Abstract
During mucosal HIV transmission, immature dendritic cells (DCs) present in the mucosa are among the first cellular targets of the virus. Previous studies have analyzed the inhibition of HIV-1 transfer from human mature DCs to T lymphocytes by neutralizing IgG, but so far no in vitro data regarding the capacity of antibodies to inhibit HIV-1 infection of immature DCs have been reported. Here, we found an increased HIV-inhibitory activity of monoclonal IgG and purified polyclonal IgG when immature monocyte-derived dendritic cells (iMDDCs) were used as target cells instead of autologous blood lymphocytes. We showed that FcγRII is involved in the mechanism for inhibiting HIV-1 infection of iMDDCs by IgG, whereas no induction of maturation was detected at concentrations of IgG that result in a 90% reduction of HIV replication. After induction of FcγRI expression on iMDDCs by IFN-γ, an augmentation of the HIV-inhibitory activity of IgG, related to the expression of FcγRI, was observed. Taken together, our results demonstrate the participation of FcγRs in HIV-1 inhibition by IgG when iMDDCs are the targets. We propose that IgG is able to efficiently inhibit HIV-1 replication in iMDDCs and should be one of the components to be induced by vaccination.
Introduction
Dendritic cells (DCs) constitute an essential component of the immune system.1 These cells, present at trace level in all organs,2 play a crucial role in bridging innate and acquired immune responses to pathogens.3 Mucosal HIV-1 transmission is the major mode of infection, and immature myeloid DCs (MDCs) present at mucosal sites are among the first cells targeted by the virus. DCs play an important role in virus transmission, dissemination, and persistence of HIV-1 infection and are considered as reservoirs for the virus in lymphoid tissues where they may contribute to the infection of newly recruited T lymphocytes.3-6 Different subsets of DCs have been found to be infected in vivo and in vitro,5-11 although the frequency of HIV-infected DCs is often 10 to 100 times lower compared with CD4+ T lymphocytes.12
It has been reported that HIV-1 proteins, such as gp-120, Nef, and Tat, can each induce maturation of MDCs, but maturation induced by whole HIV infectious particles is more controversial.5,13 Some authors have shown that plasmacytoid DCs (PDCs) can mature after in vitro infection,14 whereas maturation of MDCs seems to occur as a bystander effect due to cytokines produced by PDCs after HIV-1 exposure.15 On the other hand, once MDDCs are infected by HIV-1, their maturation induced by TLR4 or CD40L ligation was impaired.13,16 Moreover, abnormal maturation induced by LPS has been measured after exposure of iMDDCs to HIV-1 gp-120.17,18 Recently, it has been shown that LPS-induced maturation could be prevented by addition of recombinant Vpr.19 Thus, new evidence has shown that HIV-1 could interfere with DC immune responses by impairing their maturation process, their cytokine production, and their allogenic T-cell stimulatory function; this could contribute to immune dysfunction in AIDS patients.5
Apart from the classic infectious process,20 binding and uptake of viruses by DCs will induce iDCs to respond rapidly to virus exposure by several antigen-internalization pathways such as phagocytosis, receptor-mediated endocytosis, and macropinocytosis.3,21-24 These cells bind immune complexes (ICs) via Fcγ receptors (FcγRs).25 Several reports have shown, mainly in the case of tumor-antibody ICs targeted to specific FcγRs on the cell surface of DCs, a significant increase of antigen uptake, processing, and presentation on MHC molecules when FcγRs are involved.26-29 Others have also reported that opsonized antigen uptake by iDCs through FcγRs could allow a more efficient presentation to naive CD4+ T helper and CD8+ cytotoxic T lymphocytes after DC maturation than presentation of the same antigen in its soluble form.30,31 Nevertheless, no data are currently available on the mechanism(s) by which HIV-IgG ICs are captured and internalized by iDCs via FcγRs, and their capacity to allostimulate naive B and T lymphocytes after ICs degradation in specific lysosomal compartment.
Few studies have analyzed inhibition of HIV transmission from mature DCs to T lymphocytes by antibodies.4,13,32 Frankel et al have found an increased inhibitory activity of neutralizing mAbs when a virus/antibody mixture is added to mature DCs before transfer to T lymphocytes versus direct infection of T lymphocytes.32 Similarly, Ganesh et al have observed that mAb 2F5 is able to prevent transfer of HIV from mature MDDCs to T lymphocytes during the first 48 hours, whereas protection of T lymphocyte infection is no longer recorded after 4 days of culture.13 The authors concluded that antibodies cannot protect HIV-1 R5 strain transfer to autologous T lymphocytes during infectious synapse formation with mature MDDCs.13 However, these studies did not analyze the effects of anti–HIV-1 antibodies on infection of iMDDCs.
Here, we have analyzed the capacity of monoclonal IgG or polyclonal IgG purified from sera of HIV-1–infected individuals to inhibit HIV-1 replication in iMDDCs and to induce cell maturation. We have shown for the first time that anti–HIV-1 IgGs are able to efficiently inhibit HIV-1 infection of human iMDDCs without induction of maturation and demonstrate that FcγRs are involved in this mechanism of inhibition. These results strongly suggest that FcγRs play a role in the protection of HIV-1 infection of human iDCs, probably by mediating endocytosis and degradation of HIV-IgG ICs. These antibodies that efficiently inhibit in vitro HIV infection of human iDCs could participate in the protection of individuals from HIV-1 infection.
Materials and methods
Antibodies and reagents
mAbs HLA-DR–PE (G46-6), HLA-ABC–PE (G46-2.6), CD1a-PE (HI149), CD11c-PE (HL3), CD16-PE (3G8), CD40-PE (5C3), CD80-PE (L307.4), CD83-PE (HB15e), CD86-PE (FUN-1), CD89-PE (A59), CD206-PE (19.2), and DC-SIGN–PE (DCN46) were purchased from BD Pharmingen (San Diego, CA). mAbs CD4-PC5 (13B8.2), CD8-PE (B9.11), CD14-PC5 (RMO52), CD32-PE (2E1), CD64-PC5 (22), CD45RO-ECD (UCHL1), DC-LAMP–PE (104.G4), and p24-RD1 or -FITC (KC57) were purchased from Beckman-Coulter (Roissy, France). Purified mAbs anti–human FcγRIII (3G8), FcγRII (3D3), and FcγRI (10.1) (BD Pharmingen) were used to block specific FcγR. Anti–HIV-1 human mAbs to gp-120 (IgG1b1233 and 2G1234 ) and to gp-41 (2F534 ) and cyclized V3 loop peptide (ARP7041) were obtained from the NIBSC (Hertfordshire, United Kingdom). Human mAbs 4E1034 and 447-52D35 and corresponding Fab fragment were provided by Drs H. Katinger and R. Stanfield, respectively. Human polyclonal IgG and IgA were purified from sera of asymptomatic HIV-1 patients (approval obtained from the Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale [CCPPRB], and informed consent was provided according to the Declaration of Helsinki) and of HIV-seronegative donors.36 F(ab′)2 fragments of these polyclonal antibodies were prepared as described.37 Recombinant gp160 was provided by Dr R. El Habib (Aventis Pasteur, Lyon, France).
Preparation of human iMDDCs
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were obtained by Ficoll-Hypaque sedimentation. PBMCs were either stimulated by PHA for 3 days or used for isolation of blood monocytes with a one-step immunomagnetic separation procedure of CD14+ cells according to the manufacturer's instructions (EasyStep; Stem cells biotechnologies, Vancouver, BC, Canada). This purified cell fraction contained more than 99% of CD14+ monocytes as determined by flow cytometry analysis. The purified CD14+ monocytes were cultured at 2 × 106 cells/mL in RPMI 5% FCS supplemented with 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D systems, Minneapolis, MN) plus 20 ng/mL IL-4 (R&D systems) at 37°C and 5% CO2 in air. To analyze HIV neutralization by antibodies in both iMDDCs and autologous lymphocytes, 15% of unstimulated autologous peripheral blood lymphocytes (PBLs) were added to CD14+ monocyte culture for 6 days. Every 3 days, GM-CSF and IL-4 were newly added to cultures. FcγRI was induced at the cell membrane of these iMDDCs by incubation with 500 U/mL IFN-γ (NIBSC) for 24 hours.
Virus stocks
R5 HIV-1 primary isolates Bx08 and BaL (subtype B) were provided by Prof H. Fleury (Bordeaux, France) and through the National Institutes of Health (NIH; Bethesda, MD) from Drs S. Gartner, M. Popovic, and R. Gallo. Virus stocks were obtained as previously described.38,39 To eliminate the presence of bystander activation factors in virus preparation that could induce MDDC maturation, viruses were purified on sephacryl S-1000 superfin column as described.40 Purified virus preparations were aliquoted and frozen at the concentration of 10 to 15 μg/mL p24. For each iMDDC virus titration was performed at day 4 in conditions similar to neutralization.
Antibody neutralization assays
Neutralization was assessed by flow cytometry detection of intracellular p24 viral antigen after infection of PHA-stimulated PBMCs or iMDDCs, using experimental conditions similar to those previously described.37 Briefly, 25 μL purified HIV-1 (concentration ranging from 2 to 4 μg/mL p24 depending on the strain, leading to 5% infected cells at 64 hours) was preincubated for 1 hour with 25 μL mAb or polyclonal IgG or IgA purified from HIV-1 patients before the addition of 25 μL iMDDCs at 15 × 106 cells/mL (in RPMI 1640 medium with 5% FCS, supplemented with human recombinant IL-4 plus GM-CSF) or of 25 μL PHA-stimulated PBMCs at 20 × 106 cells/mL (in RPMI medium with 10% FCS plus IL-2). The numbers of infected cells were determined by the detection of intracellular p24 antigen by flow cytometry, 24 or 64 hours after the infection of PHA-stimulated PBMCs or iMDDCs, respectively. Neutralizing titers were determined as the concentration of antibodies, which results in 90% reduction of infected cells.
Flow cytometry analysis
To assess the immunophenotypic profile of iMDDCs, cells were washed once with PBS plus 3% FBS and incubated for 15 minutes at 4°C with antibodies. After washing, the stained cells were either fixed in 1.5% paraformaldehyde (Sigma, St Louis, MO) and/or further monitored for virus replication by intracellular p24 detection.37,41 Cells were characterized by flow cytometry (FACScan; Becton Dickinson, San Jose, CA).
Results
Immunophenotyping of iMDDCs
The phenotypic profile of human MDDCs was determined after 6 days of culture. After this period, cells had acquired the immunophenotype of iDCs (Figure 1). The mean percentages of MDDCs expressing the costimulatory molecules CD86 and CD80 were 40% and 47%, respectively, and only a very low percentage of these MDDCs was found positive for the maturation marker CD83. The loss of CD14 expression and the slow up-regulation of CD1a and FcγRII markers confirmed the differentiation of blood monocytes to DCs.42 MDDCs expressed different FcγRs at their cell surface. FcγRIII (CD16)–, FcγRII (CD32)–, and FcγRI (CD64)–positive cells represented about 15%, 35%, and 5% of the total MDDC population, respectively (Figure 1A).
Maturation of these cells was obtained after addition of LPS (Figure 1B) or TNF-α (not shown) for 24 hours. Addition of LPS induced a rapid up-regulation of the costimulatory molecules CD83, CD86bright, and CD40 together with a diminution of CD206+ MDDCs (Figure 1B). The expression of FcγRs decreased during this MDDC maturation. Because residual CD4+ T lymphocytes, present at less than 0.5% in purified CD14+ monocytes, may interfere with iMDDC infection, 15% of autologous PBLs were added to highly purified monocytes before differentiation into iMDDCs. In the presence or in the absence of these PBLs, the cell membrane markers of iMDDCs recorded after 6 days remained almost unchanged. After this time of coculture, CD4+ T lymphocytes represented half of the total lymphocyte population and 36% of them were positive for CD69, suggesting that these blood lymphocytes were activated.
Immunophenotyping of iMDDCs. (A) Box plot representation of the percentage of iMDDCs expressing different cell-surface molecules. The results represent median and interquartile values from 10 different HIV-seronegative donors. (B) Percentages of cells expressing CD40, CD80, CD83, CD86, CD206, and FcγRs on uninfected purified iMDDCs, on iMDDCs cocultured with 15% of autologous PBLs, or on MDDCs after lipopolysaccharide (LPS) maturation was detected. Data are from one representative experiment.
Immunophenotyping of iMDDCs. (A) Box plot representation of the percentage of iMDDCs expressing different cell-surface molecules. The results represent median and interquartile values from 10 different HIV-seronegative donors. (B) Percentages of cells expressing CD40, CD80, CD83, CD86, CD206, and FcγRs on uninfected purified iMDDCs, on iMDDCs cocultured with 15% of autologous PBLs, or on MDDCs after lipopolysaccharide (LPS) maturation was detected. Data are from one representative experiment.
HIV-1 infection of iMDDCs
The percentage of HIV-infected MDDCs was assessed by the detection of intracellular viral p24 Gag. Kinetics of HIV replication in iMDDCs were first determined. A few iMDDCs positive for p24 were detected at 48 hours, and the percentage of p24-positive cells reached 3.5 and 18 at 64 and 96 hours, respectively (Figure 2A). This p24 corresponds to new viral p24 proteins produced, as no intracellular p24 antigen was detected when 10 μM AZT was added together with or 24 hours after HIV-1 addition (Figure 2A). When AZT was added 48 hours later, the percentage of infected cells at 64 hours was identical to that measured in the absence of AZT, suggesting that the infected iMDDCs detected at 64 hours correspond to the first round of infection. This production of viral p24 in iMDDCs was delayed compared with PHA-stimulated PBMCs.41
Detection of HIV-1 replication in iMDDCs. (A) Kinetics of iMDDC infection with purified HIV-1BaL (2 μg/mL) were performed under experimental conditions described for the neutralization assay and 10 μM AZT was added at different times (0, 24, and 48 hours) to the cultures. The percentage of infected MDDCs was assessed by measurement of intracellular p24 antigen at different times after infection (up to 120 hours). Results are expressed as mean ± SD of triplicate values. (B) Dose-response between virus input and the percentage of iMDDCs positive for intracellular p24 viral antigen at 64 hours. Linear regression lines and r2 for each HIV-1 primary isolate are represented. Data are from 1 representative experiment of 4 performed independently. (C) Dot plot representation of purified iMDDCs or iMDDCs cocultured with 15% of autologous PBLs. Cells positive for intracellular p24 were detected 64 hours after HIV-1BaL infection in the presence or in the absence of mAb 2F5 at 5 μg/mL. Percentages of infected iMDDCs and autologous PBLs from total cell population are indicated in each box.
Detection of HIV-1 replication in iMDDCs. (A) Kinetics of iMDDC infection with purified HIV-1BaL (2 μg/mL) were performed under experimental conditions described for the neutralization assay and 10 μM AZT was added at different times (0, 24, and 48 hours) to the cultures. The percentage of infected MDDCs was assessed by measurement of intracellular p24 antigen at different times after infection (up to 120 hours). Results are expressed as mean ± SD of triplicate values. (B) Dose-response between virus input and the percentage of iMDDCs positive for intracellular p24 viral antigen at 64 hours. Linear regression lines and r2 for each HIV-1 primary isolate are represented. Data are from 1 representative experiment of 4 performed independently. (C) Dot plot representation of purified iMDDCs or iMDDCs cocultured with 15% of autologous PBLs. Cells positive for intracellular p24 were detected 64 hours after HIV-1BaL infection in the presence or in the absence of mAb 2F5 at 5 μg/mL. Percentages of infected iMDDCs and autologous PBLs from total cell population are indicated in each box.
Next, the relationship between virus input and the number of HIV-infected iMDDCs was assessed. Using serial dilutions of 2 purified HIV-1 primary isolates, we found a linear dose-response curve between the concentrations of virus inoculum ranging from 0.1 to 4 μg/mL p24 and the percentage of infected cells ranging from 1% to 6% (Figure 2B). The concentration of HIV-1 allowing 5% of HIV-infected iMDDCs after 64 hours was chosen for antibody neutralization experiments to be close to the experimental conditions used for single-cycle neutralization assay performed on PHA-stimulated PBMCs.41
The detection of HIV-infected cells by intracellular p24 staining used in this neutralization assay has the advantage to discriminate the infection of iMDDCs from the infection of lymphocytes. Infection of iMDDCs in the presence of autologous PBLs could thus be assessed and compared with the infection of highly purified iMDDCs. In the absence of added PBLs, 5.2% of iMDDCs were infected at 64 hours, whereas in the presence of 15% of autologous PBLs, 5.5% of iMDDCs (corresponding to 6.4% of the MDDC population) and 1.8% of PBLs (corresponding to 12% of the PBL population) were found infected (Figure 2C). After addition of neutralizing mAb 2F5 at 5 μg/mL, a strong reduction of the percentage of HIV-1BaL–infected iMDDCs was detectable (Figure 2C), whereas the percentage of HIV-1BaL–infected autologous PBLs only slightly decreased. These results showed that neutralizing mAb 2F5 was more efficient for inhibiting HIV infection of iMDDCs than blood lymphocytes.
Increased HIV-neutralizing activities of anti–HIV IgG
We extended this study to various polyclonal and monoclonal Abs. Four- to 10-fold lower neutralizing concentrations of polyclonal IgG were observed on iMDDCs compared with cocultured autologous PBLs (Table 1). These lower neutralizing concentrations were observed whether iMDDCs were purified or cultured in the presence of 15% of autologous PBLs (Table 1). The HIV-inhibitory activity of antibodies on autologous PBLs was comparable with that observed when PHA-stimulated PBMCs were used in a more “classical” neutralization assay.36 When using PHA-stimulated PBMCs, the HIV-1–infected cells were found to be essentially CD4+CD45RO+ T lymphocytes (not shown). Purified polyclonal IgG samples nos. 3 and 11 that had undetectable neutralizing activity on PHA-stimulated PBMCs decreased the percentage of HIV-infected iMDDCs by 90% at concentrations of 350 and 130 μg/mL, respectively, for HIV-1BaL and at concentrations of 275 and 165 μg/mL, respectively, for HIV-1Bx08 (Table 1). The 90% inhibitory concentrations (IC90) of 5 well-characterized neutralizing mAbs ranged between 1 and 5 μg/mL when iMDDCs were used, whereas their neutralizing concentrations were 10- to 50-fold higher on autologous PBLs or PHA-stimulated PBMCs (Tables 1, 2). Moreover, when a multiple cycle HIV-1BaL neutralization experiment was performed on highly purified iMDDCs, the concentration of mAb 2F5 resulting in 90% decrease of p24 production in the supernatant after 7 days was 1 μg/mL, a concentration similar to that determined by flow cytometry at 64 hours. On the contrary, the IC90 of polyclonal IgA samples nos. 6 and 8 were similar whether iMDDCs, autologous blood lymphocytes, or PHA-stimulated PBMCs were the targets of HIV-1 (Tables 1, 2). Polyclonal IgG or IgA purified from HIV-seronegative donors did not inhibit infection of these cells. Overall, monoclonal and polyclonal IgG exhibited more potent HIV-1–inhibitory activity when iMDDCs instead of PHA-stimulated PBMCs were used as target cells.
Determination of neutralizing titers of polyclonal Ig purified from sera of infected individuals and of neutralizing monoclonal IgG using HIV-1BaL
. | Neutralizing titer, μg/mL . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | . | Coculture . | . | . | |||
. | iMDDCs, purified at 99% . | iMDDCs, purified at 85% . | PBLs, purified at 15% . | PHA-stimulated PBMCs . | |||
| Polyclonal IgG | |||||||
| IgG no. 44 | 35 | 30 | 175 | 220 | |||
| IgG no. 8 | 40 | 30 | 250 | 155 | |||
| IgG no. 24 | 35 | 40 | 200 | 390 | |||
| IgG no. 23 | 60 | 45 | 400 | 600 | |||
| IgG no. 33 | 65 | ND | ND | 265 | |||
| IgG no. 36 | 35 | ND | ND | 350 | |||
| IgG no. 11 | 130 | 130 | — | — | |||
| IgG no. 3 | 350 | 195 | — | — | |||
| IgG control | — | — | — | — | |||
| Polyclonal IgA | |||||||
| IgA no. 8 | 215 | 290 | 250 | 360 | |||
| IgA no. 6 | 320 | 200 | 385 | 260 | |||
| IgA no. 35 | — | — | — | — | |||
| IgA control | — | — | — | — | |||
| Monoclonal IgG | |||||||
| IgG1 b12 | 1 | 3 | 15 | 25 | |||
| 2F5 IgG1k | 2 | 4 | 50 | 30 | |||
| 447-52D IgG3 | 3 | 3 | 50 | 50 | |||
| 2G12 IgG1k | 1 | 0.5 | ND | 50 | |||
| 4E10 IgG1 | 5 | ND | ND | 50 | |||
| Recombinant | |||||||
| sCD4 | 5 | 7 | 10 | 10 | |||
. | Neutralizing titer, μg/mL . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | . | Coculture . | . | . | |||
. | iMDDCs, purified at 99% . | iMDDCs, purified at 85% . | PBLs, purified at 15% . | PHA-stimulated PBMCs . | |||
| Polyclonal IgG | |||||||
| IgG no. 44 | 35 | 30 | 175 | 220 | |||
| IgG no. 8 | 40 | 30 | 250 | 155 | |||
| IgG no. 24 | 35 | 40 | 200 | 390 | |||
| IgG no. 23 | 60 | 45 | 400 | 600 | |||
| IgG no. 33 | 65 | ND | ND | 265 | |||
| IgG no. 36 | 35 | ND | ND | 350 | |||
| IgG no. 11 | 130 | 130 | — | — | |||
| IgG no. 3 | 350 | 195 | — | — | |||
| IgG control | — | — | — | — | |||
| Polyclonal IgA | |||||||
| IgA no. 8 | 215 | 290 | 250 | 360 | |||
| IgA no. 6 | 320 | 200 | 385 | 260 | |||
| IgA no. 35 | — | — | — | — | |||
| IgA control | — | — | — | — | |||
| Monoclonal IgG | |||||||
| IgG1 b12 | 1 | 3 | 15 | 25 | |||
| 2F5 IgG1k | 2 | 4 | 50 | 30 | |||
| 447-52D IgG3 | 3 | 3 | 50 | 50 | |||
| 2G12 IgG1k | 1 | 0.5 | ND | 50 | |||
| 4E10 IgG1 | 5 | ND | ND | 50 | |||
| Recombinant | |||||||
| sCD4 | 5 | 7 | 10 | 10 | |||
Purified HIV-1BaL was preincubated with serial concentrations of polyclonal Ig purified from sera of HIV-1 patients or of neutralizing monoclonal IgG. Neutralizing titers that correspond to the concentration of Ig able to reduce the percentage of infected cells by 90% were determined for each cellular subset (purified iMDDCs, coculture of iMDDCs with 15% of PBLs, or PHA-stimulated PBMCs). Data are mean of 4 experiments performed with cells from at least 4 different donors.
— indicates no neutralizing activity detected at 800 μg/mL polyclonal Ig; ND, not determined.
Determination of neutralizing titers of polyclonal Ig purified from sera of infected individuals and of neutralizing monoclonal IgG using HIV-1Bx08
. | Neutralizing titer, μg/mL . | . | |
|---|---|---|---|
. | iMDDCs, purified at 99% . | PHA-stimulated PBMCs . | |
| Polyclonal IgG | |||
| IgG no. 8 | 35 | 170 | |
| IgG no. 44 | 55 | 265 | |
| IgG no. 24 | 60 | 390 | |
| IgG no. 11 | 165 | — | |
| IgG no. 2 | 240 | — | |
| IgG no. 3 | 275 | — | |
| IgG control | — | — | |
| Polyclonal IgA | |||
| IgA no. 8 | 210 | 190 | |
| IgA no. 6 | ND | 220 | |
| IgA no. 35 | — | ND | |
| IgA control | — | — | |
| Monoclonal IgG | |||
| 2F5 IgG1 | 2 | 40 | |
| 447-52D IgG3 | 5 | 50 | |
| IgG1 b12 | 2 | 50 | |
| 2G12 IgG1 | 2 | > 50 | |
| 4E10 IgG1 | 3 | > 50 | |
. | Neutralizing titer, μg/mL . | . | |
|---|---|---|---|
. | iMDDCs, purified at 99% . | PHA-stimulated PBMCs . | |
| Polyclonal IgG | |||
| IgG no. 8 | 35 | 170 | |
| IgG no. 44 | 55 | 265 | |
| IgG no. 24 | 60 | 390 | |
| IgG no. 11 | 165 | — | |
| IgG no. 2 | 240 | — | |
| IgG no. 3 | 275 | — | |
| IgG control | — | — | |
| Polyclonal IgA | |||
| IgA no. 8 | 210 | 190 | |
| IgA no. 6 | ND | 220 | |
| IgA no. 35 | — | ND | |
| IgA control | — | — | |
| Monoclonal IgG | |||
| 2F5 IgG1 | 2 | 40 | |
| 447-52D IgG3 | 5 | 50 | |
| IgG1 b12 | 2 | 50 | |
| 2G12 IgG1 | 2 | > 50 | |
| 4E10 IgG1 | 3 | > 50 | |
Purified HIV-1Bx08 was preincubated with serial concentrations of polyclonal Ig purified from sera of HIV-1 patients or of neutralizing monoclonal IgG.
Abbreviations are explained in Table 1.
As HIV-inhibitory activity of recombinant soluble CD4 (Tables 1, 2) or of T20 fusion inhibitor (not shown and Herrera et al43 ) was similar for the different cell types used in this neutralization study, a step distinct from the binding of HIV-1 to CD4 or the fusion of HIV-1 with the cellular membrane may be involved in the increased HIV-1–inhibitory activity of IgG on iMDDCs. Kinetics of antibody addition showed that if neutralizing monoclonal antibody 2F5 or polyclonal IgG no. 11 was added 3 hours after addition of HIV to iMDDCs, the potent HIV-inhibitory activity was lost (Figure 3), suggesting that antibodies interfered with the first events of HIV-1 entry in iMDDCs.
HIV-1 inhibition by IgG is not due to iMDDC maturation
It has been previously shown that maturation of bone marrow–derived mouse DCs can be induced by antigen-IgG ICs.31 As others have indicated that HIV-1 production is lower in mature DCs than in iDCs, which may be due to a postentry block44,45 and/or to variations in coreceptor expression,8 maturation of MDDCs in the presence of HIV-1 antibody ICs may explain the increased inhibitory activities of these antibodies on iMDDCs. MDDC maturation can be induced by cellular or viral factors present in crude virus suspension; therefore, they were eliminated by gel filtration. After infection with the purified HIV-1BaL, the infected MDDCs have similar phenotype as the total population (mean ± SD of 3 different cell preparations): 40% ± 5% of MDDCs were positive for CD80; 49% ± 7% for CD86; 2% ± 1% for CD83; 84% ± 8% for DC-SIGN; 4% ± 1% for FcγRI; 42% ± 2% for FcγRII; and 17% ± 2% for FcγRIII. These percentages were similar for uninfected cells (Figure 1). Thus, infected MDDCs conserved an immunophenotypic profile of iMDDCs and did not undergo maturation.
Kinetics of antibody exposure after HIV-1 infection of iMDDCs. Virus was mixed with different concentrations of neutralizing mAb 2F5 or polyclonal IgG sample no. 11 for 1 hour prior to infection of iMDDCs (0); or antibody was added 1 hour (1), 2 hours (2), or 3 hours (3) after the infection. Percentages of HIV-1BaL–infected MDDCs in the presence of antibodies compared with control-infected cells were determined after 64 hours. Data are mean values of triplicate wells performed independently and repeated with iMDDCs from 3 different healthy donors.
Kinetics of antibody exposure after HIV-1 infection of iMDDCs. Virus was mixed with different concentrations of neutralizing mAb 2F5 or polyclonal IgG sample no. 11 for 1 hour prior to infection of iMDDCs (0); or antibody was added 1 hour (1), 2 hours (2), or 3 hours (3) after the infection. Percentages of HIV-1BaL–infected MDDCs in the presence of antibodies compared with control-infected cells were determined after 64 hours. Data are mean values of triplicate wells performed independently and repeated with iMDDCs from 3 different healthy donors.
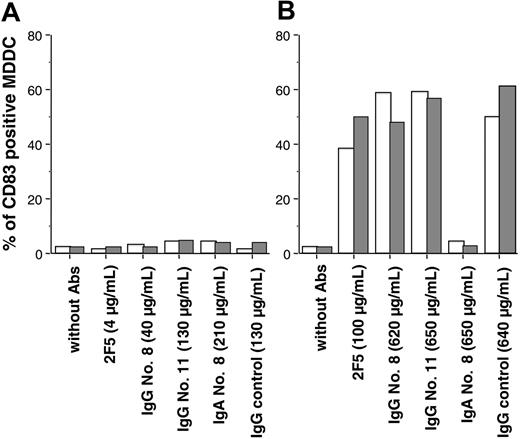
Next, we analyzed the number of MDDCs positive for CD83 in the presence of HIV-Ig ICs. After addition of monoclonal IgG as well as polyclonal IgA or IgG at concentrations equal or below the IC90, no significant change in the percentage of MDDCs positive for CD83 was detected (Table 3; Figure 4A). We also failed to observe any change in the percentage of MDDCs positive for CD86bright and for intracellular lysosomal glycoprotein DC-LAMP after addition of HIV-1BaL complexed with antibodies (not shown), whereas LPS significantly increased the percentage of MDDCs positive for CD83 (Table 3). Thus, a 90% reduction of HIV infection by antibodies was observed in the absence of induction of MDDC maturation.
Analysis of MDDC maturation after HIV inhibition by antibodies
. | % of CD83+ MDDCs . | . | % of p24-positive MDDCs . | |
|---|---|---|---|---|
. | 24 h . | 64 h . | 64 h . | |
| LPS, 10 μg/mL | 52 ± 3 | 70 ± 5 | — | |
| Control uninfected iMDDCs | 2.0 ± 0.1 | 1.8 ± 0.4 | — | |
| HIV-1BaL—infected MDDCs without Ig | 2.2 ± 0.2 | 2.2 ± 1.0 | 5.2 ± 0.2 | |
| HIV-1BaL—infected MDDCs with neutralizing monoclonal IgG | ||||
| 2F5, 5 μg/mL | 2.8 ± 0.4 | 2.7 ± 0.3 | 0.03 ± 0.01 | |
| 2G12, 5 μg/mL | 3.0 ± 0.9 | 2.8 ± 0.4 | 0.2 ± 0.05 | |
| 447-52D, 5 μg/mL | 2.8 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.02 | |
| 447-52D, 0.08 μg/mL | ND | 3.9 ± 1.0 | 1.9 ± 0.2 | |
| HIV-1BaL—infected MDDCs with purified polyclonal Ig | ||||
| IgG no. 11, 170 μg/mL | 4.0 ± 2.2 | 3.9 ± 2.2 | 0.4 ± 0.1 | |
| IgG no. 11, 40 μg/mL | ND | 2.9 ± 2.0 | 2.5 ± 0.5 | |
| IgG no. 44, 70 μg/mL | ND | 2.7 ± 1.7 | 0.07 ± 0.01 | |
| IgG no. 44, 15 μg/mL | ND | 2.0 ± 1.6 | 2.1 ± 0.4 | |
| IgG no. 8, 50 μg/mL | 3.0 ± 0.8 | 3.1 ± 1.5 | 0.06 ± 0.01 | |
| IgG no. 8, 10 μg/mL | ND | 2.4 ± 0.5 | 2.6 ± 0.5 | |
| IgG no. 8, 380 μg/mL | ND | 5.3 ± 0.4 | 0.3 ± 0.01 | |
| F(ab′)2 no. 8, 180 μg/mL | 3.0 ± 0.1 | 2.4 ± 0.6 | 1.5 ± 0.4 | |
. | % of CD83+ MDDCs . | . | % of p24-positive MDDCs . | |
|---|---|---|---|---|
. | 24 h . | 64 h . | 64 h . | |
| LPS, 10 μg/mL | 52 ± 3 | 70 ± 5 | — | |
| Control uninfected iMDDCs | 2.0 ± 0.1 | 1.8 ± 0.4 | — | |
| HIV-1BaL—infected MDDCs without Ig | 2.2 ± 0.2 | 2.2 ± 1.0 | 5.2 ± 0.2 | |
| HIV-1BaL—infected MDDCs with neutralizing monoclonal IgG | ||||
| 2F5, 5 μg/mL | 2.8 ± 0.4 | 2.7 ± 0.3 | 0.03 ± 0.01 | |
| 2G12, 5 μg/mL | 3.0 ± 0.9 | 2.8 ± 0.4 | 0.2 ± 0.05 | |
| 447-52D, 5 μg/mL | 2.8 ± 0.1 | 3.0 ± 0.1 | 0.2 ± 0.02 | |
| 447-52D, 0.08 μg/mL | ND | 3.9 ± 1.0 | 1.9 ± 0.2 | |
| HIV-1BaL—infected MDDCs with purified polyclonal Ig | ||||
| IgG no. 11, 170 μg/mL | 4.0 ± 2.2 | 3.9 ± 2.2 | 0.4 ± 0.1 | |
| IgG no. 11, 40 μg/mL | ND | 2.9 ± 2.0 | 2.5 ± 0.5 | |
| IgG no. 44, 70 μg/mL | ND | 2.7 ± 1.7 | 0.07 ± 0.01 | |
| IgG no. 44, 15 μg/mL | ND | 2.0 ± 1.6 | 2.1 ± 0.4 | |
| IgG no. 8, 50 μg/mL | 3.0 ± 0.8 | 3.1 ± 1.5 | 0.06 ± 0.01 | |
| IgG no. 8, 10 μg/mL | ND | 2.4 ± 0.5 | 2.6 ± 0.5 | |
| IgG no. 8, 380 μg/mL | ND | 5.3 ± 0.4 | 0.3 ± 0.01 | |
| F(ab′)2 no. 8, 180 μg/mL | 3.0 ± 0.1 | 2.4 ± 0.6 | 1.5 ± 0.4 | |
Percentage of CD83+ MDDCs was measured at 24 and 64 hours after HIV-1BaL infection in the presence or in the absence of different concentrations of monoclonal or polyclonal antibodies. Values represent the mean ± SD of 2 wells from 1 representative experiment of 3 performed with cells from different healthy donors.
ND indicates not determined.
Effect of monoclonal and polyclonal antibodies on MDDC maturation. CD83 expression was determined on iMDDCs 64 hours after addition of antibodies in the presence (□) or in the absence ( ) of HIV-1BaL. Abs were added at concentrations that resulted in 90% inhibition of infection (IC90) (A) or at 5- to 25-fold higher concentrations (B). Values are from one representative experiment, repeated 4 times with iMDDCs from different HIV-negative donors. In A and B, □ indicates uninfected MDDC;
) of HIV-1BaL. Abs were added at concentrations that resulted in 90% inhibition of infection (IC90) (A) or at 5- to 25-fold higher concentrations (B). Values are from one representative experiment, repeated 4 times with iMDDCs from different HIV-negative donors. In A and B, □ indicates uninfected MDDC;  , HIV-1BaL-infected.
, HIV-1BaL-infected.
Effect of monoclonal and polyclonal antibodies on MDDC maturation. CD83 expression was determined on iMDDCs 64 hours after addition of antibodies in the presence (□) or in the absence ( ) of HIV-1BaL. Abs were added at concentrations that resulted in 90% inhibition of infection (IC90) (A) or at 5- to 25-fold higher concentrations (B). Values are from one representative experiment, repeated 4 times with iMDDCs from different HIV-negative donors. In A and B, □ indicates uninfected MDDC;
) of HIV-1BaL. Abs were added at concentrations that resulted in 90% inhibition of infection (IC90) (A) or at 5- to 25-fold higher concentrations (B). Values are from one representative experiment, repeated 4 times with iMDDCs from different HIV-negative donors. In A and B, □ indicates uninfected MDDC;  , HIV-1BaL-infected.
, HIV-1BaL-infected.
Of special interest, at high concentrations of mAb 2F5 (100 μg/mL) or polyclonal IgG (650 μg/mL), a maturation signal resulting in an increased expression of CD83 (Figure 4B) and of intracellular DC-LAMP (not shown) was triggered. This maturation was observed in the presence or in the absence of infection and also with HIV-seronegative polyclonal IgG samples, demonstrating that this maturation was not related to HIV infection and was not specific to IgG directed against HIV-1. Of interest, 650 μg/mL polyclonal IgA sample no. 8 did not induce the expression of CD83 (Figure 4B) or DC-LAMP (not shown) in these cells, suggesting that this mechanism of maturation is specific to FcγRs.
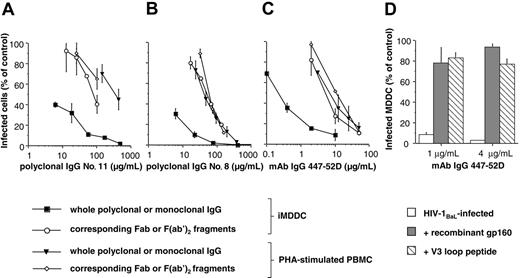
Neutralizing activities of monoclonal and polyclonal IgGs or their corresponding Fab or F(ab′)2 fragments when iMDDCs or PHA-stimulated PBMCs were used as HIV-target cells. The percentages of infected iMDDCs or PBMCs were determined by flow cytometry in the presence of increasing concentrations of purified polyclonal IgG (A-B) or neutralizing mAb 447-52D (C) and their corresponding Fab or F(ab′)2 fragments. Competition experiments were performed by addition of recombinant gp160 (at 30 μg/mL; ▦) or cyclized V3 loop peptide (at 70 μg/mL; ▧) to mAb 447-52D and purified HIV-1BaL for 1 hour before addition of iMDDCs (D). Values are mean ± SD of 3 independent wells from one representative experiment.
Neutralizing activities of monoclonal and polyclonal IgGs or their corresponding Fab or F(ab′)2 fragments when iMDDCs or PHA-stimulated PBMCs were used as HIV-target cells. The percentages of infected iMDDCs or PBMCs were determined by flow cytometry in the presence of increasing concentrations of purified polyclonal IgG (A-B) or neutralizing mAb 447-52D (C) and their corresponding Fab or F(ab′)2 fragments. Competition experiments were performed by addition of recombinant gp160 (at 30 μg/mL; ▦) or cyclized V3 loop peptide (at 70 μg/mL; ▧) to mAb 447-52D and purified HIV-1BaL for 1 hour before addition of iMDDCs (D). Values are mean ± SD of 3 independent wells from one representative experiment.
Participation of FcγRII in HIV-1 inhibition by anti–HIV IgG
As iMDDCs are antigen-presenting cells, which express several FcγRs that can internalize antigen-IgG complexes, the involvement of the Fcγ part of the IgG in HIV-1 inhibition observed with iMDDCs was assessed. F(ab′)2 fragments of polyclonal IgG purified from HIV patients were generated and their neutralizing activities determined. As shown in Figure 5, when purified iMDDCs were used as target cells, the neutralizing activities of the F(ab′)2 fragments were decreased compared with their corresponding whole polyclonal IgG. The neutralizing curves of these F(ab′)2 fragments were, however, similar to those of the F(ab′)2 fragments and their corresponding IgG obtained on PHA-stimulated PBMCs. Results were similar with the Fab fragment of the human mAb 447-52D (Figure 5C). These data suggest that the Fcγ domain of the IgG is necessary for the increased HIV-inhibitory activity observed on iMDDCs. To analyze if Abs need to bind to HIV particles, competition experiments were performed by addition of recombinant gp160 or cyclized V3 loop peptide to mAb 447-52D and HIV-1BaL. HIV-1 inhibition by this mAb was abolished by gp160 or V3 loop peptide, demonstrating that mAb needs to bind HIV-1 to inhibit infection of iMDDCs (Figure 5D).
As the Fcγ domain of the IgG could bind to FcγRs at the cell surface of iMDDCs, the participation of the FcγRs in the increased HIV inhibition recorded on iMDDCs was further evaluated. The different FcγRs were blocked by addition of mAbs directed against them. Blockade of FcγRII decreased the anti-HIV activity of human polyclonal IgG no. 8 (Figure 6A), mAb 2F5 (Figure 6B), polyclonal IgG no. 44, and mAb 2G12 (V.H., M.P., T.D., Sylvie Schmidt, A.-M.A., and C.M., unpublished data, December 2005). However, pretreatment of iMDDCs with monoclonal anti-FcγRI or –FcγRIII FcγRIII did not affect the HIV-inhibitory activities of these IgG samples (Figure 6). No change in the neutralizing activity of polyclonal IgA no. 8 was observed when iMDDCs were pretreated with these anti–human FcγRs (Figure 6E). Addition of monoclonal IgG directed against human FcγR did not change the neutralizing curves of monoclonal or polyclonal IgG when PHA-stimulated PBMCs were used as HIV-target cells (Figure 6C-D). These experiments demonstrate that FcγRII is involved in the high HIV-inhibitory activity of IgG when iMDDCs are used as target cells.
Participation of FcγRI in HIV-1 inhibition by anti–HIV IgG after IFN-γ treatment
It has been reported that FcγRI is expressed at the cell surface of some human DCs in vivo.23,46 However, Fanger et al showed that IL-4, present in the culture medium during differentiation of CD14+ monocytes into iMDDCs, down-regulates FcγRI and that FcγRI could be induced by treatment of iMDDCs with IFN-γ, without producing DC maturation.23,46 To increase FcγRI expression, we treated iMDDCs with 500 U/mL IFN-γ for 24 hours. After IFN-γ treatment, the percentage of iMDDCs positive for FcγRI was increased by 5-fold, whereas the percentage of FcγRII– or FcγRIII–positive cells remained unchanged (not shown). The percentage of cells positive for mannose receptor CD206 or for DC-SIGN did not vary, whereas the percentages of CD80+, CD83+, and CD86+ MDDCs slightly increased by 1.2- to 1.5-fold after IFN-γ treatment (not shown). These IFN-γ–treated MDDCs have conserved the morphology of iDCs. Under these conditions, a more than 10-fold decrease in the IC90 of polyclonal IgG samples nos. 8 and 11 or of mAbs 2F5, 447-52D, and 2G12 was recorded compared with IFN-γ–untreated iMDDCs (Figure 7 and not shown). This efficient HIV inhibition observed on IFN-γ–treated iMDDCs could be diminished by specific blockade of FcγRI, whereas FcγRII or FcγRIII blockade did not change HIV-inhibitory activity of polyclonal or monoclonal Abs, demonstrating that the increased HIV inhibition was due to FcγRI expression on iMDDCs. Moreover, no variation in the neutralizing activity of polyclonal IgA no. 8 was observed (Figure 7D). Overall, we describe here 2 mechanisms of HIV inhibition by IgG when iMDDCs are used as target cells: the first consists in the neutralization of HIV infectivity by Fab or F(ab′)2 parts of IgG (a mechanism that is common for T lymphocytes and MDDCs), and the second is based on the inhibition of HIV infection via FcγRII, or FcγRI when this latter receptor is expressed on iMDDCs.
Blockade of FcγRII on iMDDCs decreases HIV-inhibitory activity of polyclonal IgG sample no. 8 or neutralizing monoclonal IgG 2F5. Purified mouse monoclonal IgG1κ (10 μg/mL) directed against each human FcγRI, FcγRII, FcγRIII, or combined was added on iMDDCs (A,B,E) or PHA-stimulated PBMCs (C-D) 30 minutes prior to infection with HIV-1BaL-IgG ICs. The percentages of infected cells in the presence of purified IgG (A,C) or IgA sample no. 8 (E) or monoclonal IgG 2F5 (B,D) were determined. Values are the mean ± SD of 3 independent wells from one representative experiment.
Blockade of FcγRII on iMDDCs decreases HIV-inhibitory activity of polyclonal IgG sample no. 8 or neutralizing monoclonal IgG 2F5. Purified mouse monoclonal IgG1κ (10 μg/mL) directed against each human FcγRI, FcγRII, FcγRIII, or combined was added on iMDDCs (A,B,E) or PHA-stimulated PBMCs (C-D) 30 minutes prior to infection with HIV-1BaL-IgG ICs. The percentages of infected cells in the presence of purified IgG (A,C) or IgA sample no. 8 (E) or monoclonal IgG 2F5 (B,D) were determined. Values are the mean ± SD of 3 independent wells from one representative experiment.
Induction of FcγRI on iMDDCs by IFN-γ treatment and study of HIV-1 inhibition by monoclonal and polyclonal antibodies. The percentages of infected cells in the presence of purified IgG sample no. 8 (A) or sample no. 11 (B) of purified IgA sample no. 8 (D) or of monoclonal IgG 2F5 (C) were determined on iMDDCs treated or not with IFN-γ. In some experiments, the different anti–human FcγRs were added on IFN-γ–treated iMDDCs 30 minutes prior to incubation with HIV-1BaL-IgG ICs. Values are the mean ± SD of 3 independent wells from one representative experiment.
Induction of FcγRI on iMDDCs by IFN-γ treatment and study of HIV-1 inhibition by monoclonal and polyclonal antibodies. The percentages of infected cells in the presence of purified IgG sample no. 8 (A) or sample no. 11 (B) of purified IgA sample no. 8 (D) or of monoclonal IgG 2F5 (C) were determined on iMDDCs treated or not with IFN-γ. In some experiments, the different anti–human FcγRs were added on IFN-γ–treated iMDDCs 30 minutes prior to incubation with HIV-1BaL-IgG ICs. Values are the mean ± SD of 3 independent wells from one representative experiment.
Discussion
The aim of this study was to analyze the mechanism of HIV inhibition by antibodies when iDCs were used as target cells. For this purpose, we used DCs differentiated from human blood CD14+ monocytes with a combination of IL-4 and GM-CSF or IL-4, GM-CSF, and IFN-γ, as they exhibit immunophenotypic and functional characteristics of human blood iDCs.47 In our culture conditions, we found that iMDDCs were infected by R5 primary isolates as recorded by intracellular p24 antigen detection. Indeed, various studies have previously reported that iDCs are susceptible to and support subsequent replication of R5 HIV-1 strains,13,20,32,47,48 although less efficiently than CD4+ T lymphocytes.5 We showed that addition of AZT after 24 hours of infection still completely abrogates p24 production, suggesting that HIV replication in iMDDCs was delayed compared with PHA-stimulated PBMCs41 or monocyte-derived macrophages (MDMs).37 It has been recently shown that HIV-1 virions were internalized in iDCs as well as in mature DCs over the first hours in the absence of viral replication.49,50 At that time, the virus retained could be efficiently transferred from DCs to CD4+ T lymphocytes,48-51 suggesting that virus was protected in specific DC compartments.48 Later on, the retained HIV-1 particles were mainly destroyed in iDCs and only some may lead to productive infection.48 Thus, DCs may transfer HIV-1 to CD4+ T lymphocytes in 2 distinct phases: the first one driven from an endosomal pathway (without induction of an acidic pH lysosomal degradation) to the DC–T cell synapse49 and the second one through de novo HIV-1 production that occurs later and may be responsible for the selective transmission of R5 strains in vivo.48
In this study, we analyzed the inhibition of R5 HIV replication by monoclonal and polyclonal IgGs when iMDDCs were used as target cells. The assay used allowed us to discriminate infected iMDDCs from infected PBLs. We found that anti–HIV IgG was able to inhibit HIV-1 replication more efficiently in iMDDCs than in PBLs, whereas no change of the neutralizing activity was observed for purified polyclonal IgA. Kinetics of IgG addition indicated that IgG inhibits an early event in HIV infection, which did not favor a mechanism of antibody-dependent cellular cytotoxicity (ADCC). Natural killer (NK) cells could mediate ADCC but they were not detected in our MDDC preparation. Previous reports have shown that human DCs were unable to mediate ADCC,46,52 but more recently, it has been described that a specific subset of native DCs in blood from humans and mice has the ability to lyse tumor cells through efficient ADCC.53,54
It is well known that IgG-FcγR interaction triggers a plethora of cellular responses that include endocytosis and phagocytosis, release of inflammatory cytokines, and enhancement of antigen presentation.55,56 Although it has been reported that ligation of FcγR by antigen-IgG ICs could induce the maturation of mouse DCs,31 no evidence of maturation has been observed for human iMDDCs except when FcγRIIB is blocked.56 As maturation of DCs could decrease virus production by 10- to 100-fold, probably due to a postintegration inhibition,8,44,45 DC maturation in the presence of HIV-IgG ICs was further evaluated. First, we showed that purified HIV-1 particles were not able to induce MDDC maturation. Granelli-Piperno et al have recently found similar results with infectious HIV-1BaL particles,16 whereas others have observed an augmentation of CD83 expression in the presence of high concentrations of recombinant gp-120 or inactivated HIV particles.5,17 In the presence of concentrations of IgG that result in a 90% reduction of HIV-1 infection, no maturation of MDDCs was detected 24 or 64 hours after infection, indicating that the inhibition of HIV replication by these IgGs was not due to MDDC maturation. Nevertheless, at high concentrations of mAb 2F5 (100 μg/mL) or polyclonal IgG (650 μg/mL), an increased percentage of CD83+ and DC-LAMP–positive MDDCs was measured. Additional experiments showed that no maturation was observed with 300 μg/mL F(ab′)2 fragments of samples nos. 8 and 11 and that specific blockade of FcγRII with 10 μg/mL mouse monoclonal IgG1κ (clone 3D3) partially prevents the maturation process induced by high IgG concentration (not shown), indicating that FcγRII was involved in the maturation signal induced by high concentrations of IgG. Dhodapkar et al57 and Banki et al58 have demonstrated that cross-linking of FcγRII with immobilized IgG can induce maturation of human MDDCs via NF-κB signaling pathway. More recently, it has been described that FcγRIIB, which is an inhibitory FcγR, provides a feedback mechanism of DC maturation.57 Specific blockade of FcγRIIB allows maturation of these cells by antigen-coated IgG,57 and the outcome of FcγR engagement, tolerance, or activation, is strongly dependent on a delicate balance between the relative expression of stimulatory FcγRIIA and inhibitory FcγRIIB.56,59 ICs or opsonized particles may simultaneously engage stimulatory FcγRIIA and inhibitory FcγRIIB, which could result in an inhibition of DC maturation or cell signaling.60 Such a mechanism may explain why HIV-IgG ICs did not induce MDDC maturation at low concentrations of IgG. Alternatively, HIV-IgG or gp120-IgG ICs could preferentially engage the inhibitory FcγRIIB because of their small size.61 A recent study showed that circulating blood MDCs expressed a high level of FcγRIIB, similar to iMDDCs, whereas blood PDCs did not express this inhibitory FcγR.57 As IL-4 has been shown to promote the expression of inhibitory FcγRIIB in mice,60 high expression of FcγRIIB may be responsible for the lack of maturation observed in our study. Further experiments will be needed to test the specificity of the FcγRIIB engagement in the inhibition of DC maturation by HIV-IgG ICs as soon as specific mAbs against FcγRIIB are available.
DCs are antigen-presenting cells that have the capacity to phagocytose antigen-IgG ICs. To investigate the involvement of such a mechanism of HIV inhibition by IgG, the inhibitory activity of Fab or F(ab′)2 fragments of IgG has been determined. We found a diminution of the inhibitory activity of Fab or F(ab′)2 compared with whole IgG. By specific blockade of FcγRs, we demonstrated that FcγRII at the cell surface of iMDDCs participates in this HIV inhibition, whereas HIV-neutralizing activity of polyclonal IgA was not affected by blockade of the different FcγRs. We found FcγRII to be the predominant FcγR expressed at the cell-surface membrane of iMDDCs compared with both FcγRIII and FcγRI, as also reported by others.16,29,57,62 FcγRII is considered as a low-affinity FcγR for IgG. After induction of the high-affinity IgG FcγRI at the cell membrane of iMDDCs, an increased HIV-inhibitory activity of monoclonal or polyclonal IgG samples, by more than 10-fold, was observed, whereas neutralizing activity of polyclonal IgA remained unchanged. In these conditions, blockade of FcγRI, but not of FcγRII or of FcγRIII, diminished the HIV-inhibitory activity of these anti–HIV IgG. So, up-regulation of FcγRI expression by IFN-γ on iMDDCs could be directly correlated with this increased HIV-1 inhibition, indicating that the high IgG affinity FcγRI could be involved in the mechanism of HIV-1 inhibition by IgG. Previously, we have shown that R5 HIV-1 replication in MDMs, which expressed a high level of FcγRI, can be efficiently inhibited by anti–HIV-1 IgG and that FcγRI is implicated in the mechanism of HIV inhibition by IgG.37 In vivo, the pattern of FcγR expression in DC subsets is not well defined. Some authors have reported that FcγRI can be detected at the cell surface of some blood DCs, albeit to a lower extent than on human blood monocytes.46,57,62 Therefore, depending on the expression of FcγRs at the cell membrane of HIV-target cells, the HIV-1–inhibitory activity of IgG may vary.
It is likely that the in vitro mechanism of HIV-1 inhibition by anti–HIV IgG in iMDDC is that HIV-IgG ICs are rerouted to a lysosomal degradation pathway after internalization through FcγRs. As this HIV-inhibitory mechanism could involve IgGs other than neutralizing IgGs, induction of such IgGs should be considered in the development of candidate vaccines.
Prepublished online as Blood First Edition Paper, February 9, 2006; DOI 10.1182/blood-2005-08-3490.
Supported by grants from the European Union (QLK2-CT-1999-01321 “Eurovac”); Agence Nationale de Recherches sur le SIDA (ANRS); and National Institutes of Health (HL59725, AI36085, and AI27742).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal