo induces the activated form of CD44 and subsequent rolling interaction on immobilized HA.9,21 The initial studies by DeGrendele and colleagues8 identified a role for CD44 in lymphocyte rolling under physiologic shear stress conditions. Notably, these experiments were conducted using cell lines within which many of the principle adhesion molecules (such as E/P/L-selectin, vascular cell adhesion molecule-1 [VCAM-1], and intercellular adhesion molecule [ICAM]) were absent. To date, the question of whether CD44 mediates the trafficking of CD4+ Th1 and Th2 lymphocytes in vivo and whether HA acts as its ligand is yet to be determined.
TNFα is a global stimulus inducing the expression of P-selectin, E-selectin, VCAM-1, and ICAM-1 on endothelium. Similarly, TNFα has been clearly shown to induce CD44-mediated HA binding in human peripheral blood monocytes and in endothelial cell cultures.22-24 In this study, we use TNFα-treated wild-type and CD44-deficient animals to investigate whether CD44 plays a physiologically important role (ie, in rolling and adhesion) despite the presence of other adhesion molecules, including P-selectin, which was responsible for all rolling in a previous study.25 The results provide evidence that (1) CD44 mediates interactions between the intestinal endothelium and circulating Th1 and Th2 lymphocytes, including rolling and adhesion; and (2) CD44 expression by both the endothelium and lymphocytes is critical for these interactions to occur. Although CD44 and P-selectin are both necessary for rolling in this model, they were not counterligands in vitro, raising the possibility that there is cooperativity between these 2 pathways.
Materials and methods
Reagents and mice
CD44 antibodies were either purchased from Cedarlane (Pgp-1, HA-blocking, clone KM81; Cedarlane, Hornby, ON, Canada), or made (IRAWB14.4 and IM7) as previously described.26 11B11 (anti–IL-4), XMG1.2 (anti-IFNγ), JES5-16E3 (anti–IL-10), 4RA10 (anti–PSGL-1), and isotype control antibodies were purchased from BD Biosciences (Mississauga, ON, Canada). P-selectin/human immunoglobulin M (IgM) chimeric antibody (Ab) (a gift from John Lowe, University of Michigan, Ann Arbor). Fluorescein-conjugated HA was generated as previously described.22 TNFα was purchased from R&D Systems (Minneapolis, MN). BALB/c (Charles River Laboratories, Montreal, QC, Canada), DO11.10 (Jackson Laboratories, Bar Harbor, ME), B6129 (wild-type), and CD44-deficient mice (Jackson Laboratories) were maintained in the viral antigen–free doublebarrier unit at the University of Calgary. All mice were between 6 and 10 weeks of age. All experimental procedures were approved by the Animal Care Committee of the University of Calgary and conform to the guidelines established by the Canadian Council for Animal Care.
Lymphocyte purification and characterization of OVA-specific Th1 and Th2 cells
CD4+ T cells were isolated from pooled spleens of DO11.10 mice using mouse CD4 (L3T4) Dynabeads per the manufacturer's protocol (Dynal Biotec, Brown Deer, WI), as previously described,27 with routine purities greater than 95% for CD4+.
Th1 and Th2 cells were generated in vitro from antigen-naive CD4+ T cells as described previously.28 Briefly, purified CD4+ T cells were plated at a ratio of 1:5 (for Th1) and 1:25 (for Th2) with irradiated splenocytes (antigen-presenting cells [APCs]) and 5 μg/mL OVA peptide 323-339 for 7 days. In addition, 50 U/mL IL-12 (R&D Systems) and 10 μg/mL anti–IL-4 (11B11)29 were added to generate Th1 cells, while 1000 U/mL IL-4 (R&D Systems) and 10 μg/mL anti–IL-12 (C17.8)30 was used to generate Th2 cells.31 To ensure that the majority of ex vivo polarized cells were of a Th2 phenotype, these cells went through an identical second 7-day period of culture. Although initially we also compared Th1 cells after 2 cycles of polarization and Th2 cells after a single cycle and observed similar trends to those described in “Results,” ultimately we settled on the described protocol as it generated the best polarized Th1 and Th2 cells as determined by cytokine production. Intracellular cytokine staining was used to assess cytokine expression by polarized cells immediately prior to experimental use with the Cytofix/Cytoperm Plus with GolgiPlug Kit (BD BioSciences) following the manufacturer's instructions. Permeabilized cells were incubated with PE-conjugated anti–IL-4 (11B11; BD BioSciences), anti–IL-10 (JES5-16E3; BD BioSciences) or anti-IFNγ (XMG1.2; BD BioSciences) and analyzed with a BD Biosciences FACScan, as previously described.27 Unstimulated cells and a PE-labeled control monoclonal Ab (mAb) of the same isotype were used as controls in all experiments. To verify Th2 results, IL-4–producing cells were isolated using a mouse IL-4 Secretion Assay, Cell Enrichment and Detection kit (Miltenyi Biotech, Auburn, CA) as per the manufacturer's instructions after activating Th2 lymphocytes with PMA and ionomycin.
Intravital microscopy in the intestine
Mice were prepared for surgery using isofluorane as an inhalation anesthetic as previously described.32 Briefly, the jugular vein was cannulated for the administration of fluorescently labeled cells and antibodies. Endogenous leukocytes within the postcapillary venules of the lamina propria of the small intestine were visualized by intravenous injection of rhodamine 6G, prelabeled subsets of T lymphocytes were injected into the jugular vein, and their rolling and adhesion were visualized in the intestine. Lymphocytes were labeled with rhodamine 6G (25 μg/107 cells; Sigma Aldrich, St Louis, MO) for 5 minutes at room temperature (RT) prior to extensive washing and intravenous injection. Rhodamine 6G–associated fluorescence was visualized by epi-illumination at 510 to 560 nm, using a 590 nm emission filter. An Optiphot-2 microscope (Nikon, Mississauga, ON, Canada) with a × 25 lens (water immersion, Leitz Wetzlar L25/0.35) and a 10 × eyepiece was used. A silicon-intensified fluorescence camera (model C-2400-08; Hamamatsu Photonics, Hamamatsu City, Japan) mounted on the microscope projected the image onto a monitor. The image was recorded using a video camera (Panasonic-Digital 5100; Panasonic, Edmonton, CA) and a video recorder (Panasonic NV8950) as previously described by our laboratory.33,34 The microcirculation of the lamina propria could be assessed from the serosal side in the closed part of the intestine by changing the depth of focus of the microscope.35
Five different postcapillary venules with a diameter between 20 and 30 μm were chosen for observation over a 30- to 45-minute timespan to ensure that cell trafficking was uniformly represented per mouse and that at least 3 mice were used in each study group. All experiments were recorded for later analysis. Rolling leukocytes were defined as those cells moving at a velocity less than that of erythrocytes within a given vessel. Leukocytes were considered adherent if they remained stationary for 30 seconds or longer.
Experimental protocol
Optimal doses of TNFα (25 μg/kg) were administered intraperitoneally to mice 4 hours prior to analysis as previously described.36 Antibodies to CD44 (20 μg) or isotype control (20 μg) in a final volume of 200 μL sterile saline were administered intravenously. In some experiments, antineutrophil serum was used, RB6-8C5, a rat anti–mouse IgG2b, directed against Ly-6G (previously known as Gr-1), an antigen (Ag) on the surface of murine granulocytes. The Ab was produced by TSD BioServices (Germantown, NY) by intraperitoneal injection of RB6-8C5 hybridoma into nude mice and subsequent ascite collection. A total of 150 μg RB6-8C5 was administered during the intravital microscopy to establish the number of rolling leukocytes that were neutrophils. For control mice, an IgG2b Ab (BD BioSciences) was injected as an isotype control.
Administration of TNFα intraperitoneally induces ample endogenous leukocyte–endothelial-cell interactions (ie, slow rolling and adhesion), but does not induce emigration or any pathology. It is a model of leukocyte–endothelial cell interaction in vivo. Preliminary experiments determined that 5 minutes is required for optimal inhibition of rolling by CD44 (data not shown). For experiments requiring pretreatment, antibodies were administered at the same concentrations 5 minutes prior to lymphocyte injection.
Histology
Formalin-fixed intestinal tissue was embedded in paraffin, sectioned (3 μm), stained with hematoxylin and eosin, and incubated with biotinylated HA-binding protein (HABP, 10 μg/mL in phosphate-buffered saline [PBS]; Seikagaku, Tokyo, Japan) overnight at 4°C, as previously described.37 After washing, they were then incubated with peroxidase-labeled avidin-biotin complex (ABC) and developed with Sigma Fast DAB chromogen solution (Sigma-Aldrich). In some experiments, as a specificity control, hyaluronidase (20 U/g, hyaluronidase type IV; Sigma-Aldrich) was administered to mice prior to dissection and staining with HABP.
Parallel-plate flow chamber assay for leukocyte recruitment to hyaluronan and P-selectin
Rooster-comb HA (Sigma-Aldrich) was immobilized on plastic with 100 μL HA in PBS (2.5 mg/mL) on a 35-mm cell-culture dish (Corning, Acton, MA) and allowed to dry. All plates were blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich) in PBS for 1 hour at 37°C before use. The parallel-plate flow chamber assay was adapted from procedures previously described.38 Briefly, naive, Th1, or Th2 CD4+ T lymphocytes were diluted to 1 × 106 cells/mL in Hanks balanced salt solution (HBSS; GibcoBRL, Burlington, ON, Canada). Using a syringe pump (Harvard Apparatus, St Laurent, QC, Canada) cells were perfused across the substratum at a constant rate of 0.2 dyn/cm2 for 4 minutes and then, to mimic physiologic shear forces encountered in the vasculature, the shear rate was increased to 1 dyn/cm.2 After 1 minute, lymphocyte/HA interactions were visualized using phase-contrast microscopy on an inverted microscope fitted with a digital video camera. All experiments were recorded for later analysis. Rolling cells were defined as those traveling slower than free-flowing cells. Adherent cells were defined as those remaining stationary for at least 10 seconds. To verify that the specificity of the interaction of Th1 and Th2 cells with HA was via CD44, the cells were incubated with 10 μg/mL Pgp-1 (HA-blocking anti-CD44) for 5 minutes at 37°C prior to perfusion over immobilized HA.
We have previously observed that anti–P-selectin blocked almost all Th1 and Th2 interactions in intestinal vasculature.25 Therefore, in additional experiments, an antibody for PSGL-1, the ligand for P-selectin, was added to Th1 lymphocytes (at a concentration of 5 μg/mL, which is known to inhibit cellular interactions with P-selectin) to determine whether PSGL-1 can interact with HA. In a final series of experiments, Th1 lymphocytes were perfused over mouse P-selectin IgM fusion protein (R&D Systems). The total number of interacting cells (rolling and adhesion) per field was quantified. CD44 antibody (10 μg/mL, Pgp-1) was added to Th1 lymphocyte to determine whether CD44 can affect P-selectin–dependent rolling.
Labeling of cells for flow cytometric analysis
Lymphocytes (1 × 106) were incubated with 2 μg of antibodies to CD44 (Pgp-1, IRAWB14.4, IM7, or their respective isotype controls), washed, stained with fluorescein isothiocyanate (FITC)–conjugated polyclonal goat anti–rat Ig (BD BioSciences) and washed again. HA binding to lymphocytes was assessed using fluorochrome-tagged HA (Fl-HA) with or without a 5-minute preincubation with IRAWB14.4 for CD44 activation. All samples were resuspended in PBS/0.5% BSA/20 mM glucose/0.1% formaldehyde solution and read on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) using CellQuest Pro software (BD BioSciences).
Statistical analysis
All results are expressed as mean plus or minus standard error (SEM). An unpaired Student t test was used for comparisons between 2 means with a Welch correction where necessary, and analysis of variance followed by Newman-Keul post-hoc test was used for comparisons between more than 2 means. No statistical differences were observed between treatment groups when isotype controls were administered. Statistical significance was set at a P value less than .05.
Results
Blocking CD44 reduces TNFα-induced endogenous leukocyte rolling in vivo
To assess the role of CD44 in endogenous leukocyte rolling and adhesion following TNFα stimulation, we used intravital microscopy and an anti-CD44 blocking antibody to directly observe endogenous leukocyte–endothelial-cell interactions in the postcapillary venules of the small intestine in wild-type mice. Figure 1A shows that following administration of TNFα (0.5 mg/kg intraperitoneally for 4 hours) into mice, no change in the number of rolling endogenous leukocytes occurred (Figure 1A, bars 1 and 2). Interestingly, if a blocking antibody to CD44 was then injected intravenously into these mice, leukocyte rolling flux was reduced by more than 50% within 5 minutes (Figure 1A, bar 4). To ascertain that neutrophils were the rolling cells, antineutrophil serum was added. More than 90% of the rolling cells detached, suggesting that most rolling cells were neutrophils, with only 2 ± 1 cells continuing to roll after administration of antineutrophil serum. It has previously been shown that without stimulation of the vasculature, CD44 plays no role in rolling.37 The addition of an isotype control Ab did not alter endogenous leukocyte rolling flux (Figure 1A, bar3). As shown in Figure 1B, rolling leukocytes in TNFα-treated mice rolled at a velocity significantly slower than that in untreated mice (Figure 1B, bars 1 and 2). Blocking CD44 did not alter leukocyte velocity (Figure 1B, bar 4).
As a reduction in rolling velocity results in retention of rolling leukocytes in blood vessels, we used the aforementioned data to calculate the number of rolling leukocytes within a 100-μm length of intestinal venule by dividing the rolling flux by the rolling velocity. As shown in Figure 1C, significantly more cells rolled per 100-μm length in the intestinal vasculature of TNFα-treated mice compared with controls. Also shown in Figure 1C, the number of rolling cells per 100-μm length in TNFα-treated BALB/c mice was significantly lower when a blocking Ab to CD44 was administered. In fact, the number was now comparable with that observed in untreated animals. In TNFα-treated mice, a significant increase in the number of adherent endogenous cells within the intestinal vasculature when compared with untreated mice was also observed. This adhesion was not reversed after 5 minutes of exposure to CD44-blocking Ab (Figure 1D). As no endogenous leukocyte emigration occurred in this model, these values are not reported.
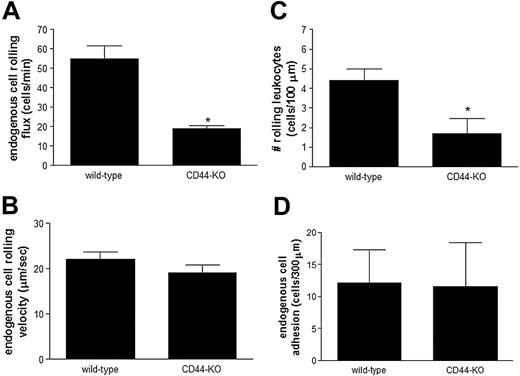
CD44-deficient mice treated with TNFα exhibit reduced leukocyte rolling
To confirm the aforementioned results, we investigated endogenous leukocyte rolling and adhesion in TNFα-treated CD44-deficient mice and compared them with their TNFα-treated wildtype controls. As shown in Figure 2A, when compared with TNFα-treated wild-type mice, the CD44-deficient mice exhibited 3-fold less endogenous leukocyte rolling in the intestinal vasculature. The remaining leukocytes, which roll independent of CD44, exhibit a rolling velocity similar to that observed in TNFα-treated wild-type mice (Figure 2B). Using these data, we identified the number of rolling leukocytes within a 100-μm length of intestinal venule for each group of animals. As shown in Figure 2C, the number of rolling cells per 100-μm length in TNFα-treated CD44-deficient mice was significantly lower than that observed in TNFα-treated wild-type controls. In fact, the number of rolling cells per 100 μm in CD44-deficient mice treated with TNFα was comparable with the numbers observed in mice not receiving TNFα (data not shown). Despite the significant reduction in endogenous leukocyte rolling flux and number of rolling cells per 100-μm length in TNFα-treated CD44-deficient mice, we observed no difference in the number of adherent leukocytes between TNFα-treated wild-type and CD44-deficient mice (Figure 2D).
CD44 mediates TNFα-induced leukocyte rolling flux. BALB/c mice were left untreated or were treated with TNFα for 4 hours prior to intravital microscopy and antibody administration. (A) Endogenous leukocyte rolling flux in control and TNFα-treated mice was determined using intravital microscopy. After basal levels of rolling flux were recorded in TNFα-treated mice, isotype controls (isotype Ab) or a blocking antibody against CD44 were administered. (B) Endogenous leukocyte rolling velocity. (C) The number of rolling endogenous leukocytes per 100 μm and (D) endogenous leukocyte adhesion was quantified. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to untreated controls; #P < .05 relative to TNFα-treated animals.
CD44 mediates TNFα-induced leukocyte rolling flux. BALB/c mice were left untreated or were treated with TNFα for 4 hours prior to intravital microscopy and antibody administration. (A) Endogenous leukocyte rolling flux in control and TNFα-treated mice was determined using intravital microscopy. After basal levels of rolling flux were recorded in TNFα-treated mice, isotype controls (isotype Ab) or a blocking antibody against CD44 were administered. (B) Endogenous leukocyte rolling velocity. (C) The number of rolling endogenous leukocytes per 100 μm and (D) endogenous leukocyte adhesion was quantified. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to untreated controls; #P < .05 relative to TNFα-treated animals.
In a separate set of experiments, antineutrophil serum was administered to both wild-type and CD44-deficient mice. Approximately 1 cell per minute remained rolling. This again suggests that most endogenous cells in this model are in fact neutrophils.
Endogenous leukocyte rolling is significantly reduced in TNFα-treated CD44-deficient mice. CD44-deficient (CD44-KO) and their wild-type controls (wild-type) mice were treated with TNFα for 4 hours. (A) Endogenous leukocyte rolling flux, (B) rolling velocity, (C) the number of cells rolling per 100 μm, and (D) leukocyte adhesion in postcapillary venules of the small intestine were determined using intravital microscopy. Data are expressed as the arithmetic means ± SEM of at least 5 animals per group. *P < .05 relative to TNFα-treated wild-type animals.
Endogenous leukocyte rolling is significantly reduced in TNFα-treated CD44-deficient mice. CD44-deficient (CD44-KO) and their wild-type controls (wild-type) mice were treated with TNFα for 4 hours. (A) Endogenous leukocyte rolling flux, (B) rolling velocity, (C) the number of cells rolling per 100 μm, and (D) leukocyte adhesion in postcapillary venules of the small intestine were determined using intravital microscopy. Data are expressed as the arithmetic means ± SEM of at least 5 animals per group. *P < .05 relative to TNFα-treated wild-type animals.
Characterization of Th1 and Th2 lymphocytes
To specifically investigate the role of CD44 in CD4+ Th1 and Th2 lymphocyte–trafficking in the inflamed intestine, we generated Th1 and Th2 cells using a well-characterized ex vivo model.27,39 To confirm the polarization of the CD4+ splenocytes into Th1 and Th2 lymphocytes, we assessed their production of cytokines following ionomycin and PMA stimulation. By intracellular cytokine staining, we observed that approximately 75% ± 6% of the activated Th1 lymphocytes produced the Th1 cytokine IFNγ, and less than 1% of these cells produced the Th2 (IL-4 and IL-10) cytokines. By contrast, most of the stimulated Th2 lymphocytes produced IL-4 and less than 1% produced IFNγ (data not shown). We previously reported that these Th1 lymphocytes can bind P-selectin and E-selectin, express LFA-1 and low levels of α4-integrin and L-selectin, but express no Mac-1 or β7-integrin. The Th2 cells bind P-selectin, express LFA-1, and express low levels of α4-and β7-integrin but no Mac-1 or L-selectin.40
CD44 mediates TNFα-induced Th1 and Th2 rolling flux and adhesion in vivo
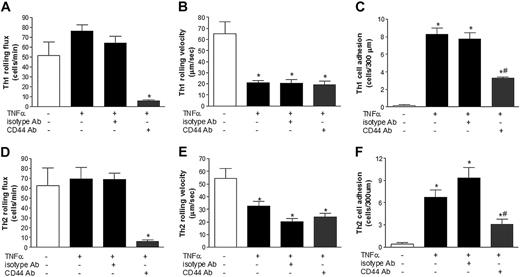
To investigate the role of CD44 in TNFα-induced Th1 lymphocyte rolling and adhesion in the intestine, we used intravital microscopy and injected Th1 lymphocytes with or without a blocking Ab to CD44. As shown in Figure 3A, we observed a slight increase in Th1 rolling flux when mice were treated with TNFα. A blocking antibody to CD44 was then injected intravenously and rolling flux was recalculated 5 minutes later. Figure 3A illustrates that by blocking CD44, Th1 lymphocyte rolling in TNFα-treated mice was reduced significantly by 94% (Figure 3A, bar 2 versus 4). The addition of an isotype control Ab induced no change (Figure 3A, bar 3). As shown in Figure 3B, Th1 lymphocytes in TNFα-treated mice rolled at a velocity significantly slower than that in untreated mice (Figure 3B, bars 1 and 2). As observed for the endogenous leukocyte populations (Figure 1), blocking CD44 did not change the velocity with which these cells rolled (Figure 3B, bar 4). With TNFα administration, we also observed a significant increase in the number of adherent Th1 lymphocytes within the intestinal vasculature (Figure 3C). But unlike the endogenous leukocyte population, when the CD44-blocking Ab was administered into the mice 5 minutes prior to Th1 lymphocyte injection, a significant reduction in lymphocyte adhesion to the intestinal vasculature (Figure 3C) was observed, clearly demonstrating for the first time that CD44 contributes to Th1 lymphocyte rolling and adhesion in vivo.
CD44 mediates Th1 and Th2 lymphocyte rolling and adhesion in TNFα-treated mice. BALB/c mice were left untreated or treated with TNFα for 4 hours prior to the injection of rhodamine 6G–labeled Th1 or Th2 lymphocytes. After basal levels of rolling flux were recorded in TNFα-treated mice, isotype controls (isotype Ab) or a blocking antibody against CD44 were administered. (A) Th1 rolling flux and (B) Th1 rolling velocity in postcapillary venules of the small intestine were determined by intravital microscopy. (C) To determine Th1 cell adhesion, isotype and a CD44-blocking Ab were administered to the mice intravenously prior to Th1 injection. In similar experiments, Th2 rolling flux (D), Th2 rolling velocity (E), and Th2 adhesion (F) were also determined. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to control group; #P < .05 relative to TNFα treatment group.
CD44 mediates Th1 and Th2 lymphocyte rolling and adhesion in TNFα-treated mice. BALB/c mice were left untreated or treated with TNFα for 4 hours prior to the injection of rhodamine 6G–labeled Th1 or Th2 lymphocytes. After basal levels of rolling flux were recorded in TNFα-treated mice, isotype controls (isotype Ab) or a blocking antibody against CD44 were administered. (A) Th1 rolling flux and (B) Th1 rolling velocity in postcapillary venules of the small intestine were determined by intravital microscopy. (C) To determine Th1 cell adhesion, isotype and a CD44-blocking Ab were administered to the mice intravenously prior to Th1 injection. In similar experiments, Th2 rolling flux (D), Th2 rolling velocity (E), and Th2 adhesion (F) were also determined. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to control group; #P < .05 relative to TNFα treatment group.
In experiments similar to those described above, we investigated the role of CD44 in Th2 lymphocyte rolling and adhesion in the intestine. As shown in Figure 3D, we observed no change in Th2 rolling flux when mice were treated with TNFα. Furthermore, blocking CD44 significantly reduced the rolling flux of Th2 lymphocytes in TNFα-treated mice by 85% (Figure 3D, bars 2 and4). The addition of an isotype control Ab caused no change (Figure 3D, bar 3). As shown in Figure 3E, rolling Th2 lymphocytes in TNFα-treated mice moved at a velocity significantly slower than that in untreated mice (Figure 3E, bars 1 and 2). As observed previously, blocking CD44 did not change the velocity with which these cells rolled in the TNFα-induced microenvironment (Figure 3E, bar 4). In response to TNFα treatment a significant increase in the number of adherent Th2 lymphocytes within the intestinal vasculature was observed when compared with untreated mice (Figure 3F). When CD44 was blocked 5 minutes prior to Th2 lymphocyte injection, a significant reduction in their adhesion to the intestinal vasculature occurred (Figure 3F), clearly demonstrating that CD44 is also involved in Th2 rolling and adhesion in vivo. There were no significant differences between the number of Th1 and the number of Th2 cells that rolled or adhered to TNFα-stimulated endothelium.
Notably, in a separate set of experiments we used intravital microscopy and observed in vivo that without polarization to either Th1 or Th2, antigen-naive CD4+ lymphocytes do not roll along the intestinal endothelium of control or TNFα-treated mice (data not shown).
Endothelial cell CD44 mediates TNFα-induced Th1 and Th2 lymphocyte rolling and adhesion in vivo
Using the CD44-deficient mice, we were able to investigate whether endothelial expression of CD44 was essential for Th1 and Th2 lymphocyte rolling and adhesion in the inflamed intestine. To test this, we set up a chimeric system in vivo within which CD44 was not expressed on the endothelium of CD44-deficient mice but was present on the injected T lymphocytes. As shown in Figure 4A, polarized Th1 cells roll on the endothelium of TNFα-treated wild-type mice with a flux of 68 ± 10 cells/minute. In CD44-deficient mice, this rolling flux was significantly reduced by 63% to 17 ± 6 cells/min. In addition, there was a 50% reduction in Th1 lymphocyte adhesion to the vasculature in TNFα-treated CD44-deficient mice when compared with their controls (Figure 4C). By contrast, no difference in Th1 rolling velocity was observed between the 2 groups (Figure 4B). Similar results were observed with Th2 lymphocytes, with a 75% reduction in Th2 lymphocyte rolling flux observed in TNFα-treated CD44-deficient mice when compared with their wild-type controls (Figure 4D). Similarly, we observed a 50% reduction in Th2 lymphocyte adhesion and no change in rolling velocity (Figure 4F and 4E, respectively), clearly demonstrating for the first time that endothelial CD44 contributes to Th1 and Th2 rolling and adhesion in vivo.
TNFα induces hyaluronan expression in vivo
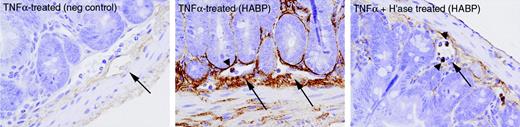
To determine whether HA is up-regulated in the intestine following TNFα treatment, and hence a plausible ligand for the lymphocytic CD44, its expression on the intestinal vasculature was investigated by immunohistochemistry. Intestinal tissue sections from mice treated with TNFα without or with hyaluronidase were stained with a hyaluronan-binding protein (HABP). As shown in Figure 5 (large arrows), HA was localized in the connective tissue of the intestine and within the blood vessels. Furthermore, staining was less intense in tissues taken from mice treated with TNFα and hyaluronidase, suggesting that HA is indeed present on endothelium and readily removed by hyaluronidase. Notably, HABP also bound to leukocytes in the circulation (Figure 5, arrowheads) and this staining was not affected by administration of hyaluronidase.
TNFα-treated CD44-deficient mice exhibit reduced Th1 and Th2 rolling flux and adhesion compared with controls. Wild-type and CD44-deficient (CD44-KO) mice were treated with TNFα for 4 hours prior to intravenous injection of rhodamine 6G–labeled Th1 or Th2 lymphocytes. Intravital microscopy determined (A) Th1 rolling flux, (B) Th1 cell rolling velocity, and (C) Th1 cell adhesion. In similar experiments, Th2 rolling flux (D), Th2 rolling velocity (E), and Th2 adhesion (F) were also determined. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to wild-type values.
TNFα-treated CD44-deficient mice exhibit reduced Th1 and Th2 rolling flux and adhesion compared with controls. Wild-type and CD44-deficient (CD44-KO) mice were treated with TNFα for 4 hours prior to intravenous injection of rhodamine 6G–labeled Th1 or Th2 lymphocytes. Intravital microscopy determined (A) Th1 rolling flux, (B) Th1 cell rolling velocity, and (C) Th1 cell adhesion. In similar experiments, Th2 rolling flux (D), Th2 rolling velocity (E), and Th2 adhesion (F) were also determined. Data are expressed as the arithmetic means ± SEM of at least 3 animals per group. *P < .05 relative to wild-type values.
Binding of hyaluronan and expression of CD44 by Th1 and Th2 lymphocytes
To investigate whether Th1 and Th2 lymphocytes can bind HA, Fl-HA and flow cytometry were used. As shown in Table 1, both Th1 and Th2 cells bound Fl-HA. We used the same antibody (Pgp-1) that blocked lymphocyte trafficking in vivo and observed that both Th1 and Th2 lymphocytes expressed CD44 (Table 1). The CD44 binding was confirmed with 2 additional antibodies to CD44 (IRAWB14.4 and IM7) with almost no mean fluorescence intensity (MFI) observed using Ag-naive T cells or isotype control antibodies (Table 1). Although CD44 expression and HA-FITC was consistently greater on Th2 than Th1 cells, in vivo inhibition of CD44 reduced rolling and adhesion similarly in Th1 and Th2 cells.
Flow cytometric data of CD44 expression and HA binding by naive, Th1, and Th2 lymphocytes
Ab . | Naive CD4+, MFI ± SEM . | Th1, MFI ± SEM . | Th2, MFI ± SEM . |
|---|---|---|---|
| F1-HA | 6 ± 1 | 9 ± 3 | 31 ± 2* |
| Pgp-1 | 24 ± 3 | 286 ± 68* | 828 ± 80* |
| Control | 5 ± 0 | 5 ± 0 | 5 ± 1 |
| IRAWB14.4 | 24 ± 3 | 304 ± 38* | 972 ± 192* |
| IM7 | 17 ± 2 | 155 ± 15* | 421 ± 64* |
| IRAWB14.4 + F1-HA | 32 ± 3 | 57 ± 5* | 95 ± 20* |
Ab . | Naive CD4+, MFI ± SEM . | Th1, MFI ± SEM . | Th2, MFI ± SEM . |
|---|---|---|---|
| F1-HA | 6 ± 1 | 9 ± 3 | 31 ± 2* |
| Pgp-1 | 24 ± 3 | 286 ± 68* | 828 ± 80* |
| Control | 5 ± 0 | 5 ± 0 | 5 ± 1 |
| IRAWB14.4 | 24 ± 3 | 304 ± 38* | 972 ± 192* |
| IM7 | 17 ± 2 | 155 ± 15* | 421 ± 64* |
| IRAWB14.4 + F1-HA | 32 ± 3 | 57 ± 5* | 95 ± 20* |
P < .05 versus naive CD4+ T cells.
Hyaluronan expression in small-intestine tissue. Tissue was collected from mice treated with TNFα without or with hyaluronidase (“H'ase”). HABP was used to stain specifically for HA. Blood vessels and leukocytes within vessels are indicated by arrows and arrowheads, respectively. Images represent × 400 magnification, and the negative control depicts the use of secondary antibody alone. One example from each group is shown.
Hyaluronan expression in small-intestine tissue. Tissue was collected from mice treated with TNFα without or with hyaluronidase (“H'ase”). HABP was used to stain specifically for HA. Blood vessels and leukocytes within vessels are indicated by arrows and arrowheads, respectively. Images represent × 400 magnification, and the negative control depicts the use of secondary antibody alone. One example from each group is shown.
IRAWB14.4 has previously been shown to rapidly activate CD44 such that binding to HA is significantly enhanced.41,42 Using IRAWB14.4, we examined Th1 and Th2 lymphocytes to bind Fl-HA. Following a 5-minute pretreatment with IRAWB14.4, Fl-HA was exposed to the cells and, as shown in Table 1, HA binding increased for both Th1 and Th2 lymphocytes.
Th1 and Th2 lymphocyte rolling and adhesion is CD44-HA dependent
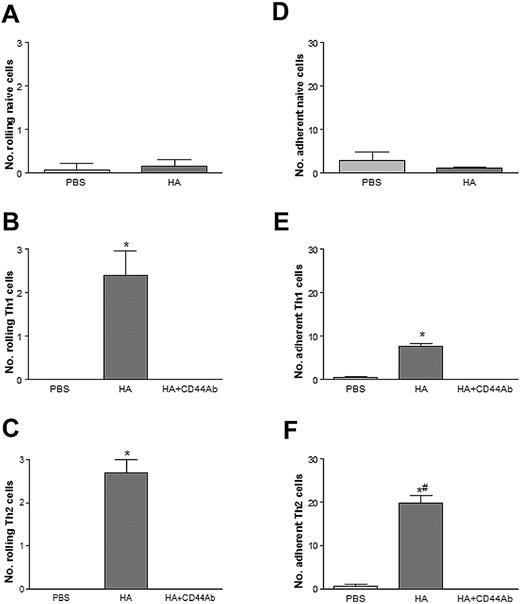
To clearly define a role for CD44 on lymphocytes in rolling and adhesion to HA, an in vitro system using a parallel-plate flow chamber wherein HA was immobilized to petri dishes was used. Naive, Th1, and Th2 cells were perfused over HA at a rate of 1.0 dyn/cm2 and assessed for their rolling and adhesion. As anticipated from the in vivo results, Figure 6A and 6D shows negligible interactions of naive CD4+ T cells on both control (ie, PBS) and HA-coated petri dishes. For Th1 lymphocytes, we observed very little if any rolling or adhesion on plates coated with PBS alone. By contrast, when the plates were coated with HA a significant increase in Th1 lymphocyte rolling and adhesion occurred (Figure 6B and 6E). If the Th1 lymphocytes were pretreated with an antibody to CD44 all of the rolling and adhesion was blocked, suggesting that CD44 expression by Th1 lymphocytes is essential for rolling on HA. Similar results were observed with the Th2 lymphocytes. That is, when compared with PBS-coated plates, HA conferred significantly more Th2 lymphocyte rolling and adhesion (Figure 6C and 6F). With the addition of an antibody to CD44 all interactions were blocked, which also suggests that CD44 expression by Th2 lymphocytes is essential for their rolling and adhesion to HA. Because 50% of Th2 lymphocytes did not produce IL-4, the IL-4–producing cells were separated and shown to express high levels of CD44. These IL-4–producing Th2 cells rolled on and adhered to immobilized HA (data not shown). Some IL-4–negative Th2 cells also expressed CD44 and also rolled on and adhered to HA (data not shown).
Th1 and Th2 lymphocytes roll on HA-coated coverslips via CD44. Rolling on HA-coated coverslips by naive (A), Th1 (B), and Th2 (C) lymphocytes. Adhesion on HA-coated coverslips by naive (D), Th1 (E), and Th2 (F) lymphocytes. Preincubation of cells with a blocking Ab to CD44 prevents both rolling and adhesion. Data are expressed as the arithmetic means ± SEM of at least 3 separate experiments. *P < .05 relative to PBS-coated plates; #P < .05 relative to Th1 adhesion to HA.
Th1 and Th2 lymphocytes roll on HA-coated coverslips via CD44. Rolling on HA-coated coverslips by naive (A), Th1 (B), and Th2 (C) lymphocytes. Adhesion on HA-coated coverslips by naive (D), Th1 (E), and Th2 (F) lymphocytes. Preincubation of cells with a blocking Ab to CD44 prevents both rolling and adhesion. Data are expressed as the arithmetic means ± SEM of at least 3 separate experiments. *P < .05 relative to PBS-coated plates; #P < .05 relative to Th1 adhesion to HA.
Given our own evidence that P-selectin mediates Th1 and Th2 lymphocyte trafficking in this identical model,25 we next investigated the contribution of CD44 and PSGL-1 to lymphocyte rolling on P-selectin and HA, respectively. As shown in Figure 7A, blocking PSGL-1 on Th1 lymphocytes had no effect on their interaction with HA (Figure 7A). Similarly, blocking CD44 did not reduce Th1 lymphocyte interactions with P-selectin (Figure 7B). Total interactions are shown in Figure 7 (rolling + adhesion) to facilitate comparisons between efficiency of interactions for P-selectin versus HA. Clearly, interactions were far more efficient on P-selectin than on HA. In fact, the lymphocytes tethered very effectively to P-selectin but not HA. In the latter case, the shear had to be reduced well below 1 dyn/cm2 before the cells engaged HA. However, once engaged the shear could be increased to 1 dyn/cm2, and the lymphocytes rolled and adhered adequately on HA.
Discussion
HA is the major glycosaminoglycan found in the extracellular matrix of many mammalian tissues, and CD44 is considered to be the principal cell-surface receptor for HA.3,43 Given the wide distribution of both CD44 and HA throughout the body, the need for stringent regulation of CD44/HA interactions might be well anticipated. Indeed, studies to date suggest that a certain threshold level of CD44 expression as well as conversion to the active form is required to engage HA. Conversion from the inactive to active form of CD44 occurs in specific cell types in response to appropriate stimuli such as TNFα, IL-1, lipopolysaccharide (LPS), super-Ag, and T-cell mitogens.23,24,44 In further support, studies also suggest that the activated form of CD44 is a transient property of activated T cells, and can therefore be used only within a confined timeframe.44 Our data now extend the work by suggesting that both CD4+ Th1 and Th2 lymphocytes use CD44 to roll and adhere on immobilized HA as well as on endothelium in vivo.
Naive T lymphocytes express low to moderate levels of CD44 and high L-selectin. Upon cell activation CD44 expression is increased and L-selectin is rapidly shed43 such that these activated T lymphocytes have reduced homing to the lymph nodes and increased probability of being delivered to areas of inflammation. Lymphocytes have been shown to roll in in vivo and in vitro flow assays, and in many instances this primary adhesion is selectin dependent.45,46 However, evidence is continually emerging for the existence of additional adhesion molecules involved in mediating lymphocyte rolling and adhesion.47,48 Relevant to this study, Siegelman and colleagues have shown that CD44 and HA mediate rolling of PMA/ionomycin-activated T cells and T-cell lines under physiologic flow both on HA-coated plates and on cultured endothelial cell lines that express HA and CD44.8,49 In a separate study by Stoop and coworkers,50 in vivo experiments demonstrate that lymphocytes harvested from naive CD44-deficient and wildtype mice exhibit similar trafficking patterns when injected into naive recipients. However, activated lymphocytes from CD44-deficient mice with collagen-induced arthritis transferred to arthritic recipients preferentially homed to the lymph nodes and were severely delayed in their entry into arthritic joints when compared with their wild-type counterparts.50
In this study, we show that polarized Th1 and Th2 cells adhere to HA under shear stress in vivo. Moreover, CD44 was the ligand for HA in the in vitro flow chamber studies. We have also shown that, in vivo, the intestinal vascular endothelium expresses HA, particularly following exposure to TNFα, and that this expression is readily reduced following administration of hyaluronidase, an HA-degrading enzyme. Our observations that hyaluronidase treatment reduced HABP binding to the vasculature is supported by hyaluronidase targeting bound HA and degrading the interstitial bonds between HA and its receptor on the endothelium. Preliminary in vivo experiments support an involvement of HA in CD4+ lymphocyte rolling and adhesion as administration of hyaluronidase prevented their interactions with the endothelium (data not shown). These results are further supported by a previous demonstration that the noncovalent interaction between CD44 and HA is sufficient to provide resistance and thereby support BW5147 T-cell line rolling and adhesion under physiologic conditions of shear stress.51 Endothelial CD44 is required for CD4+ lymphocyte rolling and adhesion in vivo inasmuch as these cells rolled less efficiently in CD44-deficient mice (lacking CD44 on the vascular endothelium) than in wild-type vessels. Using this chimeric system, our data clearly demonstrate that endothelial CD44 is important for Th1 as well as Th2 lymphocyte rolling and adhesion. Taken together, these results strongly suggest that both Th1 and Th2 lymphocytes use CD44 to roll and adhere to the intestine of TNFα-treated mice using a sandwich-type configuration between CD44 and HA, where HA acts as a bridge to mediate CD44-CD44–dependent cellular events.
P-selectin/PSGL-1 and CD44/HA are separate pathways in vitro. (A) Preincubation of Th1 cells with a blocking Ab to PSGL-1 does not inhibit interactions of Th1 cells with HA-coated coverslips. (B) Preincubation of Th1 cells with a blocking Ab to CD44 does not inhibit interactions with P-selectin–coated coverslips. Data are expressed as the arithmetic means ± SEM of 4 separate experiments.
P-selectin/PSGL-1 and CD44/HA are separate pathways in vitro. (A) Preincubation of Th1 cells with a blocking Ab to PSGL-1 does not inhibit interactions of Th1 cells with HA-coated coverslips. (B) Preincubation of Th1 cells with a blocking Ab to CD44 does not inhibit interactions with P-selectin–coated coverslips. Data are expressed as the arithmetic means ± SEM of 4 separate experiments.
Given the indisputable evidence that P-selectin mediates lymphocyte trafficking in the inflamed intestine,25,52-54 one must question the contribution of CD44. Some light may have been shed on this issue by Nandi and coworkers, who recently demonstrated that the formation of functional complexes between different adhesion molecules is important for the process of cell-cell interactions and leukocyte trafficking. More specifically, Nandi and colleagues51 demonstrated a functional coupling of CD44 and very-late antigen 4 (VLA-4) in the plasma membrane, and that through deletion of the CD44 cytoplasmic tail, the firm adhesion of VLA-4 on its ligand VCAM-1 was abrogated. These studies clearly suggest that the lymphocyte interactions with the endothelium may rely on the assembly of complexes that contain different adhesion molecules. Although Nandi et al51 reported no colocalization of CD44 with LFA-1, their work did not include Th1 or Th2 cells, where colocalization may include PSGL-1 and CD44 or even CD18 and CD44 to regulate lymphocyte rolling and adhesion. Our data suggest that a complex may exist between PSGL-1 and CD44 inasmuch as they are both required to coordinate the rolling of lymphocytes on TNFα-stimulated endothelium. Our observations that inhibiting PSGL-1 did not hinder lymphocyte adhesion to HA is in keeping with other studies that HA and CD44 are not ligands for PSGL-1 and P-selectin.8 Whether CD44 and CD18 coexist to regulate lymphocyte adhesion is still to be determined.
An alternative explanation for both P-selectin25 and CD44 antibodies blocking lymphocyte rolling in vivo may be related to 2 distinct functions for these 2 molecules. For example, P-selectin is an extremely effective molecule at the very first step (ie, tethering of the lymphocyte to endothelium under physiologic shear), but may not be able to support rolling in vivo due to discontinuous expression. Indeed, P-selectin expression has been shown to be patchy on endothelium, for example, expressed mainly at endothelial junctions55 or at clathrin-coated pits.56 In contrast, CD44 may be less effective at tethering lymphocytes at physiologic shear, but once tethered (for example, via P-selectin), CD44 may be far more effective as a rolling molecule because of its widespread distribution on endothelium. This dual function has previously been proposed for P-selectin and α4 integrin.57 Indeed, in our in vitro studies it was necessary to perfuse lymphocytes across HA at low shear to allow the cells to tether before increasing to physiologic shear. Lymphocytes tethered and rolled avidly on a continuous P-selectin substratum using this protocol, and will roll even at constant high shear.58 In other words, CD44/HA may fill in the gaps in the patchy P-selectin distribution, allowing for continuous rolling. However, this latter hypothesis needs to be tested further.
Our contention that CD44 can mediate CD4+ Th1 and Th2 lymphocyte rolling and is a viable adhesion molecule is also supported by other studies which show (1) that CD44 mediates lymphocyte adhesion to synovial vessels59 ; (2) that CD44 can act as a ligand for both L-selectin and E-selectin60,61 ; and (3) CD44 can in some situations mediate rolling over a wider shear range than that of PSGL-1.61 Taken together, these studies suggest that the paradigm of leukocyte trafficking as a multistep cascade determined by a variety of adhesion receptors continues to serve as a useful model, but needs to be refined to accommodate CD44 and other nonselectin and nonintegrin molecules. The findings presented here add new complexities that broaden the accepted concept of cellular trafficking, as we show here that primary adhesive events of CD4+ Th1 and Th2 lymphocytes, both in vitro and in vivo, are dependent upon CD44.
Prepublished online as Blood First Edition Paper, February 23, 2006; DOI 10.1182/blood-2005-09-3581.
Supported by grants from the Crohn's and Colitis Foundation of Canada (CCFC), Canadian Institutes of Health (CIHR), and a CIHR group grant. P.K. is an Alberta Heritage Foundation for Medical Research (AHFMR) Scientist and a Canadian Research Chair recipient; C.S.B. was supported by a Canadian Association of Gastroenterology (CAG) Fellowship and by the AHFMR and CIHR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Krista McRae and Lori Zbytnuik for animal assistance, Carol Gwozd for immunohistochemistry assistance, and Dr Casey Weaver for provision of DO11.10 mice.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal