Abstract
A single center, prospective clinical trial was conducted evaluating 2 cycles of induction high-dose chemotherapy for adults younger than 65 years of age with aggressive non-Hodgkin lymphoma (NHL) and 2 to 3 Age-Adjusted International Prognostic Index risk factors. Patients received one cycle of standard dose cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) followed by one cycle of dose-intensive cyclophosphamide 5.25 g/m2, etoposide 1.05 g/m2, cisplatin 105 mg/m2 (DICEP), then underwent autologous blood stem cell collection, followed by one cycle of high-dose carmustine (BCNU) 300 mg/m2, etoposide 800 mg/m2, Ara-C 1600 mg/m2, melphalan 140 mg/m2 (BEAM), and autologous stem cell transplantation (ASCT) and radiotherapy to prior bulk. From June 1998 to August 2004, 55 patients aged 20 to 63 years (median 44 years) were accrued, 51 (92%) of whom had diffuse large B-cell NHL. Poor prognostic factors included stage 4 (n = 46), elevated lactate dehydrogenase (LDH; n = 47), Eastern Cooperative Oncology Group (ECOG) performance status 2 to 4 (n = 43), bulky mass more than 10 cm (n = 34), and marrow involvement (n = 16). Only one patient experienced nonrelapse mortality. With a median follow-up of 49 months, 4-year event-free survival (EFS) and overall survival (OS) rates for all 55 patients are 72% (95% confidence interval [CI] = 60%-84%) and 79% (95% CI = 69%-90%), respectively. In conclusion, CHOP-DICEP-BEAM is feasible and gave encouraging EFS and OS for patients with poor-prognosis aggressive NHL.
Introduction
Less than one-third of patients with diffuse large B-cell lymphoma (DLBCL) and 2 to 3 adverse risk factors (stage 3-4, ECOG performance status 2-4, elevated serum lactate dehydrogenase [LDH] level) defined by the Age-Adjusted International Prognostic Index (AAIPI) achieve long-term event-free survival (EFS) following standard cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy.1 The addition of rituximab anti-CD20 monoclonal antibody therapy to CHOP (R-CHOP) results in a 15% to 20% increase in absolute EFS rate over CHOP alone for DLBCL patients over age 60 years, and those under age 60 years with 0 to 1 AAIPI risk factors.2,3 A similar degree of benefit of R-CHOP over CHOP is expected but not yet reported for DLBCL patients under 60 years of age who have 2 to 3 AAIPI factors. Despite this expected improvement in outcome, it is likely that 40% to 50% of patients with poor-prognosis DLBCL will not be cured by R-CHOP. Outcomes are even worse for patients with aggressive T-cell lymphoma histologies.4 Further improvements in initial therapy are required for poor-prognosis aggressive histology lymphoma.
High-dose chemotherapy (HDCT) and autologous stem cell transplantation (ASCT) have been shown to salvage a substantial proportion of patients with aggressive lymphoma who relapse after CHOP chemotherapy.5 Despite this fact, randomized controlled trials comparing standard-dose chemotherapy (SDCT) alone to SDCT followed by HDCT/ASCT as initial treatment for poor-prognosis DLBCL have given inconsistent results.6-14 Although the use of abbreviated induction therapy followed by a single HDCT/ASCT is not considered a viable strategy for DLBCL therapy, late HDCT consolidation may not be able to overcome drug resistance that cancer cells might develop after SDCT.15
Another HDCT approach that is worthy of study involves multiple cycles of high-dose sequential induction chemotherapy. Herein, we report the results of a single-center prospective study designed to assess feasibility, toxicity, and efficacy of 2 cycles of HDCT following just one cycle of CHOP induction therapy for patients under 65 years of age who were diagnosed with aggressive histology lymphoma and 2 to 3 AAIPI risk factors. The study was conducted prior to the widespread use of rituximab plus CHOP (R-CHOP). The rationale for various aspects of this trial include: (1) the AAIPI was used to identify poor-prognosis patients for whom standard chemotherapy regimens such as CHOP give relatively low chance for cure; (2) one cycle of CHOP was given to allow time to organize the double HDCT as well as to initiate tumor response, improving patient performance status and organ function prior to HDCT; (3) HDCT began as early as possible in the treatment course to minimize the time available for the cancer cells to develop drug resistance; (4) dose-intensive cyclophosphamide, etoposide, and cisplatin (DICEP) provided nonmyeloablative HDCT with a well-tolerated, extensively studied regimen that also mobilizes autologous blood stem cells extremely well, and may clear the marrow and bloodstream of lymphoma cells prior to stem cell collection16,17 ; and (5) BCNU, etoposide, Ara-C, and melphalan (BEAM) were used for myeloablative HDCT because there was extensive experience with this commonly used HDCT regimen, worldwide.18
Patients, materials, and methods
Patients
Eligibility criteria for the study included age between 18 and 65 years; aggressive histology lymphoma (large B-cell, peripheral T-cell, Burkitt lymphoma); AAIPI score of 2 to 3, and adequate organ function as defined by a left ventricular ejection fraction more than or equal to 50%; serum creatinine less than 150 μM; serum bilirubin less than 30 μM; control of other medical conditions such as infection, diabetes, and hypertension; white blood cell (WBC) count more than or equal to 3.5 × 109/L; and platelet count more than or equal to 100 × 109/L unless caused by lymphoma infiltration of marrow or spleen. Exclusion criteria included pregnancy or lactation; a recent history of other malignant disease except for nonmelanoma skin cancer or carcinoma-in-situ of the cervix; prior radiotherapy or chemotherapy; HIV positive; CD30+ anaplastic large cell lymphoma; and primary central nervous system lymphoma. Due to the fact that poor-prognosis lymphoma patients often require urgent treatment that cannot be delayed for study enrollment, patients were eligible for enrollment until one week after the first cycle of CHOP.
Approximately 35 patients younger than 65 years of age with newly diagnosed aggressive histology lymphoma are seen at the Tom Baker Cancer Centre each year. Of these, approximately 15 patients per year have 2 to 3 AAIPI risk factors and would meet eligibility criteria for this study. The study was approved by the Conjoint Scientific Review Board for Clinical Trials and the Health Research Ethics Board in Calgary. All patients gave written informed consent prior to participation, in accordance with the Declaration of Helsinki.
Treatment protocol
One cycle of standard CHOP chemotherapy involved 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine (maximum 2 mg) all administered intravenously on day 1, with 100 mg prednisone given orally on days 1 to 5. At 3 to 4 weeks after CHOP, patients received DICEP (dose-intensive cyclophosphamide [1.75 g/m2] daily over 2 hours on days 1 to 3, 350 mg/m2 etoposide daily over 2 hours on days 1 to 3, and 35 mg/m2 cisplatin with 25 g mannitol daily over 2 hours on days 1 to 3). Other medications administered with DICEP included 1.75g/m2 Mesna daily by continuous infusion over 24 hours on days 1 to 3, granulocyte colony-stimulating factor (G-CSF) subcutaneously daily at a dose of 300 μg/d for body weight less than 70 kg, 480 μg/d for weight 70 kg to 100 kg, or 600 μg/d for weight more than 100 kg from day 14 until blood stem cell apheresis was completed. Apheresis occurred on approximately day 19 to 21 of DICEP, after the postnadir absolute neutrophil count (ANC) was more than 5 × 109/L, the nontransfused platelet count was more than 50 × 109/L, and the peripheral blood CD34+ cell count was over 20 × 106/L. Large volume apheresis was performed via a central venous catheter until at least 3 × 106 CD34+ cells/kg were collected.
High-dose BEAM was administered 4 to 8 weeks after DICEP and consisted of 300 mg/m2 BCNU on day –6, 100 mg/m2 etoposide given intravenously every 12 hours × 8 doses on days –5 to –2, 200 mg/m2 Ara-C given intravenously every 12 hours × 8 doses on days –5 to –2, and 140 mg/m2 melphalan given intravenously on day –1. Autologous blood stem cells were infused on day 0. If actual body weight exceeded ideal body weight by more than 20%, a corrected body weight defined as ideal weight plus 40% (actual-ideal weight) was used for both DICEP and BEAM.
All blood products were irradiated to prevent transfusion-associated graft-versus-host disease. Patients received 500 mg prophylactic ciprofloxacin by mouth twice a day, 100 mg/d fluconazole, or 500 000 U mycostatin suspension as swish and swallow four times a day, 400 mg acyclovir by mouth three times a day while ANC was below 0.5 × 109/L. All patients without sulfa allergy received prophylactic sulfamethoxazole/trimethoprim twice a day every Monday and Thursday from day 5 DICEP until 6 months after BEAM. Patients received 5 U to 6 U irradiated random donor platelets for platelet counts less than 10 × 109/L and 2 U irradiated red blood cells for hemoglobin level less than 80 g/L. G-CSF (300 μg/d for those weighing < 70 kg; 480 μg/d for those weighing > 70 kg) was started day +7 after ASCT.
Meningeal prophylaxis with intrathecal chemotherapy was only recommended for those patients with epidural masses, paranasal sinus involvement, or Burkitt lymphoma. Involved field radiotherapy (IFRT) was considered following completion of BEAM/ASCT for patients who had prior sites of bulky disease more than 10 cm.
The patients were followed daily until they experienced blood count recovery and no longer required antibiotics, frequent transfusions, or intravenous hydration. Thereafter, follow-up was performed every 2 to 3 weeks until 3 months, then every 3 to 4 months for the first 2 years, and then every 6 months until 5 years after BEAM/ASCT. Annual follow-up was performed thereafter.
Restaging CT scans were performed 4 to 5 weeks after DICEP as well as 6 to 8 weeks and again 6 months after ASCT. Patients who had a positive marrow biopsy at diagnosis underwent a repeat marrow biopsy 4 to 5 weeks after DICEP, before receiving BEAM. Complete response was defined as complete disappearance of all clinical and radiologic evidence of lymphoma, or only small residual radiologic abnormalities of uncertain significance that did not progress during the first 6 months after ASCT. Partial response was defined as less than complete response, but more than 50% reduction in the sum of the products of the greatest perpendicular diameters (SPD) of measurable disease sites with no increase in size of other disease sites or development of new sites. Progressive disease was defined as the appearance of new disease masses, or a more than 25% increase from nadir in the SPD of measurable disease. Stable disease was defined as neither meeting criteria for partial/complete remission, nor progressive disease.
Statistics
The major end point of this prospective, single-arm, pilot study was feasibility of early sequential DICEP then BEAM/ASCT in patients with poor-prognosis NHL as measured by the proportion of patients who completed all planned therapy and attained complete response, relapse rates, and frequency of Bearman grade III-IV life-threatening or fatal treatment-related toxicity.19
The sample size for this study was initially based on the end point of complete response rate. This study was designed in 2 stages. If the results of the first phase were sufficiently promising, then accrual was to be continued, allowing for a more precise estimate of the probability at the end of the second phase. If toxicity was unacceptable, the trial was to be terminated. The rules for this design are determined from the following values: p0 (inactive), pA (highly active), α (the probability of not rejecting an inactive drug) and β (the probability of rejecting a highly active drug), n1 (the size of the first stage), and n (the total size of the trial). Both α and β were set equal to 0.10 (equivalent to a one-sided P value of .05 and 90% power). Assuming p0, the probability of a complete response on standard CHOP therapy, is 0.55 and pA, the probability of a complete response on the new therapy, would be 0.80, then the trial was to be terminated if at the end of 20 patients, fewer than 14 experienced a complete response. If 14 or more patients experience a complete response, 20 more patients were to be accrued. If, at the end of 40 patients, fewer than 29 experienced a complete response, then the investigation of this treatment was to be terminated.
In addition, early stopping rules were established for unacceptable treatment-related mortality. The lower bound of a 95% confidence interval (CI) on the observed proportion of toxic deaths was used as a guideline for early stopping of the study. A toxic death rate of 10% was considered to be unacceptable, the study was to be stopped at the time when the lower bound of the 95% confidence interval exceeds 10%. Using this method, the study was to be stopped if 2 deaths occurred in the first 3 patients accrued, 3 deaths in the first 4 to 8 patients accrued, 4 deaths in the first 9 to 14 patients accrued, 5 deaths in the first 15 to 20 patients accrued, 6 deaths in the first 21 to 27 patients accrued, or 7 deaths in the first 28 to 34 patients accrued.
Accrual to the study continued after the initial 40 patients satisfied adequate complete response rate and treatment-related mortality endpoints. This further accrual was approved on an annual basis by our research ethics board to more precisely estimate EFS and overall survival (OS) rates. Accrual significantly dropped after our center opened a competing American Intergroup phase 3 randomized controlled trial through the National Cancer Institute of Canada Clinical Trials Group. The study was terminated once R-CHOP became the standard initial therapy for diffuse large B-cell lymphoma in Calgary.
The study results were analyzed in September 2005, once all surviving patients had at least one year of follow-up after ASCT. The data were analyzed using GraphPad PRISM 4 (GraphPad Software, San Diego, CA). Kaplan Meier survival curves were plotted and used to determine the 4-year OS and EFS rates from the time of initial CHOP chemotherapy. Event-free survival was defined as relapse or death from any cause.
Results
Patient characteristics
From July 1998 to August 2004, 55 patients were accrued. Characteristics of these patients are summarized in Table 1.
Patient characteristics
Characteristic . | Values . |
|---|---|
| Median age, y (range) | 44 (20-63) |
| Bulky mass more than 10 cm, no. patients | 34 |
| Stage, no. patients | |
| I | 1 |
| II | 4 |
| III | 4 |
| IV | 46 |
| Extranodal sites, no. patients | |
| Pleura | 21 |
| Bone marrow | 16 |
| Liver | 15 |
| Pericardium | 10 |
| Bone | 8 |
| Lung | 8 |
| Kidney | 4 |
| Gastrointestinal tract | 3 |
| Muscle | 3 |
| Ascites | 3 |
| Skin | 1 |
| 2 or more extranodal sites, no. patients | 36 |
| Elevated serum LDH level, no. patients | 47 |
| ECOG performance status, no. patients | |
| 1 | 12 |
| 2 | 29 |
| 3 | 13 |
| 4 | 1 |
| AAIPI risk factors, no. patients | |
| 2 | 20 |
| 3 | 35 |
| Histology, no. patients | |
| DLBCL | 51 |
| Burkitt lymphoma | 1 |
| Post-MTX DLBCL | 1 |
| Follicular grade 3 lymphoma | 1 |
| γδ Hepatosplenic T-cell lymphoma | 1 |
Characteristic . | Values . |
|---|---|
| Median age, y (range) | 44 (20-63) |
| Bulky mass more than 10 cm, no. patients | 34 |
| Stage, no. patients | |
| I | 1 |
| II | 4 |
| III | 4 |
| IV | 46 |
| Extranodal sites, no. patients | |
| Pleura | 21 |
| Bone marrow | 16 |
| Liver | 15 |
| Pericardium | 10 |
| Bone | 8 |
| Lung | 8 |
| Kidney | 4 |
| Gastrointestinal tract | 3 |
| Muscle | 3 |
| Ascites | 3 |
| Skin | 1 |
| 2 or more extranodal sites, no. patients | 36 |
| Elevated serum LDH level, no. patients | 47 |
| ECOG performance status, no. patients | |
| 1 | 12 |
| 2 | 29 |
| 3 | 13 |
| 4 | 1 |
| AAIPI risk factors, no. patients | |
| 2 | 20 |
| 3 | 35 |
| Histology, no. patients | |
| DLBCL | 51 |
| Burkitt lymphoma | 1 |
| Post-MTX DLBCL | 1 |
| Follicular grade 3 lymphoma | 1 |
| γδ Hepatosplenic T-cell lymphoma | 1 |
Results of DICEP
No patient experienced treatment-related mortality, or severe, life-threatening, Bearman grade III regimen-related toxicity requiring the intensive care unit or dialysis support. Patients required a median of 2 U (range, 0-14 U) of red blood cells (RBCs) and 2 days (range, 0-6 days) of platelet transfusion after DICEP. Serious toxicities following DICEP included deep venous thrombosis (n = 1), delirium (n = 1), and pericardial effusion (n = 1). Stomatitis was mild (Bearman grade 0-I), and did not require parenteral analgesics. Documented infections included reactivated tuberculosis in a man who emigrated from Vietnam 20 years earlier (n = 1), Streptococcal bacteremia (n = 1), and cheek cellulitis (n = 1). The median number of days to an ANC more than 0.5 × 109/L and platelets more than 20 × 109/L after DICEP (counting the first day of DICEP as day 1) was 17 days (range, 10-21 days) and 16 days (range, 12-21 days), respectively. Median length of hospital stay for DICEP was 19 days (range, 3-49 days).
DICEP plus G-CSF mobilized peripheral blood CD34+ progenitor cells extremely well. Only one patient did not mobilize sufficient stem cells for ASCT (< 2 × 106/kg CD34+ cells). Of the remaining 54 patients, a median of 21.9 × 106 CD34+ cells/kg was collected (3.2-54.9 × 106 CD34+ cells/kg) with a median 14.8 L apheresis (range, 6.1-27.9 L). Fifty-one patients underwent a single apheresis procedure.
In total, 45 patients experienced a partial response and 4 a complete response to DICEP, for an overall response rate of 89%. Only one patient experienced progressive disease and 5 had stable disease to DICEP. Of 16 patients who had involved marrow before DICEP, 15 became marrow-negative after DICEP.
Results of BEAM/ASCT and IFRT
The study met predetermined criteria for continued accrual with complete responses following BEAM/ASCT with or without IFRT achieved by 17 of the initial 20 patients, 34 of the initial 40 patients, and 47 of all 55 patients (85%). Fifty-one of the 55 patients (93%) received BEAM/ASCT. Reasons for not receiving BEAM/ASCT included bulky refractory or progressive lymphoma (n = 2), reactivated tuberculosis (n = 1), or failure to mobilize stem cells (n = 1).
No patient experienced grade III-IV regimen-related toxicity within 100 days of BEAM/ASCT. Patients required a median of 4 U (range, 0-11 U) of RBCs and 2 days (range, 0-7 days) of platelet transfusion after BEAM. Serious toxicities following BEAM included Bearman grade II stomatitis (n = 29), grade II renal (n = 1), grade II CNS (n = 2), deep venous thrombosis of ovarian vein (n = 1), engraftment syndrome (n = 2), grade I-II liver (n = 2). One patient with a history of systemic lupus erythrematosus became transiently unarousable immediately following stem cell infusion and associated premedications with diphenhydramine, lorazepam, and hydrocortisone. She made a complete recovery with no neurologic sequelae. The median number of days to an ANC more than 0.5 × 109/L and platelets more than 20 × 109/L after ASCT (counting ASCT as day 0) was 11 days (range, 9-14 days) and 10 days (range, 7-15 days), respectively. Median length of hospital stay for BEAM/ASCT was 20 days (range, 7-117 days).
A total of 23 patients received IFRT after ASCT to the following sites: mediastinum with or without the neck (n = 13), abdomen or pelvis (n = 6), neck (n = 3), and leg (n = 1). The majority (n = 18) of these patients received 3500 cGy in 20 fractions. Eleven patients with bulky masses at diagnosis never received IFRT because of patient refusal, advanced extranodal disease, or early lymphoma progression. The EFS rate was not associated with use of IFRT (log rank P = .96) or tumor bulk more than 10 cm (log rank P = .27).
Reversible late complications occurring more than 100 days after ASCT included Varicella zoster (n = 11), hemorrhagic cystitis (n = 2), steroid-requiring radiation pneumonitis (n = 1), and BCNU lung toxicity (n = 1). The only patient who experienced nonrelapse mortality died from hemophagocytosis and thrombocytopenic, massive lower GI bleeding 6 months after ASCT. An autopsy was refused by this patient's family; therefore, absence of lymphoma relapse was not confirmed.
Survival data and univariate analysis of factors associated with outcome
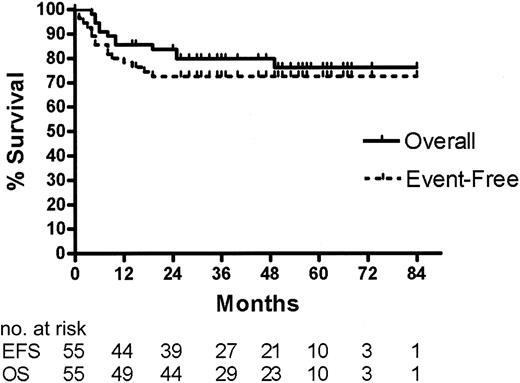
At the time of this analysis, the median follow-up of surviving patients is 49 months (range, 14-73 months). As illustrated in Figure 1, the 4-year OS rate is 79% (95% CI = 69%-90%) and the EFS rate is 72% (95% CI = 60%-84%) for all 55 patients enrolled in the study. Except for the patient described under “Results of BEAM/ASCT and IFRT,” the cause of death for all 12 patients who died was progressive lymphoma.
Discussion
In this study of 55 patients with poor-prognosis, aggressive histology lymphoma, CHOP-DICEP-BEAM/ASCT and possible IFRT to sites of bulk resulted in 4-year EFS and OS rates of 72% and 79%, respectively. Nonrelapse mortality was only 1.8% overall. These results are substantially better than those expected from SDCT alone for these patients with 2 to 3 AAIPI risk factors. DICEP plus G-CSF effectively in vivo purged contaminated marrows, and mobilized large numbers of CD34+ cells as previously reported.16
In the setting of advanced stage aggressive lymphoma, the use of IFRT to sites of prior bulk is controversial. It is difficult to determine whether IFRT contributed to the results of our study. We found that IFRT was generally well tolerated following DICEP and BEAM/ASCT and that patients who had bulky lymphoma experienced similar outcomes to those who did not have tumor bulk. The latter may have been due to the use of IFRT, but may also have been due to small patient numbers or lack of independent prognostic significance of bulk in this group of poor-prognosis patients.
This study has several limitations. It was a single center, uncontrolled study of a relatively small number of patients. The median follow-up was relatively short at only 4 years, although this is a reasonable period to evaluate EFS for aggressive histology lymphoma. The improved outcome relative to historic controls may be due to factors other than protocol sequential HDCT. Such factors possibly include selection bias, stage migration, and improvements in supportive care. Selection bias for this study, however, was decreased through the use of the standardized AAIPI as an eligibility criterion. The relevance of our findings is diminished by the 15% to 20% improvement in EFS rates reported for B-cell lymphoma patients who receive CHOP plus rituximab over CHOP alone. Ongoing studies are being conducted to determine whether further improvements in outcome may be achieved by administering R-CHOP every 14 days with G-CSF instead of the traditional standard of every 21 days.20 Although R-CHOP has become standard therapy for diffuse large B-cell lymphoma, likely 40% to 50% of patients younger than 65 years of age with 2 to 3 AAIPI factors will not be cured by R-CHOP. Studies involving HDCT/ASCT are still reasonable for this group of patients.
Event-free and overall survival for 55 patients with aggressive histology lymphoma and 2 to 3 AAIPI factors based on intention-to-treat with CHOP, DICEP, BEAM/ASCT with or without IFRT.
Event-free and overall survival for 55 patients with aggressive histology lymphoma and 2 to 3 AAIPI factors based on intention-to-treat with CHOP, DICEP, BEAM/ASCT with or without IFRT.
Conflicting results have been reported from randomized controlled trials evaluating first remission consolidation with HDCT/ASCT for poor-prognosis DLBCL.6-14 Many studies were frankly negative, whereas a few have shown significant PFS benefits from HDCT. Criticisms of these studies, however, are numerous. Many studies had inadequate statistical power, most did not use the AAIPI as an eligibility or stratification criterion, and overall they were extremely heterogeneous with respect to histologic subtypes, choice of standard and HDCT regimens, and timing of HDCT relative to number of induction chemotherapy cycles. Some studies used a nonconventional, intensive chemotherapy “control arm.” These studies reported that up to 40% of patients in the HDCT arm never received the assigned HDCT, often due to an inadequate response to abbreviated induction chemotherapy prior to planned HDCT/ASCT. The use of abbreviated induction therapy followed by a single HDCT/ASCT is not considered a viable strategy for future trials.
At the 9th International Conference on Malignant Lymphoma in Lugano, Switzerland in June 2005, the Cochrane Hematological Malignancies Group reported a meta-analysis of randomized controlled trials evaluating HDCT/ASCT as part of initial therapy for aggressive lymphoma.21 They included studies with over 20 previously untreated patients per arm, published from 1990 to 2004. Overall, 16 studies involving more than 3000 patients were identified. Funnel plot heterogeneity excluded the Groupe d'Etude des Lymphomes de l'Adulte LNH 93-3 study, wherein the dose-intensity of the control arm exceeded that of the HDCT arm.9 The meta-analysis demonstrated a significant benefit for HDCT over SDCT in terms of EFS and OS for patients with 2 to 3 AAIPI factors. No benefit of HDCT was found for lower risk patients.
A more definitive phase 3 study of late first remission HDCT consolidation for aggressive NHL and 2 to 3 AAIPI factors is currently being conducted by the American Intergroup and NCIC-CTG. This study was amended to include R-CHOP for all patients in this study. If the study meets accrual targets, it should be adequately powered to definitively address the role of late first remission consolidation with HDCT/ASCT following R-CHOP. Even if late consolidation with HDCT/ASCT does not improve EFS or OS for aggressive histology lymphoma overall, HDCT may still be worth investigating in defined subgroups based on molecular markers, or as early sequential high-dose induction therapy.22 Recent efforts such as the Leukemia-Lymphoma Molecular Profiling Project are attempting to correlate outcome with molecular markers in order to identify subgroups of lymphoma patients that may benefit from HDCT.23
A different HDCT strategy involves multiple cycles of high-dose sequential induction chemotherapy. This strategy is supported by reports of dose-dense CHOP by the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL), which conducted studies comparing CHOP-21, CHOP-14, CHOEP-21, and CHOEP-14 in patients younger than 60 years of age (NHL-B1) and older than 60 years (NHL-B2).24,25 The results suggest that OS is improved with CHOP-14 over CHOP-21 for patients over age 60 years (53% vs 41%) and patients younger than 60 years (85% vs 79%), while CHOEP improved EFS only for younger patients with normal LDH (69% vs 58%), but had no impact on OS. The Dutch-Belgian Hemato-Oncology Cooperative Group HOVON recently reported a subgroup analysis of a randomized trial that suggested possibly greater benefits of intensified CHOP-14 over standard CHOP-21 for patients with low- to intermediate-risk aggressive NHL compared with higher risk patients.26
Schmitz and colleagues reported a series of sequential phase 2 studies evaluating intensive sequential induction chemotherapy for poor-prognosis lymphoma.27 Their most recent study involving 3 cycles of standard-dose CHOEP followed by 3 cycles of intermediate-dose CHOEP resulted in a 2-year freedom from treatment failure (FFTF) rate of 23%, compared with 53% and 67% 2-year FFTF rates for 2 earlier studies that involved only one cycle of standard-dose CHOEP then 3 cycles of intermediate- or high-dose CHOEP and ASCT. Although the total amount of chemotherapy administered was similar between the studies, worse outcome was seen in the more recent study that involved lower dose intensity over the initial 60 days of treatment. These results support the concept of early sequential high-dose induction therapy over that of late HDCT consolidation.
Only one small randomized controlled trial comparing multiple cycles of early high-dose induction chemotherapy to SDCT for poor-prognosis aggressive lymphoma has been published.28 In this study, 98 patients were randomized to high-dose sequential chemotherapy or to SDCT with MACOP-B. The study revealed significantly superior EFS (76% vs 49%) and a trend to improved OS (81% vs 55%) for HDS chemotherapy over MACOP-B (methotrexate, adriamycin [doxorubicin], cyclophosphamide, vincristine, prednisone, and bleomycin). A large confirmatory phase 3 trial for this strategy has not been reported. Given our study results and the current widespread use of rituximab for B-cell NHL, further evaluation of multicycle high-dose sequential induction chemotherapy incorporating rituximab is warranted for poor-prognosis DLBCL.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-12-4898.
Supported by an Alberta Cancer Foundation Autologous Transplant Program Grant.
D.A.S. designed research, wrote protocol and submitted to Health Research Ethics Board, performed research as principal investigator, coordinated data collection, analyzed data, and wrote the article; N.B., K.V., A.B., L.S., D.G.M., A.J., C.B., and J.A.R. were co-investigators in performing research, reviewing data, and editing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Penny Brasher for statistical assistance and Jan McLaughlin for data management.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal