Abstract
From 1989 to 1996, 533 eligible patients with stage IIIB/IV Hodgkin lymphoma (HL) were randomly assigned to receive 6 cycles of hybrid MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone/Adriamycin [doxorubicin], bleomycin, vinblastine; n = 266) or ABVPP (doxorubicin, bleomycin, vinblastine, procarbazine, prednisone; n = 267). Patients in complete remission (CR) or partial response of at least 75% after 6 cycles received 2 cycles of consolidation chemotherapy (CT) (n = 208) or subtotal nodal irradiation (RT) (n = 210). A better survival probability was observed after ABVPP alone: the 10-year overall survival (OS) estimates were 90% for ABVPP×8, 78% for MOPP/ABV×8, 82% for MOPP/ABV with RT, and 77% for ABVPP×6 with RT (P = .03); and the 10-year disease-free survival (DFS) estimates were 70%, 76%, 79%, and 76%, respectively (P = .09). The 10-year DFS estimates for patients treated with consolidation CT or RT were 73% and 78% (P = .07), and OS estimates were 84% and 79%, respectively (P = .29). These results showed that RT was not superior to consolidation CT after a doxorubicin-induced CR in patients with advanced HL. An analysis of competing risks identified age more than 45 years as a significant risk factor for death, relapse, and second cancers. Prospective evaluation of late adverse events may improve the management of patients with HL.
Introduction
Chemotherapy alone with a doxorubicin-containing regimen is considered the treatment of choice for advanced-stage Hodgkin lymphoma (HL). The regimen comprising doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) continues to be the standard regimen. ABVD alternating with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone), or a hybrid regimen, MOPP/ABV(MOPP/Adriamycin [doxorubicin], bleomycin, vinblastine), are used less frequently because of the potential late effects of alkylating agents.1 The introduction of BEACOPP, a front-line intensified regimen containing bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone, has led to better tumor control and overall survival (OS).2 Ongoing trials should indicate whether this strategy is capable of replacing the ABVD regimen. Randomized trials of chemotherapy versus combined modality therapy have questioned the benefit of adjuvant radiotherapy (RT).3-7 A recent large trial showed that RT can be avoided in patients with stage III/IV HL who achieve a complete remission (CR) after 6 or 8 cycles of conventional chemotherapy (CT) but may be beneficial to patients achieving a partial response (PR) after initial chemotherapy.8
Since the late 1980s, late complications, especially second cancers and cardiovascular disease, responsible for excess mortality in patients cured of their disease, have been investigated extensively.9-13 However, compared to modern strategies for early-stage disease, there has been a delay in applying the risk-benefit approach for advanced stages. Since 1998, a 7-factor prognostic score for advanced HL has been developed and has been found to be inversely correlated with freedom from progression (FFP) and OS.14 Based on this prognostic score, treatment has been tailored to an individual's risk in ongoing trials. The risks of late toxicities must be weighed against the risk of treatment failure, but also against the results of salvage therapy and its potential toxicity. An analysis of late adverse events in patients treated in prospective trials with CT alone or CT combined with RT may help define the risks caused by each treatment modality.
In 1989, the Groupe d'Etudes des Lymphomes de l'Adulte (GELA) initiated the H89 trial to compare 2 additional cycles of chemotherapy to subtotal nodal RT as consolidation therapy for patients with stage IIIB/IV HL in complete remission (CR) or having achieved a good PR after 6 cycles of CT.15 The feasibility and results of salvage therapy, including intensified chemotherapy and high-dose chemotherapy (HDCT) followed by autologous stem-cell transplantation (ASCT) in patients who failed to respond completely or relapsed after initial treatment, have been reported previously.16 With a median follow-up of 10 years, the long-term results of the H89 trial, with special emphasis on late toxicities and the causes of death, are reported here.
Patients, materials, and methods
The H89 trial
Details regarding the design of the trial have been reported previously.15 Patients aged 15 to 65 years with untreated stage IIIB/IV Hodgkin lymphoma were eligible. The study protocol was approved by the Saint-Louis Hospital (Paris) Ethics Committee, and all patients gave their informed consent. The standardized staging evaluation has been described previously.15 The histologic slides of 93% of the patients were reevaluated by a panel of hematopathologists; the diagnosis of the treating institution was used for the remaining patients.
Treatment and follow-up
The patients were randomly assigned to one of 2 regimens: hybrid MOPP/ABV17 or ABVPP, which consisted of doxorubicin 30 mg/m2 intravenously on day 1, bleomycin 5 mg/m2 intravenously on days 1 and 8, vinblastine 5 mg/m2 intravenously on days 1 and 8, procarbazine 100 mg/m2 on days 1 through 14, and prednisone 40 mg/m2 on days 1 through 14. CT cycles were repeated every 28 days. Patients in CR or PR of at least 75% (≥ 75% of disease regression) after 6 cycles were randomized to receive either 2 more cycles of the same CT or RT for consolidation therapy. RT was delivered to the mantle field plus the para-aortic area and spleen (subtotal nodal RT) or to the inverted Y field and the spleen (total nodal irradiation), exclusively in case of iliac or inguinal involvement. Thirty grays (Gy) were delivered to all volumes, plus 5 Gy to the initially involved areas and an additional 5 Gy to the site of residual masses after CT, given in 2-Gy fractions, 5 fractions per week. Among the 210 patients randomized to RT, 152 (72%) received radiation according to the assigned volumes and doses; the planned volumes of RT were partially delivered in 12 patients (6%); the radiation doses were not completed in 31 patients (15%); and 15 patients (7%) did not receive RT. The protocol violations and the reasons for deviations already have been described.15 Patients who did not achieve a CR or PR of at least 75% after 6 cycles were not randomized for consolidation therapy. Patients in PR with 50% to 75% regression of their tumor masses after 4 to 6 cycles and documented active disease (either by biopsy or gallium-67 scan) and patients who did not respond or who progressed under treatment received 2 to 3 cycles of salvage therapy with the MINE regimen: mitoguazone, ifosfamide, vinorelbine (Navelbine; Pierre-Fabre Medicament, Boulogne, France), and etoposide, followed by BEAM (BCNU [1,3-bis[2-chloroethyl]-1-nitrosourea], cytosine arabinoside, etoposide, and melphalan) and autologous stem cell transplant (ASCT).16
Response was evaluated after 4 and 6 cycles of CT and at the completion of treatment, using hematologic and blood biochemistry studies, erythrocyte sedimentation rate measurement, and a chest radiograph. Computed tomography scans (CT scans) that were abnormal at diagnosis were repeated after 4 cycles of CT, after 6 cycles in the case of a PR, and at completion of treatment. If a previous bone marrow biopsy had been abnormal, the bone marrow was reassessed after 4 cycles. Response criteria were defined according to standard guidelines,18 except at the end of treatment, where a partial response of at least 75% was considered a CR.
Follow-up procedures included a physical examination and a complete blood count every 3 months for the first 2 years, then every 6 months for 3 years, and then once yearly thereafter. Computerized tomography scans of the chest and abdomen were performed every 6 months during the first 2 years, then at the discretion of the treating physician. Second cancers and late toxicity were recorded on a standardized form. If necessary, additional information was requested from the trial investigator. Cardiovascular and respiratory complications, reproductive functions, and thyroid dysfunctions were informally assessed during the follow-up.
Statistical methods
Follow-up started at the end of HL treatment and ended at the date of death or the date of the last examination, whichever came first. All analyses were performed on an intention-to-treat basis. Patient characteristics and response rates were compared using χ2 tests. Disease-free survival (DFS) was measured from the date of a documented CR to that of relapse, death from any cause, or the stopping date. OS was measured from the date of the first randomization (induction treatment) to either death from any cause or the stopping date. When the later date was not reached, the date was censored at the last follow-up evaluation. Survival functions were estimated by the Kaplan-Meier method and compared by the log-rank test. Late toxicities, defined as any adverse event arising after 3 months after the end of treatment, were classified in accordance with the oncology section of the World Health Organization International Classification of Diseases.19 Diagnoses used were those provided by the centers where the patient was followed up. This end point was calculated as a cumulative probability, and the effects of potential risk factors were examined using the Cox proportional-hazards model.20,21 Interactions between treatment groups also were included in the model. All statistical tests were 2-sided. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) and Splus 6.2 (MathSoft, Cambridge, MA) software.
Results
From July 1989 to December 1996, a total of 533 patients were eligible, 266 in the MOPP/ABV group and 267 in the ABVPP group. Patient characteristics at diagnosis and the distribution of prognostic factors according to the international prognostic score are listed in Table 1. These characteristics did not differ between the 4 treatment arms.
Patient characteristics and estimated outcomes
. | . | . | Patients randomized for consolidation . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Eligible patients . | Patients not randomized for consolidation . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV + RT . | ABVPP ×6 + RT . | |||
| Patients' characteristics | |||||||||
| No. patients | 533 | 115 | 92 | 116 | 114 | 96 | |||
| Men, % | 66 | 57 | 73 | 68 | 68 | 67 | |||
| Age, median, y | 32 | 36 | 32 | 32 | 32 | 32 | |||
| Age at least 45, % | 23 | 40 | 21 | 16 | 17 | 22 | |||
| Stage IIIB, % | 40 | 25 | 52 | 41 | 39 | 46 | |||
| Stage 4, % | 60 | 75 | 48 | 59 | 61 | 54 | |||
| Extranodal sites at least 2, % | 27 | 62 | 21 | 22 | 25 | 23 | |||
| Bone marrow involvement, % | 20 | 33 | 12 | 16 | 20 | 18 | |||
| ECOG performance status at least 2, % | 12 | 22 | 8 | 10 | 11 | 11 | |||
| Hemoglobin level less than 10.5 g/L, % | 29 | 48 | 24 | 34 | 21 | 22 | |||
| Albumin level less than 40 g/L, % | 23 | 78 | 20 | 30 | 22 | 22 | |||
| WBC at least 15 G/L, % | 25 | 31 | 21 | 25 | 19 | 27 | |||
| Lymphocyte count less than 0.6 G/L or less than 8% of WBC | 13 | 23 | 8 | 16 | 12 | 8 | |||
| IPS 0 to 2, % | 39 | 23 | 41 | 40 | 48 | 40 | |||
| IPS 3 or higher, % | 61 | 77 | 59 | 60 | 42 | 60 | |||
| Additional treatment for refractory and relapsing disease, no. patients | |||||||||
| MINE, ASCT | 64 | 33 | 6 | 13 | 6 | 6 | |||
| Other CT, ASCT | 26 | 7 | 3 | 8 | 3 | 5 | |||
| CT without ASCT | 46 | 20 | 6 | 9 | 4 | 7 | |||
| CT and tandem ASCT | 11 | 5 | 2 | 1 | — | 3 | |||
| HDCT allogeneic BMT | 3 | 1 | — | 1 | — | 1 | |||
| RT alone | 3 | 1 | — | 1 | — | 1 | |||
| Outcomes, % (95% confidence interval) | |||||||||
| CR rate | — | — | 91 | 99 | 95 | 91 | |||
| 10-year DFS*† | 74 (69-78) | 59 (39-79) | 76 (67-86) | 70 (61-78) | 79 (69-88) | 76 (67-85) | |||
| 10-year event-free survival‡ | 61 (57-66) | 24 (16-32) | 71 (62-81) | 67 (60-77) | 77 (68-86) | 69 (59-78) | |||
| 10-year OS§ | 75 (71-79) | 50 (41-60) | 78 (68-85) | 90 (85-96) | 82 (74-90) | 77 (68-85) | |||
. | . | . | Patients randomized for consolidation . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Eligible patients . | Patients not randomized for consolidation . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV + RT . | ABVPP ×6 + RT . | |||
| Patients' characteristics | |||||||||
| No. patients | 533 | 115 | 92 | 116 | 114 | 96 | |||
| Men, % | 66 | 57 | 73 | 68 | 68 | 67 | |||
| Age, median, y | 32 | 36 | 32 | 32 | 32 | 32 | |||
| Age at least 45, % | 23 | 40 | 21 | 16 | 17 | 22 | |||
| Stage IIIB, % | 40 | 25 | 52 | 41 | 39 | 46 | |||
| Stage 4, % | 60 | 75 | 48 | 59 | 61 | 54 | |||
| Extranodal sites at least 2, % | 27 | 62 | 21 | 22 | 25 | 23 | |||
| Bone marrow involvement, % | 20 | 33 | 12 | 16 | 20 | 18 | |||
| ECOG performance status at least 2, % | 12 | 22 | 8 | 10 | 11 | 11 | |||
| Hemoglobin level less than 10.5 g/L, % | 29 | 48 | 24 | 34 | 21 | 22 | |||
| Albumin level less than 40 g/L, % | 23 | 78 | 20 | 30 | 22 | 22 | |||
| WBC at least 15 G/L, % | 25 | 31 | 21 | 25 | 19 | 27 | |||
| Lymphocyte count less than 0.6 G/L or less than 8% of WBC | 13 | 23 | 8 | 16 | 12 | 8 | |||
| IPS 0 to 2, % | 39 | 23 | 41 | 40 | 48 | 40 | |||
| IPS 3 or higher, % | 61 | 77 | 59 | 60 | 42 | 60 | |||
| Additional treatment for refractory and relapsing disease, no. patients | |||||||||
| MINE, ASCT | 64 | 33 | 6 | 13 | 6 | 6 | |||
| Other CT, ASCT | 26 | 7 | 3 | 8 | 3 | 5 | |||
| CT without ASCT | 46 | 20 | 6 | 9 | 4 | 7 | |||
| CT and tandem ASCT | 11 | 5 | 2 | 1 | — | 3 | |||
| HDCT allogeneic BMT | 3 | 1 | — | 1 | — | 1 | |||
| RT alone | 3 | 1 | — | 1 | — | 1 | |||
| Outcomes, % (95% confidence interval) | |||||||||
| CR rate | — | — | 91 | 99 | 95 | 91 | |||
| 10-year DFS*† | 74 (69-78) | 59 (39-79) | 76 (67-86) | 70 (61-78) | 79 (69-88) | 76 (67-85) | |||
| 10-year event-free survival‡ | 61 (57-66) | 24 (16-32) | 71 (62-81) | 67 (60-77) | 77 (68-86) | 69 (59-78) | |||
| 10-year OS§ | 75 (71-79) | 50 (41-60) | 78 (68-85) | 90 (85-96) | 82 (74-90) | 77 (68-85) | |||
ECOG indicates Eastern Cooperative Oncology Group; IPS, International Prognostic Score; MINE, salvage therapy with mitoguazone, ifosfamide, navelbine, and etoposide; ASCT, autologous stem cell transplantation; HDCT, high-dose chemotherapy; BMT, bone marrow transplantation; CT, chemotherapy; and RT, radiotherapy.
Three hundred ninety-four patients in CR were analyzed.
P = .09 for the comparison of the 4 treatment arms.
P = .23 for the comparison of the 4 treatment arms.
P = .03 for the comparison of the 4 treatment arms.
Treatment efficacy
Induction. After 6 cycles of induction CT, response rates did not differ between the 2 induction regimens: patients treated with MOPP/ABV achieved a CR or PR 51% and 34% of the time, respectively, while patients treated with ABVPP achieved a CR or PR 49% and 39%, respectively.
A total of 115 patients were not randomized to receive consolidation therapy, for the following reasons: early death (n = 11), PR to induction CT requiring salvage therapy (n = 59), treatment-related toxicity (n = 13), underlying diseases (n = 3), patient refusal (n = 7), and a protocol violation (n = 22).
Consolidation. Four hundred and eighteen patients were randomized, 208 to receive consolidation CT and 210 to receive RT. After consolidation therapy, 394 of the 418 randomized patients (94%) achieved a CR, 2 patients achieved a PR, 19 patients had early disease progression, and 3 patients died during consolidation RT. The CR rate by treatment arm (induction CT plus consolidation therapy) is shown in Table 1. The CR/complete remission uncertain (CRu) rates in patients with an international prognostic score of 0 to 2 and 3 or higher were 90% and 75%, respectively (P < .001).
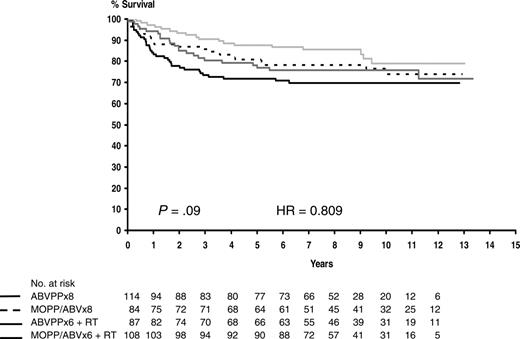
Among the 394 patients who achieved a CR, the predicted probability of survival without disease was 74% after 10 years (95% confidence interval [CI], 69%-78%). The 10-year DFS estimates for patients treated with MOPP/ABV or ABVPP were 77% (95% CI, 71%-84%) and 70% (95% CI, 64%-77%), respectively (P = .02). No difference was detected between consolidation therapies with 10-year DFS estimates of 73% (95% CI, 66%-79%) and 78% (95% CI, 71%-84%), respectively (P = .07), for patients treated with consolidation CT or RT. The 10-year DFS estimates for the 4 treatment arms are shown in Table 1, and DFS curves are shown in Figure 1. No difference was observed between the 4 treatment arms (P = .09). The 10-year DFS estimates for patients with an international prognostic score of 0 to 2 and 3 or higher were 82% (95% CI, 76%-88%) and 67% (95% CI, 61%-74%), respectively (P < .001).
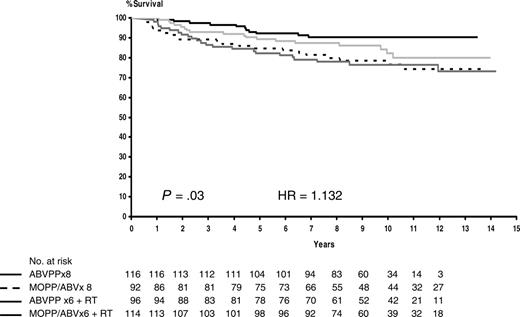
Survival. The median follow-up of surviving patients was 115 months (range, 7-172 months), with a predicted probability of survival of 75% at 10 years (95% CI, 71%-79%). The 10-year overall survival estimates for patients treated with MOPP/ABV or ABVPP were 72% (95% CI, 67%-78%) and 78% (95% CI, 73%-83%), respectively (P = .17). The 10-year overall survival estimates for patients treated with consolidation CT or RT were 84% (95% CI, 78%-89%) and 79% (95% CI, 72%-84%), respectively (P = .29). The 10-year predicted probabilities of survival were 78% for MOPP/ABV× 8, 90% for ABVPP× 8, 82% for MOPP/ABV + RT, and 77% for ABVPP×6 + RT (Table 1). The 4 treatment arms differed significantly, with a better survival probability after ABVPP alone (P = .03). Overall survival curves are shown in Figure 2. No statistical difference was observed in the 10-year predicted probability of OS and EFS after CT or RT consolidation when patients in CR or PR of at least 75% were restaged after 6 cycles and compared (data not shown). The 10-year predicted survival probability rates for patients with an international prognostic score of 0 to 2 and 3 or higher were 90% (95% CI, 85%-94%) and 67% (95% CI, 61%-72%), respectively (P < .001).
Estimated disease-free survival according to treatment arms. Broken line indicates MOPP/ABV×8 (patients at risk, n = 84; relapses or deaths, n = 20; 10-year estimate, 76%); solid black line, ABVPP × 8 (patients at risk, n = 115; relapses or deaths, n = 34; 10-year estimate, 70%); light gray line, MOPP/ABV×6 with RT (patients at risk, n = 108; relapses or deaths, n = 18; 10-year estimate, 79%); and dark gray line, ABVPP×6 with RT (patients at risk, n = 87; relapses or deaths, n = 22; 10-year estimate, 76%).
Estimated disease-free survival according to treatment arms. Broken line indicates MOPP/ABV×8 (patients at risk, n = 84; relapses or deaths, n = 20; 10-year estimate, 76%); solid black line, ABVPP × 8 (patients at risk, n = 115; relapses or deaths, n = 34; 10-year estimate, 70%); light gray line, MOPP/ABV×6 with RT (patients at risk, n = 108; relapses or deaths, n = 18; 10-year estimate, 79%); and dark gray line, ABVPP×6 with RT (patients at risk, n = 87; relapses or deaths, n = 22; 10-year estimate, 76%).
One hundred and twenty-nine patients have died. The causes of death by treatment group are given in Table 2. The major causes of death were progression of HL (46.5%), second cancers (18.5%), and treatment-related complications (17%). Other known causes of death during therapy were as follows: infection (n = 4); respiratory insufficiency after pneumopathy with hypersensitivity (n = 1); suicide (n = 1); urologic fistula (n = 1); cerebral hemorrhage (n = 1); and a solid tumor diagnosed during initial treatment (n = 2). Among 24 second cancer-related deaths, 16 occurred after MOPP/ABV and 8 after the ABVPP regimen. There were 12 intercurrent deaths after MOPP/ABV and 11 after the ABVPP regimen.
Causes of death
. | . | . | Patients randomized for consolidation . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | All patients . | Patients not randomized for consolidation . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV ×6 + RT . | ABVPP ×6 + RT . | |||
| Patients at risk, no. | 533 | 115 | 92 | 116 | 114 | 96 | |||
| Total deaths, no. | 129 | 56 | 21 | 11 | 18 | 23 | |||
| Deaths related to HL, no. | |||||||||
| Failure to achieve CR | 44 | 30 | 4 | — | 4 | 6 | |||
| After relapse | 16 | 2 | 2 | 4 | 2 | 6 | |||
| Deaths related to initial treatment, no. | 15 | 9 | 3 | — | — | 3 | |||
| Deaths related to salvage therapy, no. | 7 | 2 | 1 | 2 | 1 | 1 | |||
| Deaths related to second cancer, no. | |||||||||
| Solid tumor | 15 | 4 | 4 | — | 4 | 3 | |||
| Non-Hodgkin lymphoma | 4 | — | 1 | 1 | 2 | — | |||
| Acute leukemia - MDS | 5 | 3 | 1 | 1 | — | — | |||
| Deaths related to cardiovascular causes, no. | 1 | — | 1 | — | — | — | |||
| Deaths due to other known cause, no. | 10 | 1 | 2 | 1 | 3 | 3 | |||
| Cause of death unspecified, no. | 12 | 5 | 2 | 2 | 2 | 1 | |||
. | . | . | Patients randomized for consolidation . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | All patients . | Patients not randomized for consolidation . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV ×6 + RT . | ABVPP ×6 + RT . | |||
| Patients at risk, no. | 533 | 115 | 92 | 116 | 114 | 96 | |||
| Total deaths, no. | 129 | 56 | 21 | 11 | 18 | 23 | |||
| Deaths related to HL, no. | |||||||||
| Failure to achieve CR | 44 | 30 | 4 | — | 4 | 6 | |||
| After relapse | 16 | 2 | 2 | 4 | 2 | 6 | |||
| Deaths related to initial treatment, no. | 15 | 9 | 3 | — | — | 3 | |||
| Deaths related to salvage therapy, no. | 7 | 2 | 1 | 2 | 1 | 1 | |||
| Deaths related to second cancer, no. | |||||||||
| Solid tumor | 15 | 4 | 4 | — | 4 | 3 | |||
| Non-Hodgkin lymphoma | 4 | — | 1 | 1 | 2 | — | |||
| Acute leukemia - MDS | 5 | 3 | 1 | 1 | — | — | |||
| Deaths related to cardiovascular causes, no. | 1 | — | 1 | — | — | — | |||
| Deaths due to other known cause, no. | 10 | 1 | 2 | 1 | 3 | 3 | |||
| Cause of death unspecified, no. | 12 | 5 | 2 | 2 | 2 | 1 | |||
Estimated overall survival according to treatment arms. Broken line indicates MOPP/ABV×8 (patients at risk, n = 92; deaths, n = 21; 10-year estimate, 78%); solid black line, ABVPP×8 (patients at risk, n = 116; deaths, n = 11; 10-year estimate, 90%); light gray line, MOPP/ABV×6 with RT (patients at risk, n = 114; deaths, n = 18; 10-year estimate, 82%); and dark gray line, ABVPP×6 with RT (patients at risk, n = 96; deaths, n = 23; 10-year estimate, 77%).
Estimated overall survival according to treatment arms. Broken line indicates MOPP/ABV×8 (patients at risk, n = 92; deaths, n = 21; 10-year estimate, 78%); solid black line, ABVPP×8 (patients at risk, n = 116; deaths, n = 11; 10-year estimate, 90%); light gray line, MOPP/ABV×6 with RT (patients at risk, n = 114; deaths, n = 18; 10-year estimate, 82%); and dark gray line, ABVPP×6 with RT (patients at risk, n = 96; deaths, n = 23; 10-year estimate, 77%).
Late toxicities
Second cancers. Thirty-eight second cancers (emerging at least 3 months after the end of treatment) were observed. Ten (5 myelodysplastic syndrome [MDS]/acute myelogenous leukemia [AML], 2 non-Hodgkin lymphoma [NHL], and 3 solid tumors) occurred after additional treatment for a PR, progression, or a relapse, including HDCT with ASCT in 9 patients and CT in one patient. The other 28 patients who developed a second cancer had received the planned treatment. The rate of second cancers observed was not statistically different between patients who had received the planned treatment (9%) and those who had received salvage treatment (8%) (P = .743).
Twenty-three solid tumors occurred (lung, 9; colorectal, 2; small bowel, 1; pancreas, 2; liver, 1; esophagus, 1; head and neck, 3; breast, 1; skin, 2; and unknown origin, 1). The median time from the diagnosis of HL to solid tumors was 6 years (range, 0.5-13 years). Among the 9 lung cancers, 4 were adenocarcinoma and 2 were squamous cell carcinoma, and the histology was unavailable for 3 patients. Among the 3 cancers localized in the liver or pancreas, 2 were neuroendocrine tumors. Between the 2 skin tumors, one was a melanoma. Eight patients with solid tumors had received RT as consolidation therapy, and 4 tumors occurred in the irradiated field (3 lung, 1 pancreas). The 3-year OS rate from the diagnosis of the second cancer was 35% (95% CI, 13%-57%).
Fifteen hematological malignancies (AML, 5; myelodysplastic syndrome [MDS], 5; NHL, 5) were observed. Of the 10 patients with AML or MDS, 5 patients (2 AML, 3 MDS) were in a continuous CR of HL, and 5 patients had received salvage treatment with HDCT and ASCT after initial failure (2 patients: 1 MDS and 1 AML) or a relapse (3 patients: 1 MDS, 2 AML). The median time from HL to MDS/AML was 4.5 years (range, 2.5-6.3 years). The 3-year OS from the diagnosis MDS/AML was 33% (95%CI, 3%-64%). Of the 10 patients with AML or MDS, 5 died of the second malignancy, one died of cardiac failure after initial salvage therapy with HDCT and ASCT plus RT to the mediastinum, and 4 were alive, 3 of them without disease 43, 80, and 104 months after the second malignancy. Of the 5 patients with NHL, 4 died of NHL progression (3 aggressive and 1 follicular lymphoma) occurring 12 to 88 months after the end of HL treatment, and 1 patient is alive after marginal zone lymphoma, which occurred 126 months after treatment completion.
Nonmalignant late toxicities (occurring at least 3 months after the end of treatment) were reported in 102 patients and are listed in Table 3. Of these late toxicities, 71 were observed after the planned treatment, and 31 occurred after additional treatment, including HDCT with a hematopoietic stem cell transplant (ASCT, 21 patients; tandem ASCT, 3 patients; allogeneic SCT, 1 patient) given to patients who failed to achieve a CR after initial treatment (14 patients) or to those who relapsed (17 patients). The observed rate of nonmalignant late toxicities was similar between recipients of the planned treatment (20%) and those of salvage treatment (21%) (P = .863). Nonmalignant late toxicities resulted in 6 deaths and were the fourth cause of death, after HL, second cancers, and treatment-related death.
Second cancers and late nonmalignant toxicities
. | . | . | Patients randomized for consolidation, no. . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. events observed . | Patients not randomized for consolidation, no. . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV + RT . | ABVPP ×6 + RT . | Related deaths, no. . | |||
| Second cancers | 38 | 10 | 8 | 6 | 8 | 6 | 24 | |||
| Solid tumors | 23 | 7 | 5 | 2 | 5 | 4 | 15 | |||
| Lung | 9 | 3 | 2 | 0 | 1 | 3 | 8 | |||
| Gastrointestinal tract | 4 | 2 | 1 | 0 | 1 | 0 | 2 | |||
| Liver, pancreas | 3 | 1 | 0 | 0 | 2 | 0 | 2 | |||
| Head and neck | 3 | 1 | 1 | 1 | 0 | 0 | 2 | |||
| Breast | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Other | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| Cutaneous | 2 | 0 | 0 | 1 | 1 | 0 | 1 | |||
| AML - MDS | 10 | 3 | 2 | 2 | 1 | 2 | 5 | |||
| NHL | 5 | 0 | 1 | 2 | 2 | 0 | 4 | |||
| Nonmalignant toxicities | 102 | 17 | 16 | 20 | 29 | 20 | 6 | |||
| Infectious | 13 | 1 | 2 | 3 | 5 | 2 | 2 | |||
| Cardiovascular | 15 | 3 | 3 | 5 | 2 | 2 | 1 | |||
| Respiratory | 8 | 1 | 4 | 0 | 2 | 1 | 1 | |||
| Hypothyroidism | 21 | 3 | 1 | 2 | 9 | 6 | 0 | |||
| Hyperthyreosis | 5 | 0 | 0 | 1 | 4 | 0 | 0 | |||
| Female gonadal dysfunction | 12 | 3 | 0 | 5 | 3 | 1 | 0 | |||
| Male sterility | 13 | 2 | 1 | 3 | 4 | 3 | 0 | |||
| Prolonged cytopenia | 3 | 1 | 1 | 0 | 0 | 1 | 0 | |||
| Peripheral neuropathy | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Psychologic | 2 | 1 | 1 | 0 | 0 | 0 | 1 | |||
| Femoral osteonecrosis | 5 | 1 | 1 | 1 | 0 | 2 | 0 | |||
| Miscellaneous | 4 | 1 | 2 | 0 | 0 | 1 | 1 | |||
. | . | . | Patients randomized for consolidation, no. . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. events observed . | Patients not randomized for consolidation, no. . | MOPP/ABV ×8 . | ABVPP ×8 . | MOPP/ABV + RT . | ABVPP ×6 + RT . | Related deaths, no. . | |||
| Second cancers | 38 | 10 | 8 | 6 | 8 | 6 | 24 | |||
| Solid tumors | 23 | 7 | 5 | 2 | 5 | 4 | 15 | |||
| Lung | 9 | 3 | 2 | 0 | 1 | 3 | 8 | |||
| Gastrointestinal tract | 4 | 2 | 1 | 0 | 1 | 0 | 2 | |||
| Liver, pancreas | 3 | 1 | 0 | 0 | 2 | 0 | 2 | |||
| Head and neck | 3 | 1 | 1 | 1 | 0 | 0 | 2 | |||
| Breast | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Other | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| Cutaneous | 2 | 0 | 0 | 1 | 1 | 0 | 1 | |||
| AML - MDS | 10 | 3 | 2 | 2 | 1 | 2 | 5 | |||
| NHL | 5 | 0 | 1 | 2 | 2 | 0 | 4 | |||
| Nonmalignant toxicities | 102 | 17 | 16 | 20 | 29 | 20 | 6 | |||
| Infectious | 13 | 1 | 2 | 3 | 5 | 2 | 2 | |||
| Cardiovascular | 15 | 3 | 3 | 5 | 2 | 2 | 1 | |||
| Respiratory | 8 | 1 | 4 | 0 | 2 | 1 | 1 | |||
| Hypothyroidism | 21 | 3 | 1 | 2 | 9 | 6 | 0 | |||
| Hyperthyreosis | 5 | 0 | 0 | 1 | 4 | 0 | 0 | |||
| Female gonadal dysfunction | 12 | 3 | 0 | 5 | 3 | 1 | 0 | |||
| Male sterility | 13 | 2 | 1 | 3 | 4 | 3 | 0 | |||
| Prolonged cytopenia | 3 | 1 | 1 | 0 | 0 | 1 | 0 | |||
| Peripheral neuropathy | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Psychologic | 2 | 1 | 1 | 0 | 0 | 0 | 1 | |||
| Femoral osteonecrosis | 5 | 1 | 1 | 1 | 0 | 2 | 0 | |||
| Miscellaneous | 4 | 1 | 2 | 0 | 0 | 1 | 1 | |||
Among the cardiovascular complications, coronary insufficiency occurred in 3 patients, with 2 myocardial infarctions resulting in 1 death. Abnormal left ventricular function was observed in 3 patients, and another patient had cardiac failure. Venous thrombosis was observed in 5 patients, ischemic arteriopathy in 1 patient, and 2 patients had lymphoedema of the legs. Among infectious complications were 7 cases of herpes zoster, 2 of viral meningitis, 1 of herpetic encephalopathy, 2 of pneumopathies, and 1 of acute respiratory distress syndrome, resulting in 2 deaths. Among the pulmonary complications, 3 patients developed respiratory insufficiency, 2 had functional impairment, 2 had radiation pneumonitis, and 1 patient died of pneumopathy secondary to drug hypersensitivity. The other most frequently reported late toxicities were thyroid dysfunctions (19 of 26 patients receiving RT), male sterility (13 patients), and early menopause (12 patients), with a possible role of iliac RT in 2 cases and salvage therapy with ASCT in 4 cases. Femoral osteonecrosis occurred in 5 patients (2 of 5 received RT).
Competing risk analysis
After initial treatment, patients may experience several competing events such as a relapse or progression of HL, death, a second cancer, or nonmalignant late toxicities summarized by treatment group in Table 4. Figure 3 shows the cumulative incidence of competing risks. The 102 reported nonmalignant late toxicities resulted in a 24% ± 5% cumulative incidence probability at 10 years (including respiratory, 1.5% of events; cardiac, 3%; infectious, 2.75%), and the 38 second cancers resulted in a 9% ± 3% cumulative incidence probability at 10 years.
Results of initial treatment, outcome, and late toxicities by treatment group
. | Not randomized for consolidation . | . | . | MOPP/ABV×8 . | . | . | ABVPP×8 . | . | . | MOPP/ABV×6 + RTx . | . | . | ABVPP×6 + RT . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | ||||||||||
| Initial treatment | 39 | 29 | 47 | 84 | 3 | 5 | 114 | 0 | 2 | 108 | 1 | 5 | 86 | 2 | 8 | ||||||||||
| Follow-up | |||||||||||||||||||||||||
| Alive first CR | 27 | 19 | 1 | 64 | 0 | 0 | 81 | 0 | 1 | 91 | 1 | 0 | 66 | 1 | 0 | ||||||||||
| Alive second CR | 4 | 4 | 2 | 6 | 1 | 0 | 21 | 0 | 1 | 4 | 0 | 0 | 6 | 0 | 0 | ||||||||||
| Alive with disease | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| Died | 8 | 5 | 43 | 14 | 2 | 5 | 11 | 0 | 0 | 13 | 0 | 5 | 14 | 1 | 8 | ||||||||||
| Late toxicities | |||||||||||||||||||||||||
| Cancer | 8 | 2 | 0 | 7 | 0 | 0 | 5 | 0 | 0 | 8 | 0 | 0 | 6 | 0 | 0 | ||||||||||
| Nonmalignant | 7 | 8 | 8 | 14 | 3 | 0 | 18 | 0 | 1 | 24 | 0 | 0 | 15 | 0 | 2 | ||||||||||
. | Not randomized for consolidation . | . | . | MOPP/ABV×8 . | . | . | ABVPP×8 . | . | . | MOPP/ABV×6 + RTx . | . | . | ABVPP×6 + RT . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | Primary CR . | CR after salvage . | Failure . | ||||||||||
| Initial treatment | 39 | 29 | 47 | 84 | 3 | 5 | 114 | 0 | 2 | 108 | 1 | 5 | 86 | 2 | 8 | ||||||||||
| Follow-up | |||||||||||||||||||||||||
| Alive first CR | 27 | 19 | 1 | 64 | 0 | 0 | 81 | 0 | 1 | 91 | 1 | 0 | 66 | 1 | 0 | ||||||||||
| Alive second CR | 4 | 4 | 2 | 6 | 1 | 0 | 21 | 0 | 1 | 4 | 0 | 0 | 6 | 0 | 0 | ||||||||||
| Alive with disease | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| Died | 8 | 5 | 43 | 14 | 2 | 5 | 11 | 0 | 0 | 13 | 0 | 5 | 14 | 1 | 8 | ||||||||||
| Late toxicities | |||||||||||||||||||||||||
| Cancer | 8 | 2 | 0 | 7 | 0 | 0 | 5 | 0 | 0 | 8 | 0 | 0 | 6 | 0 | 0 | ||||||||||
| Nonmalignant | 7 | 8 | 8 | 14 | 3 | 0 | 18 | 0 | 1 | 24 | 0 | 0 | 15 | 0 | 2 | ||||||||||
There were 115 patients not randomized for consolidation; 92 who received MOPP/ABV×8; 116 who received ABVPP×8; 114 who received MOPP/ABV×6 plus RTx; and 96 who received ABVPP×6 plus RT.
To account for competing risks between death, relapse, and late toxicities, the regression analysis included age at HL diagnosis, sex, induction CT, consolidation therapy, and ASCT at relapse as explanatory covariates. The only prognostic factors for death were the ABVPP plus RT (RR = 8.9, P = .006) and age over 45 years (RR = 5.1, P < .001). Age also was significantly associated with relapse (RR = 2.8, P < .001). Male sex (RR = 3.6, P = .04), age over 45 years (RR = 4.8, P < .001), and salvage treatment including HDCT and ASCT (RR = 3.1, P = .01) were risk factors for a second cancer, whereas only female sex was associated with nonmalignant late toxicities (RR = 1.5, P = .01).
Discussion
Our results, with a median follow-up of 10 years, show that survival probability rates are similar to those reported in the previous analysis and confirm that RT does not have an advantage over CT as consolidation therapy for patients with stage IIIB/IV HL after 6 cycles of an induction doxorubicin-containing regimen. The comparison between 2 induction regimens showed that ABVPP was inferior to MOPP/ABV in terms of DFS. In addition, the 4 treatment arms differed significantly in terms of OS, as previously reported. The analysis of the causes of deaths shows that after treatment with MOPP/ABV alone or in combination with RT, the number of second cancer-related deaths observed was greater than that observed after the ABVPP regimen. Moreover, the survival advantage conferred by the ABVPP regimen was not confirmed after consolidation with RT. For patients treated with 6 cycles of ABVPP and RT, the number of deaths observed after failure or a relapse was higher than that observed in the other treatment arms, and the multivariate analysis showed that ABVPP plus RT consolidation was an independent prognostic factor for death (RR, 8.9; P = .006). However, the H89 trial was initiated more than 15 years ago, and we admit that neither the ABVPP regimen nor hybrid MOPP/ABV are currently in widespread use. Compared with ABVD, the relative dose intensity of doxorubicin in the ABVPP regimen is 40% lower, and it contains procarbazine. A randomized comparison of ABVD and hybrid MOPP/ABV showed a greater incidence of acute toxicity and an increased risk of second malignancies after the hybrid regimen.22
Compared with the commonly used ABVD regimen, our results at 10 years are similar to those reported with ABVD alone or combined with consolidative RT.23 However, a longer follow-up is needed to confirm the benefit compared with the results obtained after ABVD at 15 years of follow-up.24 Taken together, our results are consistent with the conclusions of recent studies that have addressed the use of adjuvant RT.25 The study by the German Hodgkin Lymphoma Study Group6 showed that there is no difference between adding further chemotherapy or radiotherapy as consolidation treatment after CR with standard chemotherapy. In the Southwest Oncology Group's randomized trial of involved-field RT versus no further treatment, no overall benefit from RT was observed in patients with bulky, nodular sclerosis histology. However, the relapse-free survival rate was significantly higher after radiotherapy.5 The study from the Tata Mountain Hospital26 showed a survival advantage and better event-free survival following consolidation RT in patients achieving a CR after induction with 6 cycles of ABVD chemotherapy over surveillance. Other studies did not demonstrate a benefit of consolidative RT after CR.3,27,28 More recently, a meta-analysis focused on second cancer risk questioned the role of consolidation therapy for patients who achieved a CR after initial CT.29 A large trial highlighted the fact that RT may be beneficial to patients achieving a PR after chemotherapy.8 However, our data do not demonstrate a benefit of consolidation RT over consolidation CT for patients who achieved a PR of at least 75% after 6 cycles of CT.
Cumulative incidence of competing risks after treatment in 533 patients with advanced-stage Hodgkin lymphoma.
Cumulative incidence of competing risks after treatment in 533 patients with advanced-stage Hodgkin lymphoma.
Like the strategy developed for early-stage HL, treatments of patients with advanced-stage HL are currently being based on risk factors and on better knowledge of treatment-related morbidity and mortality. The aim of the H89 randomized study that included patients with stage IIIB/IV HL was to contribute to a better understanding of late adverse events. This was an ideal setting where long-term treatment effects could be isolated, as the randomization of induction and consolidation between CT alone or CT combined with radiotherapy allowed an equal distribution of all the known (eg, age, sex) and unknown confounding variables (eg, genetics, social factors). The homogeneity of the long-term follow-up required by the H89 protocol provided a largely representative population. Furthermore, the H89 prospectively integrated intensive salvage therapy with HDCT for patients who failed to respond completely to initial treatment and for those who relapsed with adverse prognostic factors, which give to the present analysis good generalization properties with a wide range of disease courses and no selection biases.16 To estimate the risk of late toxicity, we used the cumulative incidence probability, which takes into account the presence of competing risks between death and late toxicities and differs from the Kaplan-Meier estimate in that patients must both survive and be diagnosed with a late toxicity. It is of interest to emphasize that a proportion of our patients who achieved a CR after 6 cycles may have been overtreated, if we consider the results of the recent European trial.8 In our study population, the cumulative incidence of late toxicities would not necessarily have been the same had treatment been stopped after 6 cycles in patients who achieved a CR. However, in contrast to registry data, our results must be interpreted in the light of weaknesses inherent in the small sample size and a shorter follow-up than that usually given for registry data.
We have reported a total of 38 second cancers, resulting in a 9 ± 3 cumulative incidence probability at 10 years. These results are in line with those of registries that reported that the main cause of death among HL patients was lymphoma, but the risk of death from second primary cancers (colon, lung, breast, and thyroid) is higher, in particular in older patients, when the long-term follow-up exceeds 10 years.10 We failed to find a role attributable to RT, but we confirm the report of the Societe Francaise de Greffe de Moelle by Andre et al30 that highlighted an increased risk of second cancers due to ASCT.
The 102 nonmalignant late toxicities reported here resulted in a 24 ± 5 cumulative incidence probability at 10 years. As in the paper by Knobel et al,31 most of them were endocrine or general symptoms, confirming that causes of chronic fatigue are highly prevalent among HL survivors. We were also able to individualize a 5% cumulative incidence of respiratory and cardiac late toxicities, which is rather high in this 32-year-old median age population, although left ventricular function and respiratory functions were not systematically assessed. We failed to isolate risk factors and question the absence of an association between respiratory and cardiac complications and radiochemotherapy. One explanation could be the lack of follow-up, as the risk of cardiovascular disease continued to increase up to 30 years after the diagnosis.
After treatment, patients with HL may experience cure without any late adverse events, disease progression, late toxicities, or death. Our study allowed us to identify possible risk factors for these competing events, such as age over 45 years, which was significant for death, relapse, and second cancers. In our study, the cumulative probability of second cancers and the 18.5% of deaths related to second malignancies emphasize the risk of these fatal complications. As follow-up has attained almost 10 years, the risk of developing a solid tumor continues to loom, and new events are expected during the second decade of follow-up. In our experience, long-term nonmalignant complications occurred relatively frequently, but they may have been underestimated. They should be evaluated prospectively using the benefit-risk approach. Better knowledge of late adverse events may help improve the management of patients, allowing therapy to be tailored to specific risks in individual patients, minimizing treatment-related complications and improving information given to each patient, as well as follow-up recommendations for lifelong medical surveillance.
Appendix
The clinicians and pathologists who actively participated in this study were listed in the first publication of the H89 trial.15
Prepublished online as Blood First Edition Paper, February 14, 2006; DOI 10.1182/blood-2005-11-4429.
A complete list of the clinicians and pathologists who participated in this study was included in the first publication of the H89 trial.15
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the GELA investigators who contributed to the completion of this study. We thank Nicolas Nio for assistance with data management, and Lorna Saint Ange for editing.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal