Abstract
Although infants with acute lymphoblastic leukemia (ALL) and a germline MLL gene have a better prognosis than comparable infants with a rearranged MLL gene, their optimal therapy is controversial. In 2 consecutive studies, conducted between 1996 and 2002, we treated 22 cases of infant ALL with germline MLL using chemotherapy alone. The 5-year event-free survival rate was 95.5% with a 95% confidence interval of 86.9 to 100%. All 21 infants with precursor B-cell ALL have been in first complete remission for 3.5 to 8.8 years. Most treatment-related toxicities were predictable and well tolerated, and neither secondary malignancies nor physical growth impairments have been observed. These results indicate that chemotherapy of the type described here is both safe and highly effective against infant precursor B-cell ALL with MLL in the germline configuration.
Introduction
Infants younger than 1 year of age with acute lymphoblastic leukemia (ALL), who represent 2.5% to 5% of all childhood ALL cases, still show generally poor responses to treatment.1,2 This inferior outcome is closely associated with young age, negative CD10 on leukemic cells, and positive MLL gene rearrangements.3,4 Whether infants with germline MLL can be treated less aggressively than those with rearrangement of this gene is still unclear, because most study groups have enrolled infants on the same therapeutic protocol regardless of their MLL gene status.5-11 In those trials, the event-free survival rate for infants with ALL and positive CD10 expression or lack of 11q23 abnormalities ranged from 52% to 79%, suggesting a worse outcome than seen in childhood ALL in general, even though some infants with a rearranged MLL gene might have been inadvertently included in the better-risk cohort.10-13
The Japan Infant Leukemia Study Group segregated infants with ALL into 2 subgroups according to their MLL gene status in 2 consecutive studies. Infants with a rearranged MLL gene received intensive chemotherapy followed by hematopoietic stem cell transplantation, whereas those with a germline MLL were treated with chemotherapy alone.14,15 As reported here, a highly promising outcome was obtained in the latter subgroup, providing a rationale for the design of future studies focusing on infant ALL.
Study design
Between December 1995 and December 2002, all consecutive infants with ALL and age younger than 12 months were registered and treated on 2 protocols designated MLL96 and MLL98. Written informed consent, provided according to the Declaration of Helsinki, was obtained from the parents or guardians of the patients, and the institutional review boards approved all aspects of this investigation. Each patient was evaluated with respect to the characteristics of leukemic cells, including immunophenotype, cytogenetics, and MLL gene rearrangement. Each patient with positive CD10 expression was assigned to the chemotherapy subgroup, after confirmation of the MLL gene status by Southern blot analysis or fluorescence in situ hybridization. If a rearrangement was found, the patient was excluded from the chemotherapy subgroup. The treatments used in these 2 studies were identical, consisting of induction, consolidation, and central nervous system (CNS) prophylaxis, intensification, reinduction, and maintenance phases (Table 1). The total duration of therapy was 83 to 85 weeks.
Treatment plan for infant ALL with a germline MLL gene
Phase and drug . | Site, duration . | Dosage . | Time of dose(s) . |
|---|---|---|---|
| Induction | |||
| DEX | Intravenous | 10 mg/m2 | Days 1-14 |
| PSL | By mouth or intravenous | 60 mg/m2 | Days 15-28 |
| VCR | Intravenous | 0.05 mg/kg | Days 1, 8, 15, 22 |
| CPA | Intravenous, 1-2 h | 1200 mg/m2 | Day 2 |
| DXR | Intravenous, 1 h | 25 mg/m2 | Days 3, 5 |
| ASP | Intravenous, 3-4 h | 10 000 U/m2 | Days 16, 18, 20, 23, 25, 27 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15, 29 |
| VP-16 | Intravenous, 1-2 h | 100 mg/m2 | Days 29-32 |
| Ara-C | Intravenous, 4 h | 500 mg/m2 | Days 29-32 |
| Consolidation and CNS prophylaxis | |||
| MTX | Intravenous, 24 h | 3 g/m2 | Days 1, 15, 29 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15, 29 |
| CPA | Intravenous, 1-2 h | 500 mg/m2 | Days 2, 16, 30 |
| ASP | Intravenous or intramuscular | 10 000 U/m2 | Days 2, 16, 30 |
| PSL | By mouth or intravenous | 60 mg/m2 | Days 1-3 |
| Intensification | |||
| VCR | Intravenous | 0.05 mg/kg | Days 1, 8, 15 |
| DNR | Intravenous | 25 mg/m2 | Days 1, 8, 15 |
| Ara-C | Intravenous, 1 h | 60 mg/m2 | Days 2-7, 9-14 |
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15 |
| Maintenance* | |||
| Regimen A | |||
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| MTX | By mouth | 30 mg/m2 | Days 1, 8 |
| VP-16 | Intravenous, 1-2 h | 150 mg/m2 | Day 14 |
| Ara-C | Intravenous, 4 h | 200 mg/m2 | Day 14 |
| Regimen B | |||
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| MTX | By mouth | 30 mg/m2 | Days 1, 8 |
| PSL | By mouth | 60 mg/m2 | Days 15-28 |
| VCR | Intravenous | 0.05 mg/kg | Days 15, 22, 29 |
| MTX | Intravenous, 5 h | 300 mg/m2 | Day 15 |
| TIT | Intrathecal | Age-adjusted† | Every 6 weeks |
Phase and drug . | Site, duration . | Dosage . | Time of dose(s) . |
|---|---|---|---|
| Induction | |||
| DEX | Intravenous | 10 mg/m2 | Days 1-14 |
| PSL | By mouth or intravenous | 60 mg/m2 | Days 15-28 |
| VCR | Intravenous | 0.05 mg/kg | Days 1, 8, 15, 22 |
| CPA | Intravenous, 1-2 h | 1200 mg/m2 | Day 2 |
| DXR | Intravenous, 1 h | 25 mg/m2 | Days 3, 5 |
| ASP | Intravenous, 3-4 h | 10 000 U/m2 | Days 16, 18, 20, 23, 25, 27 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15, 29 |
| VP-16 | Intravenous, 1-2 h | 100 mg/m2 | Days 29-32 |
| Ara-C | Intravenous, 4 h | 500 mg/m2 | Days 29-32 |
| Consolidation and CNS prophylaxis | |||
| MTX | Intravenous, 24 h | 3 g/m2 | Days 1, 15, 29 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15, 29 |
| CPA | Intravenous, 1-2 h | 500 mg/m2 | Days 2, 16, 30 |
| ASP | Intravenous or intramuscular | 10 000 U/m2 | Days 2, 16, 30 |
| PSL | By mouth or intravenous | 60 mg/m2 | Days 1-3 |
| Intensification | |||
| VCR | Intravenous | 0.05 mg/kg | Days 1, 8, 15 |
| DNR | Intravenous | 25 mg/m2 | Days 1, 8, 15 |
| Ara-C | Intravenous, 1 h | 60 mg/m2 | Days 2-7, 9-14 |
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| TIT | Intrathecal | Age-adjusted† | Days 1, 15 |
| Maintenance* | |||
| Regimen A | |||
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| MTX | By mouth | 30 mg/m2 | Days 1, 8 |
| VP-16 | Intravenous, 1-2 h | 150 mg/m2 | Day 14 |
| Ara-C | Intravenous, 4 h | 200 mg/m2 | Day 14 |
| Regimen B | |||
| 6-MP | By mouth | 75 mg/m2 | Days 1-14 |
| MTX | By mouth | 30 mg/m2 | Days 1, 8 |
| PSL | By mouth | 60 mg/m2 | Days 15-28 |
| VCR | Intravenous | 0.05 mg/kg | Days 15, 22, 29 |
| MTX | Intravenous, 5 h | 300 mg/m2 | Day 15 |
| TIT | Intrathecal | Age-adjusted† | Every 6 weeks |
Reinduction regimen is the same as that for induction.
DEX indicates dexamethasone; PSL, prednisolone; VCR, vincristine; CPA, cyclophosphamide; DXR, doxorubicin; ASP, L-asparaginase; TIT, triple intrathecal therapy; VP-16, etoposide; Ara-C, cytarabine; MTX, methotrexate; DNR, daunorubicin; 6-MP, 6-mercaptopurine. The dose of each drug except VCR was reduced by one third in patients younger than 2 months and by one fourth in those 2 to 4 months of age.
Each cycle consisted of two courses of regimen A, followed by regimen B. Each regimen was given over 2 weeks. The 12-week course was repeated 4 times. The total period of maintenance therapy becomes almost 56 weeks.
Doses were adjusted according to the patient's age at administration as follows: 90 days old or younger, MTX 3 mg, hydrocortisone (HDC) 10 mg, Ara-C 6 mg; younger than 1 year old, MTX 6 mg, HDC 10 mg, Ara-C 12 mg; 1 year and older, MTX 8 mg, HDC 15 mg, Ara-C 20 mg.
The present analysis was performed on October 31, 2005. Overall survival (OS) was defined as the time from diagnosis to death due to any cause or to the date of last contact. Event-free survival (EFS) was defined as the time from diagnosis until the date of an adverse event or, if no such event occurred, until the date of last contact. Induction failure (including early death or resistant leukemia), relapse, death during complete remission, and the development of a second malignancy were considered adverse events. OS and EFS rates were estimated by the Kaplan-Meier method. The 95% confidence intervals (CIs) for Kaplan-Meier estimates of survival were calculated by the use of standard errors.
Results and discussion
A total of 101 infants with ALL were registered in the MLL96 or MLL98 study; 79 with rearranged MLL were assigned to the hematopoietic stem cell transplantation (HSCT) subgroup and 22 with germline MLL to the chemotherapy subgroup. In the latter, all but one patient, who had been treated on an acute myeloid leukemia (AML)–oriented protocol, received chemotherapy alone (Table 1). The male-female ratio was 20:2, and the median age at diagnosis was 9.8 months (range, 3.8-12.0 months). Only 3 patients were younger than 6 months old at diagnosis. The median white blood cell count was 21.8 × 109/L (range, 2.8-574.1 × 109/L). Neither CNS involvement nor severe hepatosplenomegaly was observed. By immunophenotyping, 21 patients had precursor B-cell pheno-type with positive CD10 antigen expression; one infant with T-lineage ALL (T-ALL) had hyperleukocytosis at diagnosis (574.1 × 109/L). By cytogenetic analysis, 15 of the patients including the infants with T-ALL had normal karyotypes, whereas one had hyperdiploidy, one had inv(11)(p13q23), one had t(1; 19)(q32;p13) and 4 had other chromosomal abnormalities without an 11q23 translocation.
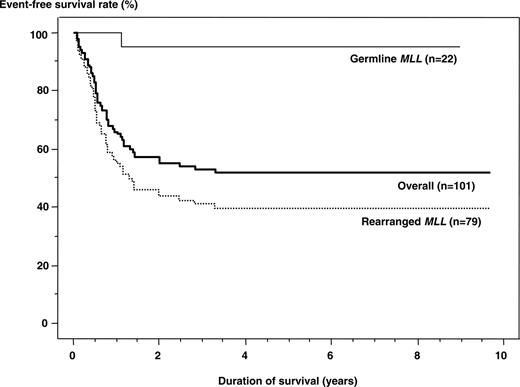
All 22 patients achieved complete remission (CR) after induction therapy. Subsequently, the 20 patients with precursor B-cell ALL remained in first CR for 3.5 to 8.8 years (median, 7 years). The 5-year EFS and OS rates for the 21 patients who were treated on the same protocol were identical, 95.2% (95% CI, 86.7%-100%). By the intent-to-treat convention, adding the patient who received AML-oriented chemotherapy and remains in CR, the EFS and OS estimates are 95.5% (95% CI, 86.9%-100%). The infant with T-ALL suffered a relapse and died after HSCT. Comparison of EFS rates by MLL gene status demonstrated a significantly better result for the patients with germline MLL (P < .001; Figure 1).
The principal grade 3 nonhematologic toxicities (National Cancer Institute–CTCAE [Common Terminology Criteria for Adverse Events] system) were as follows: induction phase—liver dysfunction (n = 11), bacterial infection (n = 8), convulsion (n = 3), diarrhea (n = 3), and allergic reaction to L-asparaginase (n = 1); consolidation phase—liver dysfunction (n = 2), bacterial infection (n = 5), and diarrhea (n = 2); intensification phase— liver dysfunction (n = 2) and bacterial infection (n = 3); reinduction phase—liver dysfunction (n = 6) and bacterial infection (n = 7); and maintenance phase—liver dysfunction (n = 7) and bacterial infection (n = 1). Grade 4 liver dysfunction and hematologic toxicity were observed in 1 and 4 patients during maintenance phase, respectively. Long-term sequelae were also evaluated. Body heights and weights reached the normal ranges in all patients; median standard deviation (SD) scores for height and weight were 0.1 SD (–1.0 to 0.9 SD) and –0.1 SD (–1.1 to 1.0 SD), respectively. A second malignancy was not detected in any patient.
Event-free survival rates for infants with ALL treated in the MLL96 or MLL98 study.Outcome was significantly better in patients with germline MLL (95.5%) than in those with rearranged MLL (39.7%; P < .001). The overall result was 52.0%.
Event-free survival rates for infants with ALL treated in the MLL96 or MLL98 study.Outcome was significantly better in patients with germline MLL (95.5%) than in those with rearranged MLL (39.7%; P < .001). The overall result was 52.0%.
Our results demonstrate the efficacy of chemotherapy alone in infants with ALL and a germline MLL gene. In previous studies with a less favorable outcome, an 11q23 translocation or negative CD10 expression was substituted for a demonstrated MLL gene rearrangement.10-13 More precise determination of MLL gene status in the present study may have enabled us to select a “true” germline MLL subgroup, contributing to the excellent results. Hilden et al,16 using reverse transcription-polymerase chain reaction to detect gene rearrangement, also reported a superior outcome in infants with germline MLL treated with chemotherapy alone, as did Pui et al17 in infants without the t(4;11). These results support our conclusion that infant ALL with germline MLL may be highly curable with chemotherapy alone. Two large multicenter trials of chemotherapy for infant ALL (INTERFANT99 and POG/COG9407) are nearing completion, and it will be important in the future to compare their experience with ours to identify the protocol elements that are most critical in securing a high EFS rate with acceptable toxicity.
Despite the relatively small number of patients in this analysis, the plan of chemotherapy we described appears to be well tolerated and to yield a very high survival rate. Although rare, infant ALL carries one of the highest risks for treatment failure among all lymphoid leukemias. Thus, international cooperation is needed to compare the advantages and disadvantages of emerging therapies in controlled clinical trials for this disease.
Appendix
The members of the Japan Infant Leukemia Study Group are as follows: Hokkaido Children's Hospital and Medical Center (Takanori Oda); Hirosaki University (Yoshihiro Takahashi); Chiba University (Takeyuki Sato); Gunma Children's Hospital (Yasuhide Hayashi); Yamanashi University (Kanji Sugita); Kanagawa Children's Medical Center (Tsuyuko Hayashi); Tokyo Medical and Dental University (Daisuke Tomizawa, Shuki Mizutani); University of Tokyo (Katsuyoshi Koh); Showa University (Keiichi Isoyama); Keio University (Tetsuya Mori); Niigata Cancer Center Niigata Hospital (Atsushi Ogawa); Kanazawa University (Takahiro Uehara); National Nagoya Hospital (Keizo Horibe); Japanese Red Cross Nagoya First Hospital (Kohji Kato); Mie University (Masahiro Hirayama); Shiga Medical School (Shigeru Ohta); Kyoto Katsura Hospital (Yoshihiro Wakazono); Kyoto University (Tatsutoshi Nakahata); Osaka Medical College (Tomoko Kuno); Hyogo Children's Hospital (Yoshiyuki Kosaka); Okayama University (Megumi Oda); Hiroshima University (Takashi Sato); National Kyushu Cancer Center (Jun Nagayama); University of Miyazaki (Hiroshi Moritake); and Saga University (Eiichi Ishii, Chairman).
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-11-4728.
A complete list of the participating members of the Japan Infant Leukemia Study Group appears in the “Appendix.”
Supported by the Japan Children's Cancer Association and a Grant-in-Aid for Cancer Research from the Ministry of Health and Labor of Japan.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank John Gilbert for critical comments and editorial assistance and all members of the Committee of the Japan Infant Leukemia Study Group for their contributions to exact follow-up and data collection in each case.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal