Comment on Huang et al, page 4721
In this issue of Blood, Huang and colleagues show that ICAM-2 mediates the transendothelial migration of neutrophils in vivo, that it can function independently of PECAM-1, and that ICAM-2, like PECAM-1, participates in the diapedesis process in a cytokine-specific manner.
Diapedesis describes the movement of leukocytes through the intact blood vessel wall. For more than 15 years adhesion molecules and chemotactic factors that allow leukocytes to interact with the endothelium at sites of leukocyte exit have been systematically identified and analyzed. Thus, the adhesion and signaling cascade that controls leukocyte capturing is well understood. Yet, the molecular mechanism by which leukocytes actually overcome the barrier of the blood vessel wall is largely unknown.
Two completely different routes have been described. Leukocytes move through endothelial junctions between neighboring cells, and they can use an alternative transcellular route through endothelial cells. Although in vitro and in vivo evidence for the latter pathway exist, the transcellular route seems to be used with low efficiency. Whatever relevance it has in vivo, it is remarkable that more and more endothelial membrane proteins that are involved in this process turn out to be located at endothelial cell contacts. Platelet endothelial cell adhesion molecule-1 (PECAM-1) was the first such protein shown to mediate neutrophil transmigration in vitro and in vivo.1 FIG1
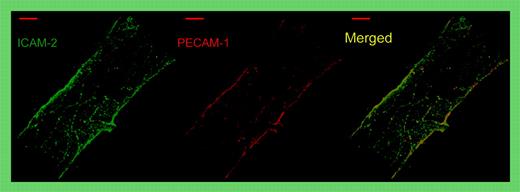
Whole-mount double staining of a representative venule in the mouse cremaster muscle for ICAM-2 (green) and PECAM-1 (red). Note that both proteins are concentrated and superimposed at endothelial cell contacts. Merge shown on the right. See the complete figure in the article beginning on page 4721.
Whole-mount double staining of a representative venule in the mouse cremaster muscle for ICAM-2 (green) and PECAM-1 (red). Note that both proteins are concentrated and superimposed at endothelial cell contacts. Merge shown on the right. See the complete figure in the article beginning on page 4721.
In this issue of Blood, Huang and colleagues use intravital microscopy to show that intercellular adhesion molecule-2 (ICAM-2) is a second membrane protein at endothelial cell contacts that mediates transmigration of neutrophils in vivo (see figure). While a role in the transmigration process had been implicated before from in vitro studies,2 the current paper is the first that analyzes this directly in vivo and that compares 2 membrane proteins, ICAM-2 and PECAM-1, in this process in vivo. The authors found that a monoclonal antibody (mAb) against ICAM-2 strongly inhibited neutrophil recruitment into inflamed peritoneum beyond the inhibitory effect found in PECAM-1–deficient mice, suggesting that both proteins act at different steps in the diapedesis process. Interestingly, blocking ICAM-2 reduced neutrophil extravasation in the peritonitis as well as in the cremaster model to the same low levels in wild-type (wt) and in PECAM-1–deficient mice. Thus, PECAM-1 had no role in ICAM-2–independent leukocyte transmigration. Together, these results suggest that ICAM-2 and PECAM-1 act in the same molecular pathway, possibly in sequential steps.
In addition, the authors found that ICAM-2 is only relevant for leukocyte recruitment if the inflammation was triggered by interleukin-1β (IL-1β), but not if the tissue was stimulated by tumor necrosis factor-α (TNF-α) or thioglycollate. Remarkably, the same cytokine specificity was found for PECAM-1, as was reported by the same group several years ago. Because IL-1β exclusively activates endothelium, whereas TNF-α, in addition, activates leukocytes, it is possible that leukocyte activation may circumvent the necessity for ICAM-2 and PECAM-1. Although other possibilities certainly exist, these results provide further evidence that PECAM1 and ICAM-2 function in the same molecular cascade.
Published in vitro studies and yet-unpublished in vivo studies indicate that there are several more endothelial-cell contact proteins involved in diapedesis. CD99 acts in vitro at a step after PECAM-1.3 Junctional adhesion molecule-A (JAM-A) participates in stimulus specific ways,4 whereas endothelial cell–selective adhesion molecule (ESAM) participates in a cytokine-independent way (F. Wegmann, B. Petri, A. G. Khandoga, C. Moser, A. Khandoga, S. Volkery, H. Li, I. Nasdala, O. Brandan, R. Fässler, S. Butz, F. Krombach, and D. V., manuscript submitted). It will require many more studies such as the one by Huang and colleagues to understand the cascades and interplay of the various endothelial cell junction proteins in leukocyte diapedesis. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal