Abstract
Establishing a CD8+ T cell–mediated immune correlate of protection in HIV disease is crucial to the development of vaccines designed to generate cell-mediated immunity. Historically, neither the quantity nor breadth of the HIV-specific CD8+ T-cell response has correlated conclusively with protection. Here, we assess the quality of the HIV-specific CD8+ T-cell response by measuring 5 CD8+ T-cell functions (degranulation, IFN-γ, MIP-1β, TNF-α, and IL-2) simultaneously in chronically HIV-infected individuals and elite nonprogressors. We find that the functional profile of HIV-specific CD8+ T cells in progressors is limited compared to that of nonprogressors, who consistently maintain highly functional CD8+ T cells. This limited functionality is independent of HLA type and T-cell memory phenotype, is HIV-specific rather than generalized, and is not effectively restored by therapeutic intervention. Whereas the total HIV-specific CD8+ T-cell frequency did not correlate with viral load, the frequency and proportion of the HIV-specific T-cell response with highest functionality inversely correlated with viral load in the progressors. Thus, rather than quantity or phenotype, the quality of the CD8+ T-cell functional response serves as an immune correlate of HIV disease progression and a potential qualifying factor for evaluation of HIV vaccine efficacy.
Introduction
CD8+ T cells play an important role in controlling HIV and SIV disease progression. Evidence for this is based on several important observations and correlative studies. First, their presence during SIV infection leads to decreased viral replication and slower disease progression in rhesus macaques.1,2 Second, resolution of acute viremia is coincident with a major expansion of HIV-specific CD8+ T cells.3,4 Third, during primary and chronic infection, immunologic pressure mediated by SIV- and HIV-specific CD8+ T cells is often manifested by viral escape mutation.5-9 Finally, there are strong correlations between the expression of certain HLA class I alleles, lack of escape, and nonprogressive HIV infection.5,10-13 Ultimately, the CD8+ T-cell response to HIV in most infected patients is insufficient to maintain durable control of HIV viral load and disease progression.
Despite extensive efforts, the definition of a CD8+ T cell–mediated immune correlate of protection in HIV infection has proven elusive, because the precise mechanisms involved in CD8+ T cell–mediated control of most chronic viral infections, including HIV, are largely unknown. Three attributes of HIV-specific CD8+ T cells could be involved in controlling HIV-1 viral replication: frequency, breadth of epitope recognition, and functional quality. MHC class I tetramer-based studies initially demonstrated that the frequency of HIV-specific CD8+ T cells was inversely correlated with viral load.14 Subsequent correlative studies examining the frequency of functional HIV-specific CD8+ T cells and HIV viral load have been inconclusive.15-19 Similarly, the breadth of epitopes recognized by HIV-specific CD8+ T cells does not correlate with control of viral replication or protection from disease progression.18 How HIV-specific CD8+ T-cell response quality factors into the control of HIV remains unclear, but initial studies examining 2 CD8+ T-cell functions concordantly suggest an important relationship between response quality and immune control.20,21
CD8+ T cells are capable of a multitude of functions, including cytolysis and production of several cytokines and chemokines. Therefore, measurement of only one or 2 CD8+ T-cell functions may not provide an adequate measure of CD8+ T-cell response quality. Recent technologic advancements in flow cytometry now permit the simultaneous examination of multiple T-cell functions.22,23 We used polychromatic flow cytometry to assess simultaneously 5 CD8+ T-cell functions, including degranulation (CD107a mobilization24 ) and cytokine (IFN-γ, TNF-α, and IL-2) and chemokine production (MIP-1β) to determine whether HIV-specific CD8+ T-cell response quality is an important factor in HIV disease progression. We first examined a group of 79 HIV-infected individuals to establish the functional profile of HIV-specific CD8+ T cells and compared this to the CD8+ T-cell functional profile in a well-defined group of HIV-infected nonprogressors. Our findings indicate that HIV-infected nonprogressors have a qualitatively different, and presumably superior, HIV-specific CD8+ T-cell response compared to that of HIV-infected progressors and that this response constitutes a correlate of protection and a target functional CD8+ T-cell response to induce with candidate HIV vaccines.
Patients, materials, and methods
Patients
The progressor group consisted of HIV-infected subjects recruited from 3 different sites: Case Western Reserve University, Cleveland, OH (C1-15); Hospital Carlos III, Madrid, Spain (S1-29); University of Alabama, Birmingham, AL (A1-35). Only patients not receiving antiretroviral therapy (ART) were recruited, although some subjects had a previous history of highly active antiretroviral therapy (HAART; Table 1). All assays using this group were performed blinded to viral load, CD4 count, and disease history. The viral load ranged from less than 50 to 708 000 copies/mL, and the CD4 counts from 4 to 1428 cells/mm3. Thus, these subjects represent the normal spectrum of disease progression expected in untreated HIV infection. The precise infection time in these individuals is unknown. Because these subjects are considered a representative sample of HIV-infected subjects, as many as about 5% of the subjects may be nonprogressors. For example, subject C3 appears to fit our definition of a nonprogressor; however, there are not supporting longitudinal data to be sure this subject fits these criteria. Several subjects subsequently initiated HAART following the initial sampling date; viral load in these individuals dropped below the limit of detection (< 50 copies/mL), with one exception (70 copies/mL). Samples from these subjects were collected approximately 6 months to 1 year following inception of HAART. A second group consisted of extensively characterized HIV-infected nonprogressors11,25,26 (NP1-9) recruited at the National Institutes of Health. The criteria for nonprogression were durable maintenance of normal CD4+ T-cell counts/ratios and viral load less than or equal to 125 RNA copies/mL in the absence of therapeutic intervention or opportunistic infections for duration of infection. All subjects gave informed consent as required. This study was reviewed and approved by the National Institutes of Health Institutional Review Board. Peripheral blood mononuclear cells (PBMCs) were isolated by Hypaque-Ficoll (Pharmacia, Uppsala, Sweden) density centrifugation and were frozen at –140°C at 10 to 20 × 106 cells/mL in 90% fetal calf serum (FCS) and 10% dimethylsulfoxide (DMSO; Sigma-Aldrich, St Louis, MO) until use.
Characteristics of HIV-infected patients
Patient . | Viral load, RNA copies/mL . | CD4 count, cells/mm3 . | Therapy history* . | Sample month† . |
|---|---|---|---|---|
| C1 | 27 000 | 466 | Naive | 42 |
| C2 | 19 000 | 273 | Naive | 33 |
| C3 | < 50 | 677 | 3.9 y | 222 |
| C4 | 18 000 | 398 | Naive | 6 |
| C5 | 708 000 | 514 | 3 y | 118 |
| C6 | 59 000 | 361 | Naive | 13 |
| C7 | 17 000 | 636 | 1.3 y | 91 |
| C8 | 71 000 | 391 | Naive | 7 |
| C9 | 400 | 718 | Naive | 83 |
| C10 | 44 000 | 428 | Naive | 25 |
| C11 | 18 000 | 508 | Naive | 21 |
| C12 | 22 000 | 384 | Naive | 117 |
| C13 | 14 000 | 413 | Naive | 167 |
| C14 | 21 000 | 693 | Naive | 29 |
| C15 | 35 000 | 478 | Naive | 14 |
| S1 | 2 350 | 368 | 4 mo | 74 |
| S2 | 147 435 | 300 | Unknown | 43 |
| S3 | 26 918 | 810 | 6 mo | 21 |
| S4 | 35 937 | 299 | Naïve | 0 |
| S5 | 150 | ND | 7 mo | 76 |
| S6 | 500 000 | 209 | Unknown | 77 |
| S7 | 204 | 1056 | Naive | 27 |
| S8 | 190 | 700 | Naive | 16 |
| S9 | 1 054 | 684 | Naive | 23 |
| S10 | 10 366 | 162 | Naive | 0 |
| S11 | 54 194 | 459 | Naive | 0 |
| S12 | 172 852 | 196 | Naive | 1 |
| S13 | 2 153 | 450 | Naive | 0 |
| S14 | 73 780 | 690 | Naive | 12 |
| S15 | 70 414 | 406 | Naive | 1 |
| S16 | 15 315 | 700 | Unknown | 0 |
| S17 | 613 | 594 | Naive | 39 |
| S18 | 99 180 | 210 | Naive | 0 |
| S19 | 500 000 | 768 | Naive | 3 |
| S20 | 181 | 750 | Naive | 41 |
| S21 | 63 905 | 810 | Naive | 34 |
| S22 | 60 310 | 465 | Naive | 0 |
| S23 | 17 448 | 962 | 3 mo | 51 |
| S24 | 706 | 1431 | Naive | 0 |
| S25 | 42 423 | 759 | Unknown | 0 |
| S26 | 8 941 | 671 | Naive | 0 |
| S27 | 62 948 | 783 | Naive | 6 |
| S28 | 69 668 | 850 | Naive | 0 |
| S29 | 10 694 | 1428 | Naive | 20 |
| A1 | 23 069 | 405 | 10 mo | 182 |
| A2 | 4 917 | 1071 | 4 y | 79 |
| A3 | 3 012 | 689 | Naive | 0 |
| A4 | 2 319 | 818 | Naive | 109 |
| A5 | 146 | 877 | Naive | 54 |
| A6 | 13 979 | 419 | 10 mo | 21 |
| A7 | 72 662 | 570 | Naive | 209 |
| A8 | 185 720 | 22 | Naive | 1 |
| A9 | 41 459 | 167 | 3 y | 63 |
| A10 | 148 000 | 36 | Naive | 219 |
| A11 | 18 993 | 286 | Naive | 1 |
| A12 | 49 755 | 372 | Naive | 21 |
| A13 | 5 107 | 716 | 3 y | 131 |
| A14 | 377 | 759 | 1.3 y | 176 |
| A15 | 24 985 | 252 | Naive | 99 |
| A16 | 10 391 | 563 | 1 y | 90 |
| A17 | 48 680 | 294 | 1.9 y | 63 |
| A18 | 16 121 | 531 | 2.5 y | 59 |
| A19 | 47 551 | 838 | 1.3 y | 76 |
| A20 | 65 548 | 388 | Naive | 101 |
| A21 | 103 | 503 | Naive | 30 |
| A22 | 63 382 | 426 | Naive | 13 |
| A23 | 128 540 | 431 | 1.5 y | 123 |
| A24 | 68 380 | 150 | 8 mo | 95 |
| A25 | 6 369 | 857 | Naive | 119 |
| A26 | 659 | 619 | Naive | 139 |
| A27 | 19 700 | 542 | 3 y | 80 |
| A28 | 2 981 | 1049 | Naive | 189 |
| A29 | 30 553 | 835 | Naive | 27 |
| A30 | 64 | 975 | Naive | 172 |
| A31 | 21 159 | 571 | 1.8 y | 108 |
| A32 | 5 821 | 694 | Naive | 10 |
| A33 | 239 300 | 4 | Naive | 58 |
| A34 | 128 460 | 228 | Naive | 136 |
| A35 | 10 333 | 513 | Naive | 41 |
| NP1 | 124 | 1830 | Naive | 122 |
| NP2 | < 50 | 311 | Naive | 219 |
| NP3 | < 50 | 908 | Naive | 216 |
| NP4 | < 50 | 1042 | Naive | 222 |
| NP5 | < 50 | 1645 | Naive | 165 |
| NP6 | 59 | 972 | Naive | 103 |
| NP7 | < 50 | 785 | Naive | 72 |
| NP8 | < 50 | 1506 | Naive | 179 |
| NP9 | < 50 | 806 | Naive | 57 |
Patient . | Viral load, RNA copies/mL . | CD4 count, cells/mm3 . | Therapy history* . | Sample month† . |
|---|---|---|---|---|
| C1 | 27 000 | 466 | Naive | 42 |
| C2 | 19 000 | 273 | Naive | 33 |
| C3 | < 50 | 677 | 3.9 y | 222 |
| C4 | 18 000 | 398 | Naive | 6 |
| C5 | 708 000 | 514 | 3 y | 118 |
| C6 | 59 000 | 361 | Naive | 13 |
| C7 | 17 000 | 636 | 1.3 y | 91 |
| C8 | 71 000 | 391 | Naive | 7 |
| C9 | 400 | 718 | Naive | 83 |
| C10 | 44 000 | 428 | Naive | 25 |
| C11 | 18 000 | 508 | Naive | 21 |
| C12 | 22 000 | 384 | Naive | 117 |
| C13 | 14 000 | 413 | Naive | 167 |
| C14 | 21 000 | 693 | Naive | 29 |
| C15 | 35 000 | 478 | Naive | 14 |
| S1 | 2 350 | 368 | 4 mo | 74 |
| S2 | 147 435 | 300 | Unknown | 43 |
| S3 | 26 918 | 810 | 6 mo | 21 |
| S4 | 35 937 | 299 | Naïve | 0 |
| S5 | 150 | ND | 7 mo | 76 |
| S6 | 500 000 | 209 | Unknown | 77 |
| S7 | 204 | 1056 | Naive | 27 |
| S8 | 190 | 700 | Naive | 16 |
| S9 | 1 054 | 684 | Naive | 23 |
| S10 | 10 366 | 162 | Naive | 0 |
| S11 | 54 194 | 459 | Naive | 0 |
| S12 | 172 852 | 196 | Naive | 1 |
| S13 | 2 153 | 450 | Naive | 0 |
| S14 | 73 780 | 690 | Naive | 12 |
| S15 | 70 414 | 406 | Naive | 1 |
| S16 | 15 315 | 700 | Unknown | 0 |
| S17 | 613 | 594 | Naive | 39 |
| S18 | 99 180 | 210 | Naive | 0 |
| S19 | 500 000 | 768 | Naive | 3 |
| S20 | 181 | 750 | Naive | 41 |
| S21 | 63 905 | 810 | Naive | 34 |
| S22 | 60 310 | 465 | Naive | 0 |
| S23 | 17 448 | 962 | 3 mo | 51 |
| S24 | 706 | 1431 | Naive | 0 |
| S25 | 42 423 | 759 | Unknown | 0 |
| S26 | 8 941 | 671 | Naive | 0 |
| S27 | 62 948 | 783 | Naive | 6 |
| S28 | 69 668 | 850 | Naive | 0 |
| S29 | 10 694 | 1428 | Naive | 20 |
| A1 | 23 069 | 405 | 10 mo | 182 |
| A2 | 4 917 | 1071 | 4 y | 79 |
| A3 | 3 012 | 689 | Naive | 0 |
| A4 | 2 319 | 818 | Naive | 109 |
| A5 | 146 | 877 | Naive | 54 |
| A6 | 13 979 | 419 | 10 mo | 21 |
| A7 | 72 662 | 570 | Naive | 209 |
| A8 | 185 720 | 22 | Naive | 1 |
| A9 | 41 459 | 167 | 3 y | 63 |
| A10 | 148 000 | 36 | Naive | 219 |
| A11 | 18 993 | 286 | Naive | 1 |
| A12 | 49 755 | 372 | Naive | 21 |
| A13 | 5 107 | 716 | 3 y | 131 |
| A14 | 377 | 759 | 1.3 y | 176 |
| A15 | 24 985 | 252 | Naive | 99 |
| A16 | 10 391 | 563 | 1 y | 90 |
| A17 | 48 680 | 294 | 1.9 y | 63 |
| A18 | 16 121 | 531 | 2.5 y | 59 |
| A19 | 47 551 | 838 | 1.3 y | 76 |
| A20 | 65 548 | 388 | Naive | 101 |
| A21 | 103 | 503 | Naive | 30 |
| A22 | 63 382 | 426 | Naive | 13 |
| A23 | 128 540 | 431 | 1.5 y | 123 |
| A24 | 68 380 | 150 | 8 mo | 95 |
| A25 | 6 369 | 857 | Naive | 119 |
| A26 | 659 | 619 | Naive | 139 |
| A27 | 19 700 | 542 | 3 y | 80 |
| A28 | 2 981 | 1049 | Naive | 189 |
| A29 | 30 553 | 835 | Naive | 27 |
| A30 | 64 | 975 | Naive | 172 |
| A31 | 21 159 | 571 | 1.8 y | 108 |
| A32 | 5 821 | 694 | Naive | 10 |
| A33 | 239 300 | 4 | Naive | 58 |
| A34 | 128 460 | 228 | Naive | 136 |
| A35 | 10 333 | 513 | Naive | 41 |
| NP1 | 124 | 1830 | Naive | 122 |
| NP2 | < 50 | 311 | Naive | 219 |
| NP3 | < 50 | 908 | Naive | 216 |
| NP4 | < 50 | 1042 | Naive | 222 |
| NP5 | < 50 | 1645 | Naive | 165 |
| NP6 | 59 | 972 | Naive | 103 |
| NP7 | < 50 | 785 | Naive | 72 |
| NP8 | < 50 | 1506 | Naive | 179 |
| NP9 | < 50 | 806 | Naive | 57 |
ND indicates not determined.
Time since most recent documented ART. All patients were off therapy at the time of sampling. Patients with undocumented therapy history prior to sampling are listed as unknown.
Sample month refers to the time between HIV diagnosis and the sampling date. For patients S16 and S25, the diagnosis date was unknown, so a value of 0 was used.
Peptides
Peptides (15mers overlapping by 11) corresponding to full-length HIV-1 gag, pol, env, nef, tat, rev, vif, vpr, and vpu were used at 2 μg/mL each peptide. The gag, pol, env, and nef peptides corresponded to the HXBc2/Bal R5 chimeric strain, and the tat, rev, vif, vpr, and vpu were clade B consensus sequence (NIH AIDS Research and Reference Reagent Program). All peptides were synthesized as free acids and were more than 80% pure. Lyophilized peptides were reconstituted to 100 mg/mL in 100% DMSO for the peptide mixes. Five mixes were prepared, one each for full-length gag, pol, env, and nef; tat, rev, vif, vpr, and vpu peptides were combined into one mix (TRVVV). Optimized peptides derived from CMV, EBV, and influenza were the kind gift of Dr Guido Ferrari (Duke University, Durham, NC).
Antibodies
Directly conjugated antibodies were obtained from the following: BD Biosciences (San Jose, CA): IL-2 (APC), CD3 (Cy7APC), IFN-γ (FITC), MIP-1β (PE), and TNF-α (Cy7PE); Beckman Coulter (Hialeah, FL): CD8 (Texas red-PE) and CD45RO (Texas red-PE). The following antibodies were conjugated in our laboratory (http://drmr.com/abcon/index.html): CD4 Cascade Blue, CD14 Cy5PE, CD19 Cy5PE, CD8 Qdot 655, CD27 Cascade Blue, CD27 Qdot 605, CD4 Cy5.5 PE, CD57 Qdot 565, CD57 Cascade Blue, and CD107a Alexa 680. The unconjugated monoclonal antibodies (mAbs) were obtained from BD Biosciences. Cascade Blue and Alexa 680 were obtained from Molecular Probes (Eugene, OR). Cy5 was obtained from Amersham Biosciences (Pittsburgh, PA). Quantum Dots were obtained from Quantum Dot Corporation (Hayward, CA).
Cell stimulation and staining
Purified PBMCs were thawed, resuspended to 2 × 106 cells/mL in complete RPMI media (RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 1.7 mM sodium glutamate), and rested overnight at 37°C with DNAse I (10 U/mL; Roche Diagnostics, Indianapolis, IN). The following morning, the PBMCs were examined for viability by trypan blue exclusion (typically ≥ 90% viable) and adjusted to 1 × 106 cells/mL. Costimulatory antibodies (αCD28 and 49d, 1 μg/mL; BD Biosciences), monensin (Golgistop, 0.7 μL/mL; BD Biosciences), brefeldin A (10 μg/mL; Sigma-Aldrich), and αCD107a-Alexa 680 (pretitered volume) were added, and the cells aliquoted at 1 mL to each tube containing 5 μL of each peptide mix. An unstimulated and positive control (Staphylococcus enterotoxin B, 1 μg/mL; Sigma-Aldrich) were included in each assay. Cells were incubated for 5.5 hours at 37°C. Following incubation, the cells were washed (PBS containing 1% bovine serum albumin and 0.1% sodium azide) and stained with surface antibodies. The cells were washed and fixed using the Cytofix/Cytoperm kit (BD PharMingen, San Diego, CA) according to instructions. Following fixation, the cells were washed twice in the perm buffer and stained with antibodies against intracellular markers. Following staining, the cells were washed, fixed (PBS containing 1% paraformaldehyde), and stored at 4°C until analysis.
Flow cytometry
Cells were analyzed on a modified LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA). Between 200 000 and 1 000 000 events were collected per sample. Data analysis was performed using FlowJo version 6.0 (TreeStar, San Carlos, CA). Initial gating used a forward scatter area (FSC-A) versus height (FSC-H) plot to remove doublets. Subsequently, the events were gated through a side scatter (SSC) versus Cascade Blue plot to remove dead cells (Cascade Blue bright), as a surrogate marker of viability (M.R., unpublished observations, 2003). CD14/19+ events were removed to reduce background staining of CD107a. Finally, the events were subjected to a lymphocyte gate by a FSC-A versus SSC plot. Following this, events are sequentially gated on CD3+, CD8+, and CD4– events versus IFN-γ to account for down-regulation. Following identification of CD8+ T cells, a gate was made for each respective function using combinations that provided optimal separation. After the gates for each function were created, we used the Boolean gate platform to create the full array of possible combinations, equating to 32 response patterns when testing 5 functions. Note that the CD4 Cascade Blue conjugate serves 2 purposes: (1) The bright events, resulting from the nonspecific entry and binding of Cascade Blue to an unknown molecule in permeable (ie, dead) cells, are removed, and (2) the CD4+ events (Cascade Blue dim positive) are removed further into the gating analysis. Thus, the Cascade Blue–anti-CD4 antibody conjugate serves both as a dead cell marker and specific label. Data are reported after background correction. Nonspecific background becomes extremely low when examining combinations of functions, nearly always reaching 0 events for 3 or more functions simultaneously. The background is higher for single positive responses, particularly for MIP-1β, because some CD8+ T cells constitutively express MIP-1β. The cutoff for a positive response is dependent on the particular combination from 10 events (4 and 5 functions) to as many as a few hundred events (1 function).
Statistical analysis
Unless otherwise noted, all data were background-subtracted using the 28/49d stimulation. A lower threshold corresponding to 2 SDs above background was built for each cytokine pattern based on a Poisson model, and values below this threshold were set to 0. For comparisons in which the interest was mainly in response patterns and not magnitude, the proportion of each individual response pattern within the total response was calculated by dividing each background-subtracted value by the sum of all patterns with at least one positive cytokine. Comparisons between groups were based on Wilcoxon rank sum test. A stricter criterion for significance (P < .01) was used to address the question of multiple comparisons. Correlations between log viral load and CD8+ T-cell responses were calculated using a linear regression model (least-squares fit).
Results
Concurrent measurement of 5 separate CD8+ T-cell functions
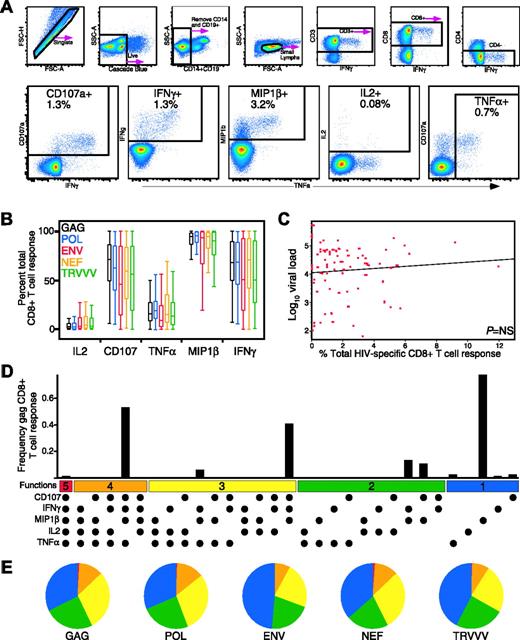
To measure the complexity of the HIV-specific T-cell response, we developed a flow cytometric assay to measure simultaneously and independently CD107a mobilization and IFN-γ, MIP-1β, TNF-α, and IL-2 production. Figure 1A shows representative data from an HIV-infected subject stimulated with overlapping HIV gag peptides. In this subject, HIV Gag-specific CD8+ T cells eliciting each of the 5 functions were measurable (CD107a, 3.5%; IFN-γ, 2.5%; MIP-1β, 4.6%; IL-2, 0.2%; TNF-α, 0.6%). The full complexity of the response was then examined using a Boolean gating algorithm, yielding 32 unique response patterns comprising every combination of the 5 individual measurements (Figure 1D). The frequency of each individual response pattern was then assessed and the contribution of each combination toward the total response determined.
Hierarchy of the HIV-specific CD8+ T-cell functional response
Using this panel, we assessed the HIV-specific CD8+ T-cell response functionality by measuring responses against the total HIV genome in 79 chronically infected individuals (termed “progressors,” Table 1; see “Patients and methods” for selection criteria). Overall, MIP-1β dominated the HIV-specific CD8+ T-cell response for every HIV antigen (P < .001; Figure 1B). CD107a expression and IFN-γ production were the next highest responses, followed by TNF-α, and finally IL-2 production. In some subjects, cells expressing IFN-γ accounted for only 60% of the response, and in no subject was IFN-γ the most highly expressed function (data not shown). This indicates that measurement of only IFN-γ underestimates the total HIV-specific CD8+ T-cell frequency.
Detection of 5 concurrent T-cell functions and characterization of HIV-specific CD8+ T-cell functionality in HIV-infected progressors. (A) Gating scheme for identification of multifunctional CD8+ T-cell responses. Shown are representative data of the HIV Gag-specific response from subject A26, an HIV-infected progressor, after a 6-hour in vitro stimulation. See “Patients, materials, and methods” for a detailed explanation of the procedure. (B) MIP-1β is the dominant HIV-specific CD8+ T-cell response (P < .001 for all stimulation conditions). Box plots represent the 10th, 25th, 50th, 75th, and 90th percentiles of the contribution of the indicated functional response (x-axis) toward the total CD8+ T-cell response against the indicated HIV peptide mix (color coded as shown) within the 79 progressors. The responses from each subject were standardized to allow comparison of the proportions of the total response expressing each function irrespective of the others. (C) Total CD8+ T-cell response frequency does not correlate with viral load. The red dots represent the total response summed across all functional combinations for each antigen for each progressor (n = 79). The x-axis denotes CD8+ T-cell frequency, and the y-axis denotes log10 viral RNA load. (D) The CD8+ T-cell response to HIV-Gag is composed of multiple functional subpopulations and is largely restricted to cell populations with limited functionality. The black bars represent the total CD8+ T-cell response frequency to Gag in subject A26 expressing the particular combination of functions shown. Each dot denotes CD107a, IFN-γ, MIP-1β, IL-2, and/or TNF-α positivity. The panel also contains horizontal bars of different colors showing those combinations of 5, 4, 3, 2, or 1 function for reference. Responses shown are background subtracted using the 28/49d negative control. (E) The functional profile of HIV-specific CD8+ T-cells is limited in HIV-infected progressors. Each pie chart represents the mean response across the 79 subjects to the 5 different HIV-antigen stimulations. For simplicity, responses are grouped by number of functions, matched to the colored bars in panel D. As shown, more than 75% of the average response to each antigen expresses fewer than 4 functions.
Detection of 5 concurrent T-cell functions and characterization of HIV-specific CD8+ T-cell functionality in HIV-infected progressors. (A) Gating scheme for identification of multifunctional CD8+ T-cell responses. Shown are representative data of the HIV Gag-specific response from subject A26, an HIV-infected progressor, after a 6-hour in vitro stimulation. See “Patients, materials, and methods” for a detailed explanation of the procedure. (B) MIP-1β is the dominant HIV-specific CD8+ T-cell response (P < .001 for all stimulation conditions). Box plots represent the 10th, 25th, 50th, 75th, and 90th percentiles of the contribution of the indicated functional response (x-axis) toward the total CD8+ T-cell response against the indicated HIV peptide mix (color coded as shown) within the 79 progressors. The responses from each subject were standardized to allow comparison of the proportions of the total response expressing each function irrespective of the others. (C) Total CD8+ T-cell response frequency does not correlate with viral load. The red dots represent the total response summed across all functional combinations for each antigen for each progressor (n = 79). The x-axis denotes CD8+ T-cell frequency, and the y-axis denotes log10 viral RNA load. (D) The CD8+ T-cell response to HIV-Gag is composed of multiple functional subpopulations and is largely restricted to cell populations with limited functionality. The black bars represent the total CD8+ T-cell response frequency to Gag in subject A26 expressing the particular combination of functions shown. Each dot denotes CD107a, IFN-γ, MIP-1β, IL-2, and/or TNF-α positivity. The panel also contains horizontal bars of different colors showing those combinations of 5, 4, 3, 2, or 1 function for reference. Responses shown are background subtracted using the 28/49d negative control. (E) The functional profile of HIV-specific CD8+ T-cells is limited in HIV-infected progressors. Each pie chart represents the mean response across the 79 subjects to the 5 different HIV-antigen stimulations. For simplicity, responses are grouped by number of functions, matched to the colored bars in panel D. As shown, more than 75% of the average response to each antigen expresses fewer than 4 functions.
We next addressed whether by measuring additional CD8+ T-cell functions beyond IFN-γ a relationship between HIV-specific CD8+ T-cell frequency and viral load might be identified. No relationship between the HIV-specific CD8+ T-cell frequency, either in total (Figure 1C) or to individual HIV antigens (data not shown), and viral load was found, which confirms and extends our previous observation that the absolute frequency of HIV-specific CD8+ T cells does not correlate with viral load.15
We examined the functional hierarchy of the HIV-specific CD8+ T-cell response in the progressors to HIV-Gag, as shown in Figure 1D. The response hierarchy was limited, with the major responding populations being mainly segregated to few of the possible functional combinations, most with 3 or fewer simultaneous functions. In this individual, the Gag-specific response was composed of 3 major populations: cells expressing 4 (CD107a+IFN-γ+MIP-1β+TNF-α+), 3 (CD107a+IFN-γ+MIP-1β+), and one function (MIP-1β+). Cells expressing all 5 measured functions were nearly absent in the responding cell population, driven primarily by a paucity of IL-2 production. This pattern of limited functionality extended throughout nearly the entire progressor cohort against HIV-Gag, Pol, Env, Nef, and TRVVV (Figure 1E), with the vast majority of responses to any antigen being restricted to 3 functions or fewer. Although this response pattern was largely shared between the different antigens among the cohort, there were also differences. For example, CD107a+IFN-γ+MIP-1β+TNF-α+ and CD107a+MIP-1β+ CD8+ T cells were significantly more frequent for Gag and Pol-specific responses than Env-specific responses (P < .001). Furthermore, MIP-1β+ populations sometimes dominated the response to particular HIV antigens. This was often observed for HIV Env, where the MIP-1β+ response sometimes represented nearly 90% of the Env-specific response. Therefore, not only are there different functional responses to a single HIV antigen, but individual HIV proteins can stimulate qualitatively diverse response profiles.
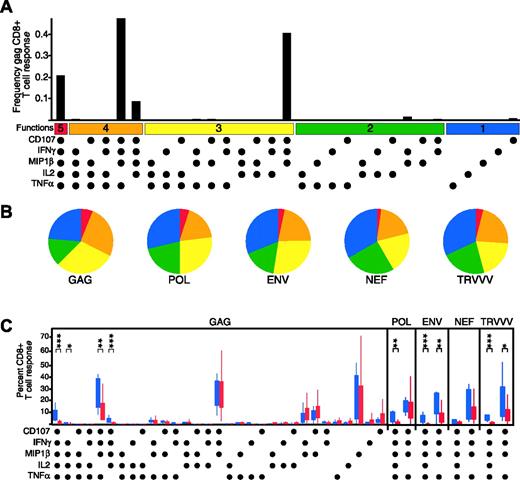
HIV-infected nonprogressors maintain a higher degree of CD8+ T-cell functionality
Having observed consistent patterns of limited functionality in response to HIV antigens in the progressors, we hypothesized that nonprogressive HIV infection may be associated with a qualitatively different CD8+ T-cell functional profile. We therefore performed a similar analysis of HIV-specific CD8+ T cells in 9 HIV-infected nonprogressors (Table 1). Figure 2A shows the response to HIV-Gag in a representative HIV-infected nonprogressor. The response to HIV-Gag was similar to the progressors, in that many of the same functional populations were present. However, the response was notably shifted in functionality, such that cells positive for all 5 functions measured were detectable (CD107a+IFN-γ+MIP-1β+IL-2+TNF-α+ [“5+”]) and a larger proportion of the response was positive for 4 measured functions (Figure 2A-B). This enhanced functionality extended to responses against all HIV antigens in the nonprogressors (Figure 2B). The frequency of HIV Gag-specific CD8+ T cells was higher in nonprogressors than progressors (P < .001, data not shown). Although the total frequency of Pol-specific CD8+ T cells was not significantly different, more responding cells produced IL-2 (P < .001, data not shown). Similarly, more Env-specific CD8+ T cells in the nonprogressors produced IL-2 and TNF-α (P < .01, data not shown).
We compared the functional profiles, irrespective of magnitude, of the CD8+ T-cell responses by expressing each response component as a proportion of that total response (Figure 2C). The left panel of Figure 2C compares the entire Gag-specific response between the progressors and nonprogressors. The HIV-Gag response profiles appeared mostly similar between the groups, in that much of the response was limited in functionality. However, responses in the nonprogressors (blue box plots) had a higher degree of functionality (4+ or 5+ different functions simultaneously) than in the progressors (red box plots). The presence of 5+ HIV Pol-, Env-, and TRVVV-specific CD8+ T cells was also a discriminating factor between nonprogressors and progressors (Figure 2C right). Additionally, Env- and TRVVV-specific CD8+ T cells expressing various combinations of 4 functions were more prevalent in the nonprogressors.
Recent data have suggested that HIV-infected nonprogressors and progressors can be differentiated by the proportion of CD8+ T cells that produce IFN-γ and IL-2.27 To confirm this, we compared the proportion of IFN-γ+IL-2+ cells between the groups for each peptide pool; these proportions were significantly different for Env and Gag (P < .001), but not Pol, Nef, or TRVVV (data not shown). Next, we examined whether the additional measurement of CD107a, TNF-α, and MIP-1β helped to discriminate between the 2 groups. We used a likelihood ratio test to compare a logistic regression model predicting progression status (nonprogressor versus progressor) using mean IFN-γ+IL-2+ proportion across peptide pools versus a similar model including both the mean IFN-γ+IL-2+ and mean CD107a+IFN-γ+MIP-1β+IL-2+TNF-α+ proportions. The test was significant at P = .001, indicating that measuring responses by 5 functions provides a better differentiation between progressors and nonprogressors than measuring only IFN-γ and IL-2.
HIV-specific CD8+ T-cell functionality discriminates HIV-infected nonprogressors. (A) HIV Gag-specific CD8+ T-cell responses in HIV-infected nonprogressors are highly functional. The black bars represent the CD8+ T-cell response frequency to Gag in a representative nonprogressor (subject NP8) expressing the particular combination of functions shown. Each dot denotes CD107a, IFN-γ, MIP-1β, IL-2, and/or TNF-α positivity. The panel also contains horizontal bars of different colors showing those combinations of 5, 4, 3, 2, or 1 function for reference. Responses shown are background subtracted using the 28/49d negative control. (B) HIV-specific CD8+ T cells are highly functional in HIV-infected nonprogressors. Each pie chart represents the mean response across the 9 nonprogressors to the 5 different HIV-antigen stimulations. For simplicity, responses are grouped by number of functions, matched to the colored bars in panelA. As shown, responses with 5 functions can be detected to each HIV antigen in the nonprogressors, and a large proportion of the responding cells express 4 functions. (C) HIV-specific CD8+ T cells from nonprogressors (blue boxes) have a qualitatively different functional profile compared to progressors (red boxes). The box plots represent the 10th, 25th, 75th, and 90th percentiles of the proportion of the respective functional response toward the total CD8+ T-cell response against HIV Gag (left) or other HIV antigens (right panels). For simplicity, only the 5+ and 4+ populations lacking IL-2 production are shown for Pol, Env, Nef, and TRVVV responses; no differences were found for those functional combinations not shown. The responses from the cohorts were standardized so that the profiles could be compared irrespective of any frequency differences. Asterisks are placed above response pairs that are significantly different: ***P ≤ .001; **P ≤ .01. Marginal differences (P ≤ .05) are designated as a single asterisk. Each dot denotes a positive response for the function indicated at the bottom left.
HIV-specific CD8+ T-cell functionality discriminates HIV-infected nonprogressors. (A) HIV Gag-specific CD8+ T-cell responses in HIV-infected nonprogressors are highly functional. The black bars represent the CD8+ T-cell response frequency to Gag in a representative nonprogressor (subject NP8) expressing the particular combination of functions shown. Each dot denotes CD107a, IFN-γ, MIP-1β, IL-2, and/or TNF-α positivity. The panel also contains horizontal bars of different colors showing those combinations of 5, 4, 3, 2, or 1 function for reference. Responses shown are background subtracted using the 28/49d negative control. (B) HIV-specific CD8+ T cells are highly functional in HIV-infected nonprogressors. Each pie chart represents the mean response across the 9 nonprogressors to the 5 different HIV-antigen stimulations. For simplicity, responses are grouped by number of functions, matched to the colored bars in panelA. As shown, responses with 5 functions can be detected to each HIV antigen in the nonprogressors, and a large proportion of the responding cells express 4 functions. (C) HIV-specific CD8+ T cells from nonprogressors (blue boxes) have a qualitatively different functional profile compared to progressors (red boxes). The box plots represent the 10th, 25th, 75th, and 90th percentiles of the proportion of the respective functional response toward the total CD8+ T-cell response against HIV Gag (left) or other HIV antigens (right panels). For simplicity, only the 5+ and 4+ populations lacking IL-2 production are shown for Pol, Env, Nef, and TRVVV responses; no differences were found for those functional combinations not shown. The responses from the cohorts were standardized so that the profiles could be compared irrespective of any frequency differences. Asterisks are placed above response pairs that are significantly different: ***P ≤ .001; **P ≤ .01. Marginal differences (P ≤ .05) are designated as a single asterisk. Each dot denotes a positive response for the function indicated at the bottom left.
Relationship between functionality and viral load
Having observed enhanced functionality in the nonprogressors, we revisited the relationship between CD8+ T-cell response and viral load in the progressors. Rather than focusing on total HIV-specific CD8+ T-cell frequency, which was not correlated with viral load (Figure 1C), we examined the relationship between the frequency and percent of the HIV-specific T-cell response positive for all 5 measured functions and viral load in the progressors. Both the frequency and percent (Figure 3A-B) of HIV Gag-specific CD8+ T cells expressing all 5 functions correlated inversely with viral load (P < .001). We next calculated the percent and total frequency of all 5+ responses against all HIV antigens in each progressor toward the total HIV-specific CD8+ T-cell response (Figure 3C and 3D, respectively), and found these were inversely correlated with viral load (P < .001 and P < .01, respectively). The frequency of the largest single 5+ response to any HIV antigen in each progressor also correlated inversely with viral load (Figure 3E; P < .01). Finally, we examined the relationship between Nefspecific 5+ responses and viral load (Figure 3F). Despite having found no difference in Nef-specific responses between progressors and nonprogressors, a strong inverse correlation (P < .001) between Nef-response functionality and viral load was found. We addressed concerns regarding influential points by excluding the most extreme points and refitting the regression models. The significance of all the regressions decreased, as expected with the exclusion of an extreme point, although only the relationship between total frequency and viral load dropped below significance (P < .09). Perhaps more importantly, for all of the statistically significant regression terms for single peptide measures of 5+ frequency or proportion, the coefficient either increased in absolute value or changed only slightly when the most influential point was removed (data not shown). This suggests that the removed point, while influencing the P value as expected, did not substantially change the estimates of the relationship between the variables. These data suggest that rather than total magnitude, the frequency of particular functional subsets of the HIV-specific CD8+ T-cell responses are important in control of viral load.
The magnitude and proportion of the HIV-specific CD8+ T-cell response positive for all 5 functions is inversely correlated with viral load. Each dot represents data from a single progressor. The dotted line represents the linear regression (least-squares fit) line for predicting viral load based on the respective CD8+ T-cell response. Respective P values are shown in the top right of each graph. In the graphs, frequency refers to the magnitude of the antigen-specific response with a specific functional profile within the total CD8 pool, and percent refers to the proportion of the total antigen-specific response that bears a specific functional profile. The graphs depict: (A) frequency of 5+ Gag-specific cells versus viral load (slope =–30.6, r 2 = 0.15); (B) percent of Gag-specific response that is 5+ versus viral load (slope =–13.2, r 2 = 0.16); (C) percent of the total response (all antigens) that is 5+ versus viral load (slope =–23.8, r 2 = 0.19); (D) summed frequency of all 5+ responses from each antigen versus viral load (slope =–12.3, r 2 = 0.09); (E) the maximum frequency of any single 5+ response from any single antigen in each progressor versus viral load (each progressor is only represented by a single point; slope =–19.3, r 2 = 0.08); and (F) the percent of the Nef-specific response that is 5+ versus viral load (slope =–23.6, r 2 = 0.19). Note that the low r 2 values are primarily a function of the large number of values at 0 in the progressors, which cluster at higher viral loads.
The magnitude and proportion of the HIV-specific CD8+ T-cell response positive for all 5 functions is inversely correlated with viral load. Each dot represents data from a single progressor. The dotted line represents the linear regression (least-squares fit) line for predicting viral load based on the respective CD8+ T-cell response. Respective P values are shown in the top right of each graph. In the graphs, frequency refers to the magnitude of the antigen-specific response with a specific functional profile within the total CD8 pool, and percent refers to the proportion of the total antigen-specific response that bears a specific functional profile. The graphs depict: (A) frequency of 5+ Gag-specific cells versus viral load (slope =–30.6, r 2 = 0.15); (B) percent of Gag-specific response that is 5+ versus viral load (slope =–13.2, r 2 = 0.16); (C) percent of the total response (all antigens) that is 5+ versus viral load (slope =–23.8, r 2 = 0.19); (D) summed frequency of all 5+ responses from each antigen versus viral load (slope =–12.3, r 2 = 0.09); (E) the maximum frequency of any single 5+ response from any single antigen in each progressor versus viral load (each progressor is only represented by a single point; slope =–19.3, r 2 = 0.08); and (F) the percent of the Nef-specific response that is 5+ versus viral load (slope =–23.6, r 2 = 0.19). Note that the low r 2 values are primarily a function of the large number of values at 0 in the progressors, which cluster at higher viral loads.
We further tested the importance of the 5+ HIV-specific CD8+ T-cell response by creating a linear regression model to predict viral load as a function of both CD4 count and the 5+ HIV-specific CD8+ T-cell response frequency (sum of Gag-, Nef-, and Envspecific 5+ responses). After controlling for CD4 count, we found that the frequency of the 5+ response strongly correlated with viral load (P < .001, r2 = 0.39, slope =–19.6). Similar relationships were observed when testing this model using the individual 5+ response frequencies specific for Gag, Env, or Nef, but not Pol or TRVVV (data not shown). Thus, the 5+ HIV-specific CD8+ T-cell response frequency is predictive of viral load independent of CD4 T-cell count.
Influence of generalized immunosuppression, HLA type, and memory phenotype on functionality
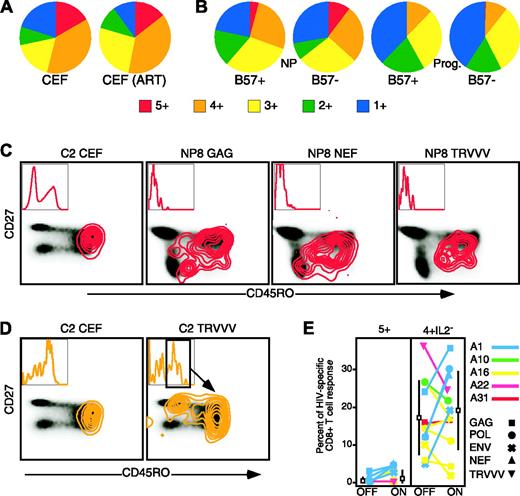
Limited CD8+ T-cell functionality in the HIV progressors could directly result from a number of factors, including generalized immunosuppression and direct effects of viral load. In this scenario, we would expect to observe limited CD8+ T-cell functionality in the progressors against non-HIV antigens, such as peptides derived from CMV, EBV, and influenza (CEF). Rather than limited in nature, the functionality of the CEF-specific CD8+ T cells was quite high in a group of HIV-infected progressors, with over 50% of the CD8+ T-cell response to CEF being positive for 4 and 5 functions (Figure 4A). This level of functionality is characteristic of CEF-specific responses in HIV-seronegative individuals (data not shown). Moreover, if active viral replication were causing a generalized effect, artificial control of viral load through HAART would likely alleviate this effect. We therefore examined CEF-specific responses in a subset of the untreated individuals (see Figure 4E for subject identification) who initiated ART after initial entry into the study. No change in functionality against CEF peptides was apparent in these subjects 6 months to 1 year after initiation of ART. Thus, limited functionality of HIV-specific CD8+ T cells is restricted to the HIV-specific CD8+ T cells and is not a generalized characteristic of all CD8+ T cells in HIV-infected individuals.
HLA-B57 has been strongly associated with elite nonprogressor status.10,11 Six of the nonprogressors were HLA-B57+; however, there were no definitive functional differences between the HLA-B57+ and B57– nonprogressors (Figure 4B). Moreover, 6 of the progressors were HLA-B57+; however, there was again no difference in functionality between the HLA-B57+ and B57– progressors. These results indicate that enhanced functionality in the nonprogressors is not associated with the presence of HLA-B57 as a presenting allele.
Maintenance of HIV-specific CD8+ T cells of a central memory phenotype in the nonprogressors could also result in differential functionality. Indeed, 12-color analysis of 5+ function CEF-specific cells from progressor C2 (Figure 4C) showed a central memory phenotype (CD45RO+CD27+). However, analysis of a third marker indicated that this functional population could be subdivided based on CD57 expression. There was surprising variability in the memory phenotype of the 5+ function Gag, Nef, and TRVVV-specific CD8+ T cells from NP8 (Figure 4C). Although uniformly CD57–, identical functional responses against the different antigens spanned memory cell phenotypes considered to be central memory, effector memory (CD45RO+CD27–), and effector (CD45RO–CD27–). We observed similar phenotypic heterogeneity in responses positive for all functions except IL-2 (“4+IL-2–”; orange contours, Figure 4D). The 4+IL-2– CEF-specific cells in progressor C2 also were CD45RO+CD27+, but greatly skewed toward CD57 expression. In the same subject, 4+IL-2– TRVVV-specific cells could be subdivided into multiple populations based on CD45RO, CD27, and CD57 levels. Similar variability was observed in the 4+IL-2– Gag- and Nef-specific CD8+ T cells in subject NP8. These results demonstrate that memory phenotype is not necessarily predictive of functionality and that the presence of the 5+ function cells in the nonprogressors is not simply due to an overrepresentation of cells with a central memory phenotype.
Enhanced functionality in nonprogressors is not due to the presence of HLA-B57 or maintenance of central memory cells. (A) CMV-, EBV-, and influenzaspecific CD8+ T cells in the progressors are not restricted in functionality either off (left) or after initiation of ART (right). Each pie chart represents the average CD8+ T-cell response from a group of 5 progressors either prior to or after initiation of HAART. The colors correspond to the number of functions expressed, as shown. (B) HLA-B57+ (n = 6) and B57– (n = 3) nonprogressors were separated (left 2 pie charts), and the functionality of their HIV Gag-specific CD8+ T-cell responses compared with each other, and to HLA-B57+ (n = 6) and B57– (n = 6) progressors (right 2 pie charts). The colors in each pie chart correspond to the number of functions depicted by colored squares. (C) 5+ and (D) 4+ IL-2– responding cells can have varied memory phenotype. Twelve-color flow cytometry was performed to characterize the memory phenotype of responding CD8+ T cells. Plots depict CD8+ T cells responding with either the 5+ (red contours) or 4+IL-2– (orange contours) functional profile overlaid onto a density plot of the memory phenotype, as determined by CD27 and CD45RO, of the total CD8+ T-cell population. Inset in each plot is a histogram representing CD57 expression in the responding cells. (E) Initiation of ART does not improve CD8+ T-cell functionality in most individuals. Plots show the proportion of the total response against the indicated HIV peptide mix (indicated at top of each plot together with stimulus) prior to (OFF) and 6 to 12 months after (ON) initiation of ART. Each subject is represented by a colored line/symbol, and the symbols indicate the particular stimuli tested. The black box represents the mean response on or off therapy, with the SD of the mean shown as a black bar.
Enhanced functionality in nonprogressors is not due to the presence of HLA-B57 or maintenance of central memory cells. (A) CMV-, EBV-, and influenzaspecific CD8+ T cells in the progressors are not restricted in functionality either off (left) or after initiation of ART (right). Each pie chart represents the average CD8+ T-cell response from a group of 5 progressors either prior to or after initiation of HAART. The colors correspond to the number of functions expressed, as shown. (B) HLA-B57+ (n = 6) and B57– (n = 3) nonprogressors were separated (left 2 pie charts), and the functionality of their HIV Gag-specific CD8+ T-cell responses compared with each other, and to HLA-B57+ (n = 6) and B57– (n = 6) progressors (right 2 pie charts). The colors in each pie chart correspond to the number of functions depicted by colored squares. (C) 5+ and (D) 4+ IL-2– responding cells can have varied memory phenotype. Twelve-color flow cytometry was performed to characterize the memory phenotype of responding CD8+ T cells. Plots depict CD8+ T cells responding with either the 5+ (red contours) or 4+IL-2– (orange contours) functional profile overlaid onto a density plot of the memory phenotype, as determined by CD27 and CD45RO, of the total CD8+ T-cell population. Inset in each plot is a histogram representing CD57 expression in the responding cells. (E) Initiation of ART does not improve CD8+ T-cell functionality in most individuals. Plots show the proportion of the total response against the indicated HIV peptide mix (indicated at top of each plot together with stimulus) prior to (OFF) and 6 to 12 months after (ON) initiation of ART. Each subject is represented by a colored line/symbol, and the symbols indicate the particular stimuli tested. The black box represents the mean response on or off therapy, with the SD of the mean shown as a black bar.
Effect of artificial control of viral load on CD8+ T-cell functionality
Initiation of ART has multiple effects on the immune system, including reduction of generalized immune activation and partial reconstitution of peripheral CD4+ T cells,28-30 which could potentially influence, and possibly improve, HIV-specific CD8+ T-cell functionality. Because generalized immune activation subsides rapidly after therapy initiation, and long-term antiretroviral therapy often results in a dramatic decrease in HIV-specific CD8+ T-cell frequency31,32 we studied the effects of short-term (∼6 months to 1 year) therapy on CD8+ T-cell functionality in a subset of the HIV-infected progressor cohort who initiated ART. Limited improvement of HIV-specific CD8+ T-cell functionality (5+ and 4+IL-2– response proportion) was observed (Figure 4E), and modest improvement in one subject (A23) was observed for Gag-specific responses (yet not for Nef-specific responses). This relative lack of improvement occurred despite moderate (70 cells/mm3) to substantial (400 cells/mm3) increases in CD4 count during therapy in 4 of 5 subjects. Taken together, these results suggest that CD8+ T-cell functionality is not directly influenced by generalized immune activation or active viral replication.
Discussion
Here we examined the HIV-specific CD8+ T-cell functional hierarchy in HIV-infected progressors and nonprogressors. Our results demonstrate that the functional profile of HIV-specific CD8+ T cells is highly complex, but limited compared to the functional profile of CD8+ T cells specific for other viruses in the same subject. Importantly, we find that HIV-infected nonprogressors possess a fundamentally different HIV-specific CD8+ T-cell response with enhanced functionality. In addition, we show that highly functional CD8+ T-cell responses are commonly found in response to other viral infections known to be effectively controlled by cellular immunity, suggesting that the association between polyfunctional CD8+ T cells and viral control may extend beyond HIV.
HIV-specific CD8+ T cells are composed of several major populations with unique functional patterns. In progressors, this profile is largely limited to the expression of various combinations of CD107a, IFN-γ, and MIP-1β. Little TNF-α is produced, and even less IL-2. Nonprogressors have a different profile, in that they retain the capacity to produce TNF-α and IL-2 in conjunction with the other functions. Although there were some differences between the response profiles for different HIV antigens, by and large the functional profiles were similar regardless of the HIV antigen and total response frequency.
Similar to a recent report,22 we found that MIP-1β production, and not IFN-γ, dominates the HIV-specific CD8+ T-cell response. Every major responding HIV-specific CD8+ T-cell population produced MIP-1β, suggesting this chemokine may be the best single indicator of HIV-specific CD8+ T-cell frequency. It is unclear if the response to every epitope follows the same functional hierarchy we have defined here. Thus, it remains to be determined if mapping the breadth of viral responses is appropriate using only a single T-cell function. These results also have important ramifications for the interpretation of previous findings that have relied on quantification of HIV-specific CD8+ T-cell responses by measuring IFN-γ production. Here, even by measuring additional responses to more accurately quantify responding HIV-specific CD8+ T cells, there was still no correlation with viral load. Some components of the CD8+ T-cell response may be irrelevant in the control of viral load, whereas others may have a profound antiviral effect. The most dramatic difference in functionality that we observed was the loss of the 5+ population in the progressors. Indeed, we find that both the frequency and proportion of the 5+ population correlates inversely with viral load in the progressors. Thus, the total magnitude of the HIV-specific response is not necessarily associated with viral control; instead, the magnitude of specific functional subpopulations within the total response is associated with control of viral load.
The 5+ functional population is likely of significant immunologic importance because it represents up to 25% of the total response to CMV, EBV, and influenza, viral infections thought to be effectively controlled by CD8+ T cells. This population could directly eliminate virally infected cells (assuming it expresses or up-regulates perforin) and suppress viral replication, while maintaining itself without CD4+ T-cell help through autocrine production of IL-2. This latter ability may be especially important in the setting of diminished CD4+ T-cell help in HIV infection, as was recently suggested.27 Alternatively, these cells may represent a self-renewing progenitor population of antigen-specific CD8+ T cells responsible for long-term maintenance of effector CD8+ T cells, without any direct antiviral ability themselves. Because these cells cannot simply be defined by the commonly used cell surface memory phenotyping markers CD27, CD45RO, and CD57, further experiments will be necessary to determine their exact phenotypic lineage and role in immunologic control.
We find that diminished HIV-specific CD8+ T-cell functionality in progressors is not an indirect effect of generalized immune activation or immunosuppression based on 2 observations. First, highly functional responses against other viruses (CMV, EBV, influenza) are maintained in the presence of high HIV load and are not further improved after initiation of ART. Second, reduction in immune activation that occurs after short-term ART does not directly improve functionality in the HIV-specific CD8+ T cells. Functionality is also not a direct effect of the presence of HIV because initiation of antiretroviral therapy results in substantial improvement of HIV-specific CD8+ T-cell functionality in only a minority of subjects. Because maturational state partially dictates functionality, it is unlikely that long-term therapy will improve the functionality of pre-existing HIV-specific CD8+ T cells, especially in the absence of HIV-specific CD4+ T-cell reconstitution, unless new HIV-specific CD8+ T cells arise from recent thymic emigrants. Further experiments will be necessary to determine whether functionality can be improved after long-term ART and therapy interruption, or if functionality of existing HIV-specific CD8+ cells could possibly be manipulated through therapeutic intervention.
Why do the nonprogressors maintain HIV-specific CD8+ T-cell responses with improved functionality? We have shown here that it is not simply due to the presence HLA-B57 that, along with HLA-B27, is often overrepresented in nonprogressor cohorts.10,11 Furthermore, HIV-specific CD8+ T cells with the 5+ (or 4+) functional profile are not restricted to central memory phenotype. In fact, we observed a surprising disconnect between memory phenotype and CD8+ T-cell functionality, which calls into question whether the differential CD8+ T-cell maturation status in HIV infection plays a role in disease progression.33 Perhaps because the nonprogressors maintain cognate CD4+ T-cell help (M.R.B. and R.A.K., manuscript in preparation), they are better able to maintain highly functional HIV-specific CD8+ T cells. This, in part, would explain why there is little improvement in functionality after initiation of therapy in the progressors because reconstitution of the HIV-specific CD4+ T cell pool is limited.34
Taken together, these results provide an unprecedented assessment of the functionality of HIV-specific CD8+ T cells and clearly demonstrate that HIV-infected nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells compared to progressors, indicating that the quality, and not quantity, of HIV-specific CD8+ T cells is a clinical correlate of protection from disease progression. These findings are crucial to the development and monitoring of HIV vaccine candidates and therapeutic agents designed to stimulate HIV-specific immunity, and provide a benchmark for future analysis of human antiviral T-cell responses.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI10.1182/blood-2005-12-4818.
Supported by NIH grants AI 36219 (M.M.L.) and AI 49126 (P.A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank David Price and Daniel Douek for review of this manuscript, and Joanne Yu and Pratip Chattophadyay for reagent manufacture and validation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal