Abstract
Platelets play a fundamental role in maintaining hemostasis and have been shown to participate in innate and adaptive immunity. However, the role of platelets in the immune response to injury remains undefined. We tested the importance of platelets in the host response to serious injury in a newly developed platelet-deficient mouse model. Wild-type and platelet-depleted C57BL/6J mice underwent a 25% full-thickness total body surface area thermal or sham injury. Platelet-deficient mice showed survival of 51% at 48 hours after injury compared with 94% to 100% survival in experimental control mice (P < .001). Necropsy and histology ruled out hemorrhage and hypovolemia as causes of death. Percentages of peripheral blood monocytes (P < .01) and neutrophils (P < .05) were increased between 36 and 48 hours after thermal injury in platelet-deficient mice compared with control mice. Plasma levels of TNFα (P < .001), IL-6 (P < .001), and MCP-1 (P < .05) were also elevated by 24 hours whereas levels of TGFβ1 were reduced between 24 and 36 hours following injury in platelet-depleted mice (P < .001) compared with control mice. Our findings demonstrate for the first time that platelets play a critical protective role during the host response to injury. Moreover, our findings suggest that platelets and, more importantly, platelet-derived TGFβ1 modulate the systemic inflammatory response occurring after injury.
Introduction
Platelets are the smallest but most abundant cell type found in the circulation and range in numbers from 150 × 109/L to 400 × 109/L in humans.1 They are enucleated cells derived from the fragmentation of megakaryocytes and contain preformed compartmentalized proteins as well as messenger RNA.1,2 While platelets primarily function to maintain hemostasis, they also participate in tissue repair and wound remodeling and in antimicrobial host defense.1,3-5 Thus, platelets can be perceived as nomadic “sentinels” capable of responding instantly to chemical changes in their environment and acting as a first line of defense after injury or bacterial invasion.
The activation of platelets in response to injury or bacterial infection initiates the formation of platelet aggregates and the expression of cell adhesion molecule receptors and costimulatory molecules such as P-selectin (CD62P), CD40, and CD154.5-12 Activated platelets are also capable of releasing proinflammatory cytokines such as interleukin-1 beta (IL-1β) and immune-regulatory cytokines such as transforming growth factor beta-1 (TGFβ1).1,7,13 Platelets also play a role in the innate immune response, since they express the pattern recognition molecules referred to as toll-like receptors (TLRs), which recognize a broad range of microbial antigens.14,15 The ability of platelets to respond to their environment and their subsequent expression and release of cell surface molecules and cytokines suggest that they may play a role in inflammatory responses. In support of this idea, platelets have been shown to participate in inflammation of the vessel wall during the development of atherosclerosis where they interact with the endothelium and infiltrating monocytes, in the synovium during arthritis development, and in the mucosal capillaries during active inflammatory bowel disease.12,16-20
We undertook this study because we believe that platelets may participate in the intercellular communication network between injured tissue and the immune system. This may occur as part of the innate immune response, through the expression of platelet TLRs, or through other specific ligand-receptor interactions. Thus, an injury-induced change in the number of circulating platelets or platelet-derived products or alterations in platelet function may be detrimental. This has been suggested by the clinical observation that platelet deficiency correlates with a higher mortality after severe trauma and after sepsis.21,22 However, the underlying mechanisms associated with these observations have not been closely examined. Thus, the purpose of this study was to determine the effect of injury on platelets and to evaluate the role of platelets in the host response to injury. Using polyclonal antibodies to deplete platelets, we developed a platelet-deficient mouse model to test the influence of platelets on the host response to injury. We demonstrate that platelets play a significant protective role in survival following injury. We also show that injury leads to measurable changes in the numbers of circulating platelets. Finally, we demonstrate that platelets appear to control innate immune system reactivity after thermal injury, since platelet-deficient mice display significantly higher levels of circulating proinflammatory cytokines (TNFα, IL-6, and MCP-1), significantly lower levels of TGFβ1, and changes in peripheral blood cell subsets compared with wild-type (WT) mice.
Materials and methods
Animals
Six- to 8-week-old pathogen-free C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and acclimated for at least 1 week prior to use. All mice were maintained in an accredited virus antibody–free facility in accordance with guidelines of the National Institutes of Health and the Harvard Medical Area Standing Committee on Animals.
Mouse injury model
Groups of C57BL/6J mice were either sham or thermal injured in accordance with an injury protocol that has been approved by the National Institutes of Health and Harvard Medical Area Standing Committee on Animals.23 Briefly, 8- to 10-week-old C57BL/6J mice were anesthetized with ketamine (125 mg/kg) + xylazine (6 mg/kg), and the dorsal fur was shaved. Mice were placed in a template exposing a 25% total body surface area. The exposed skin was immersed in 90°C water for 9 seconds. This has been shown to produce an anesthetic full-thickness burn. Control mice were anesthetized, shaved, and exposed to 24°C water. After sham or thermal injury, mice were resuscitated by intraperitoneal injection of 1 mL sterile normal saline solution. The resulting survival rate from this form of injury is at least 95%.24
Time course study
Sham- and thermal-injured mice were killed at sequential intervals after thermal injury immediately after carbon dioxide asphyxiation. Whole blood was collected by cardiac puncture into 1-mL syringes containing 50 μL 169 mM EDTA (ethanolamine diamine tetra-acetic acid; Sigma, St Louis, MO). The blood was transferred to sterile 2.0-mL polypropylene microcentrifuge tubes containing an additional 50 μL EDTA. Duplicate 30-μL aliquots were removed for analysis of platelet numbers, leukocyte numbers, and subsets, using the Hemavet 850 (Drew Scientific, Farmington, CT). Plasma was prepared from the remaining anticoagulated blood samples by centrifugation at 16 000g for 20 minutes at 4°C. Plasma samples were stored for cytokine determination.
Affinity purification of rabbit anti–mouse platelet antibody
Rabbit anti–mouse platelet antiserum was purchased from Inter-Cell Technologies (Jupiter, FL) and was affinity purified on a protein G column (Pharmacia, Uppsala, Sweden). Briefly, the affinity column was prepared by washing with 20–column bed volumes of Tris (tris(hydroxymethyl)aminomethane)–buffered saline containing sodium azide (50 mM Tris-HCl, pH = 7.4; 150 mM NaCl; 0.05% NaN3). The rabbit Ig–containing antiserum was loaded on to the column, which was then washed with 20 volumes of PBS (pH = 7.4). The Ig was eluted with 50 mM glycine-HCl (pH = 3.0; Sigma) into tubes containing neutralization buffer (1 M Tris-HCl, pH = 8.0; 1.5 mM NaCl; 1 mM EDTA; Sigma). Fractions containing the antiplatelet Ig were pooled and sterilized by 0.22-μm filtration (Millipore, Burlington, MA). The sterile Ig–containing fractions were mixed with END-X B52 beads (Associates of Cape Cod Incorporated, Falmouth, MA) to ensure that the resulting antiplatelet Ig (αPLT-Ig) was endotoxin free. The concentration of the purified antiplatelet Ig was determined using the Bradford Protein reagent (Sigma) that included a standard curve prepared with serial dilutions of known concentrations of affinity-purified normal rabbit Ig. Aliquots were prepared and stored at –40°C until use.
Platelet depletion in vivo
Mice were treated with either an affinity-purified normal rabbit immunoglobulin (CTL-Ig; Inter-Cell Technologies) or the affinity-purified rabbit anti–mouse platelet polyclonal immunoglobulin (αPLT-Ig) diluted in nonpyrogenic sterile normal saline (0.9% [wt/vol] sodium chloride; Hospira, Lake Forest, IL) at a final concentration of 2.5 mg/kg body weight. Mice were killed 72 hours later and bled as described above for “Time course study.”
Development of a platelet-depleted thermal-injury model
Groups of mice were pretreated with either CTL-Ig or αPLT-Ig 72 hours prior to sham or thermal injury. After thermal injury, control and experimental mice were followed for survival. At each time point tested, mice were killed by carbon dioxide asphyxiation. Whole blood was drawn by cardiac puncture as described for “Time course study.” All mice used in these studies underwent necropsy to determine cause of death. Approximately 10% to 15% of the experimental αPLT-Ig–treated thermal-injured mice were excluded from the study when any degree of subcutaneous, peritoneal, or gastric hemorrhage was found. Whole blood and plasma samples from the remaining mice underwent analysis.
Cytokine determinations
Plasma levels of TNFα, IL-6, MCP-1, IL-10, IL-12p70, and IFNγ were determined by flow cytometry using the BD Cytometric Bead Array (CBA; Becton Dickinson, Waltham, MA) mouse inflammation kit following the manufacturer's recommendations. Plasma levels of TGFβ1 were determined by a TGFβ1-specific enzyme-linked immunosorbent assay (ELISA) following the manufacturer's recommendations (R&D Systems, Minneapolis, MN). Briefly, plasma samples were acid treated to liberate the TGFβ1, neutralized with sodium hydroxide (NaOH; Fisher Scientific, Hampton, NH), and pH adjusted to 7.2 to 7.4 as suggested by the manufacturer.
Data analysis
The GraphPad Prism 4.0 for Windows software program (GraphPad Software, San Diego, CA) was used for all statistical calculations. Data from survival studies were analyzed using the Kaplan-Meier and log-rank tests. Hematologic and plasma cytokine values were analyzed using the nonparametric Mann-Whitney test. Differences were considered significant when the P value was less than or equal to .05.
Results
The effect of thermal injury on circulating platelet numbers
Experiments were performed to determine the effect of thermal injury on circulating platelets at different times after injury (Figure 1A). Platelet numbers in thermal-injured wild-type (WT) mice were significantly reduced by 1.5 hours after injury, resulting in a transient but mild thrombocytopenia when compared with WT sham-injured controls (P < .05). This reduction in platelet numbers persisted for at least 12 hours (P < .05) and then returned to normal or elevated levels over time from 24 to 168 hours after injury (data not shown).
Hematocrit levels were also measured in the same blood samples from thermal-injured and sham-injured mice to determine whether the observed reduction in platelet numbers in thermal-injured mice occurred as a consequence of hemorrhage or hemodilution. Hematocrit levels from thermal-injured mice were significantly higher at the earliest time point (0.5 hours) when compared with sham controls (P < .05; Figure 1B). Hematocrit levels, however, were within normal limits at all other time points examined (1.0-18.0 hours). This suggests that the observed reduction in circulating platelets was not simply due to hemorrhage or hemodilution. To further determine whether platelet-deficient thermal-injured mice displayed hemorrhage, we performed necropsy of sham versus burn mice at 0.5 hours after injury. We did not observe any signs of hemorrhage in thermal-injured platelet-deficient mice.
Platelet numbers decrease following thermal injury. Groups of mice were either sham treated (n = 4, ○) or thermal injured (n = 8, •). At each time point tested, mice were killed, whole blood was removed by cardiac puncture, and platelet numbers were determined (A). Normal range for platelet numbers is 592 × 109/L (592 × 103/μL) to 2972 × 109/L (2972 × 103/μL), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Values below the dotted line represent thrombocytopenia. Hematocrits were determined from the same blood sample at each time point (B). The gray vertical bar represents the normal limits for hematocrit (.35-.45 [35.1%-45.4%]), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Data are representative of 2 independent experiments (mean ± SEM). Data were analyzed using the Mann-Whitney test. Differences were considered significant when *P < .05.
Platelet numbers decrease following thermal injury. Groups of mice were either sham treated (n = 4, ○) or thermal injured (n = 8, •). At each time point tested, mice were killed, whole blood was removed by cardiac puncture, and platelet numbers were determined (A). Normal range for platelet numbers is 592 × 109/L (592 × 103/μL) to 2972 × 109/L (2972 × 103/μL), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Values below the dotted line represent thrombocytopenia. Hematocrits were determined from the same blood sample at each time point (B). The gray vertical bar represents the normal limits for hematocrit (.35-.45 [35.1%-45.4%]), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Data are representative of 2 independent experiments (mean ± SEM). Data were analyzed using the Mann-Whitney test. Differences were considered significant when *P < .05.
Development of a mouse model of platelet depletion following thermal injury
Since thermal injury significantly alters circulating platelet numbers, we wanted to develop a model to test whether platelets might modulate the host response to injury. We reasoned that developing a platelet-deficient mouse would allow us to investigate this question. Using a unique rabbit anti–mouse platelet polyclonal antibody (αPLT-Ig), mice were made platelet deficient by a single intraperitoneal injection of the antibody. We found that circulating platelet numbers were reduced for up to 72 hours when compared with mice treated with control rabbit Ig (CTL-Ig, P = .002; Figure 2A). To determine whether platelet depletion caused hemorrhage, hematocrit levels were determined in platelet-deficient versus control mice. We found that hematocrit levels were similar in both CTL-Ig– and αPLT-Ig–treated mice (Figure 2B), thereby indicating that platelet depletion of the degree produced in the present study does not cause reduction of hematocrit.
Platelet depletion does not alter hematocrit. C57BL/6J mice were treated with CTL-Ig (□) or αPLT-Ig (▪) and killed 72 hours later. Anticoagulated whole blood was collected by cardiac puncture, and platelet numbers (A) and hematocrit (B) were determined (mean ± SEM). Data were analyzed using the Mann-Whitney test. Differences were considered significant when *P < .05.
Platelet depletion does not alter hematocrit. C57BL/6J mice were treated with CTL-Ig (□) or αPLT-Ig (▪) and killed 72 hours later. Anticoagulated whole blood was collected by cardiac puncture, and platelet numbers (A) and hematocrit (B) were determined (mean ± SEM). Data were analyzed using the Mann-Whitney test. Differences were considered significant when *P < .05.
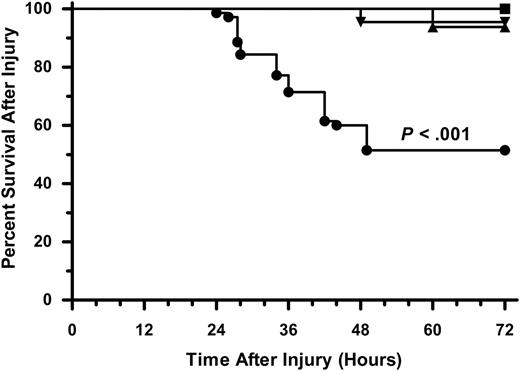
The effect of platelet depletion on the survival of mice following thermal injury. A total of 124 C57BL/6J mice in 3 independent experiments were treated with CTL-Ig or αPLT-Ig 72 hours before burn or sham injury and followed for survival (CTL-Ig Sham, n = 16, ▪; CTL-Ig Burn, n = 22, ▾; αPLT-Ig Sham, n = 16, ▴; αPLT-Ig Burn, n = 70, •). Data were analyzed using the Kaplan-Meier and log-rank test. Differences were considered significant when P < .05.
The effect of platelet depletion on the survival of mice following thermal injury. A total of 124 C57BL/6J mice in 3 independent experiments were treated with CTL-Ig or αPLT-Ig 72 hours before burn or sham injury and followed for survival (CTL-Ig Sham, n = 16, ▪; CTL-Ig Burn, n = 22, ▾; αPLT-Ig Sham, n = 16, ▴; αPLT-Ig Burn, n = 70, •). Data were analyzed using the Kaplan-Meier and log-rank test. Differences were considered significant when P < .05.
Survival of mice to thermal injury after platelet depletion
Thrombocytopenia has been shown to correlate with a poor clinical prognostic outcome in human trauma patients.21,22 Therefore, we were interested in testing whether platelet-deficient mice demonstrated differences in survival following thermal injury. Mice were treated 3 days prior to burn or sham injury with either CTL-Ig (n = 38) or αPLT-Ig (n = 86) and survival was followed for 7 days. As shown in Figure 3, platelet-deficient mice had markedly lower survival of 51% at 48 hours compared with survival rates of between 94% and 100% in αPLT-Ig–treated sham-injured mice and in thermal-injured control mice (P < .001). Histologic analysis of tissue from the lung, liver, spleen, and kidney demonstrated no discernable cause of death in any of the animals used for statistical analysis (not shown). As noted in “Development of a platelet-depleted thermal-injury model,” the 10% to 15% of animals demonstrating any hemorrhage at necropsy was excluded.
Hematologic parameters in mice following thermal injury
To determine the mechanisms through which platelets might protect mice following thermal injury, we assessed hematologic parameters including sequential determinations of platelet numbers, hematocrit, absolute leukocyte numbers, and percentages of leukocyte subsets after injury in groups of sham- and thermal-injured control and platelet-deficient mice.
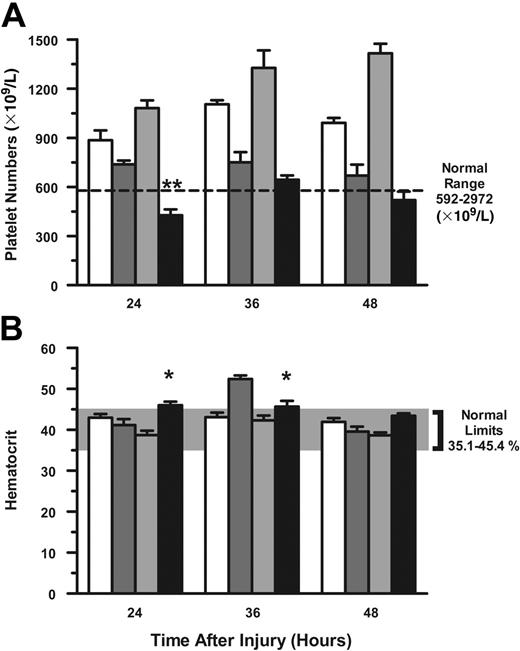
Platelets. Since the majority of platelet-depleted mice died between 24 and 48 hours after injury, we evaluated platelet numbers during these time points. Platelet numbers remained significantly lower in αPLT-Ig–treated mice 24 hours after thermal injury compared with CTL-Ig thermal-injured mice (P < .01; Figure 4A). In those mice that survived 72 hours after thermal injury, platelet numbers recovered and were significantly greater than those of CTL-Ig–treated burn mice (P < .01; data not shown).
Hematocrit. We then evaluated hematocrit levels in sham- and thermal-injured mice that were treated with either CTL-Ig or αPLT-Ig to determine whether hemorrhage accounted for the reduction in platelet numbers and mortality. Normal limits for hematocrit are between .35 (35.1%) and .45 (45.4%). Hematocrit levels at 24 to 48 hours after injury were within normal limits in both CTL-Ig– and αPLT-Ig–treated mice (Figure 4B). However, hematocrit levels at 36 hours were significantly higher in CTL-Ig thermal-injured mice when compared with αPLT-Ig–treated thermal-injured mice (P < .05).
Leukocytes. Whole blood leukocyte numbers and leukocyte subsets were evaluated in sham- and thermal-injured mice. As shown in Table 1, absolute white blood cell counts and lymphocyte counts were markedly greater at 24 hours in thermal-injured mice that were treated with αPLT-Ig when compared with CTL-Ig–treated thermal-injured mice (P < .025). However, absolute numbers of total leukocytes and lymphocytes did not remain elevated at 36 and 48 hours. Numbers of circulating neutrophils and monocytes were not different in αPLT-Ig– and CTL-Ig–treated mice at any time point.
Hematologic analysis of whole blood samples from platelet-depleted and control mice following thermal injury
. | . | Absolute cell numbers, × 109/L, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Animal group, by time following injury . | No. mice* . | White blood cells . | Lymphocytes . | Monocytes . | Neutrophils . | |||
| 24 h after injury | ||||||||
| CTL-Ig Sham | 8 | 9.82 ± 0.42 | 8.64 ± 0.42 | 0.44 ± 0.03 | 1.16 ± 0.26 | |||
| CTL-Ig Burn | 7 | 9.34 ± 1.27 | 6.44 ± 1.24 | 0.84 ± 0.14 | 2.54 ± 0.35 | |||
| αPLT-Ig Sham | 8 | 10.29 ± 0.75 | 8.93 ± 0.76 | 0.40 ± 0.04 | 0.68 ± 0.05 | |||
| αPLT-Ig Burn | 9 | 14.50 ± 0.88† | 10.49 ± 0.99† | 1.17 ± 0.15 | 2.02 ± 0.17 | |||
| 36 h after injury | ||||||||
| CTL-Ig Sham | 4 | 6.62 ± 0.48 | 5.72 ± 0.60 | 0.26 ± 0.05 | 0.60 ± 0.05 | |||
| CTL-Ig Burn | 4 | 13.80 ± 1.29 | 10.89 ± 0.49 | 1.26 ± 0.11 | 2.11 ± 0.41 | |||
| αPLT-Ig Sham | 4 | 7.19 ± 0.26 | 6.37 ± 0.21 | 0.22 ± 0.02 | 0.48 ± 0.05 | |||
| αPLT-Ig Burn | 8 | 13.30 ± 0.99 | 8.75 ± 0.62 | 1.79 ± 0.24 | 2.42 ± 0.25 | |||
| 48 h after injury | ||||||||
| CTL-Ig Sham | 8 | 11.30 ± 0.58 | 9.76 ± 0.52 | 0.50 ± 0.03 | 0.94 ± 0.15 | |||
| CTL-Ig Burn | 7 | 5.51 ± 0.34 | 3.70 ± 0.33 | 0.34 ± 0.04 | 0.61 ± 0.06 | |||
| αPLT-Ig Sham | 8 | 9.94 ± 0.87 | 8.82 ± 0.86 | 0.45 ± 0.04 | 0.61 ± 0.06 | |||
| αPLT-Ig Burn | 5 | 6.24 ± 0.51 | 4.51 ± 0.45 | 0.87 ± 0.06 | 0.85 ± 0.04 | |||
. | . | Absolute cell numbers, × 109/L, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Animal group, by time following injury . | No. mice* . | White blood cells . | Lymphocytes . | Monocytes . | Neutrophils . | |||
| 24 h after injury | ||||||||
| CTL-Ig Sham | 8 | 9.82 ± 0.42 | 8.64 ± 0.42 | 0.44 ± 0.03 | 1.16 ± 0.26 | |||
| CTL-Ig Burn | 7 | 9.34 ± 1.27 | 6.44 ± 1.24 | 0.84 ± 0.14 | 2.54 ± 0.35 | |||
| αPLT-Ig Sham | 8 | 10.29 ± 0.75 | 8.93 ± 0.76 | 0.40 ± 0.04 | 0.68 ± 0.05 | |||
| αPLT-Ig Burn | 9 | 14.50 ± 0.88† | 10.49 ± 0.99† | 1.17 ± 0.15 | 2.02 ± 0.17 | |||
| 36 h after injury | ||||||||
| CTL-Ig Sham | 4 | 6.62 ± 0.48 | 5.72 ± 0.60 | 0.26 ± 0.05 | 0.60 ± 0.05 | |||
| CTL-Ig Burn | 4 | 13.80 ± 1.29 | 10.89 ± 0.49 | 1.26 ± 0.11 | 2.11 ± 0.41 | |||
| αPLT-Ig Sham | 4 | 7.19 ± 0.26 | 6.37 ± 0.21 | 0.22 ± 0.02 | 0.48 ± 0.05 | |||
| αPLT-Ig Burn | 8 | 13.30 ± 0.99 | 8.75 ± 0.62 | 1.79 ± 0.24 | 2.42 ± 0.25 | |||
| 48 h after injury | ||||||||
| CTL-Ig Sham | 8 | 11.30 ± 0.58 | 9.76 ± 0.52 | 0.50 ± 0.03 | 0.94 ± 0.15 | |||
| CTL-Ig Burn | 7 | 5.51 ± 0.34 | 3.70 ± 0.33 | 0.34 ± 0.04 | 0.61 ± 0.06 | |||
| αPLT-Ig Sham | 8 | 9.94 ± 0.87 | 8.82 ± 0.86 | 0.45 ± 0.04 | 0.61 ± 0.06 | |||
| αPLT-Ig Burn | 5 | 6.24 ± 0.51 | 4.51 ± 0.45 | 0.87 ± 0.06 | 0.85 ± 0.04 | |||
Anticoagulated whole blood from experimental or control mice was analyzed for absolute cell numbers. Mice per group per time point are listed. Statistical significance of the data (mean ± SEM) was analyzed using the Mann-Whitney test. Differences were considered significant when P < .05.
Animals per group from 3 independent experiments.
P < .025 for CTL-Ig Burn compared with αPLT-Ig Burn.
Platelet numbers remain depressed following thermal injury in platelet-deficient mice. C57BL/6J mice were treated as described in Figure 3. Anticoagulated whole blood was collected by cardiac puncture, and platelet numbers (A) and hematocrit (B) were determined at the times listed for groups CTL-Ig Sham (n = 16; open bars), CTL-Ig Burn (n = 22; dark gray bars), αPLT-Ig Sham (n = 16; light gray bars), and PLT-Ig Burn (n = 70; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05; **P < .01.
Platelet numbers remain depressed following thermal injury in platelet-deficient mice. C57BL/6J mice were treated as described in Figure 3. Anticoagulated whole blood was collected by cardiac puncture, and platelet numbers (A) and hematocrit (B) were determined at the times listed for groups CTL-Ig Sham (n = 16; open bars), CTL-Ig Burn (n = 22; dark gray bars), αPLT-Ig Sham (n = 16; light gray bars), and PLT-Ig Burn (n = 70; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05; **P < .01.
Because absolute numbers of both leukocytes and leukocyte subsets were unremarkable at later time points (36 and 48 hours), we evaluated the percentages of each leukocyte subset in control and experimental mice (Table 2). The percentages of circulating lymphocytes were similar in thermal-injured mice treated with either CTL-Ig or αPLT-Ig at 24 and 48 hours, whereas the percentages of lymphocytes were significantly lower in the αPLT-Ig group at 36 hours when compared with the CTL-Ig group (P < .05). While the percentages of monocytes were similar in the 2 groups 24 hours after thermal injury, a marked increase in the percentages of monocytes in the αPLT-Ig–treated group was observed when compared with the CTL-Ig–treated group at 36 (P < .01) and 48 (P < .005) hours. Although the percentage of neutrophils was lower at 24 hours after injury in the αPLT-Ig–treated group, this percentage was significantly increased at 36 hours (P < .05) followed by significant decrease at 48 hours (P < .01).
Percentage of leukocyte subsets from platelet-depleted and control mice following thermal injury
Animal group, by time following injury . | . | Percent leukocyte subset, × 109/L, mean ± SEM . | . | . | ||
|---|---|---|---|---|---|---|
| . | No. mice* . | Lymphocytes . | Monocytes . | Neutrophils . | ||
| 24 h after injury | ||||||
| CTL-Ig Sham | 8 | 85.75 ± 1.59 | 4.15 ± 0.31 | 10.38 ± 1.40 | ||
| CTL-Ig Burn | 7 | 68.19 ± 2.48 | 9.68 ± 1.06 | 24.60 ± 3.76 | ||
| αPLT-Ig Sham | 8 | 87.37 ± 1.54 | 3.92 ± 0.30 | 8.00 ± 1.32 | ||
| αPLT-Ig Burn | 10 | 75.05 ± 2.30 | 8.64 ± 0.67 | 16.92 ± 2.03 | ||
| 36 h after injury | ||||||
| CTL-Ig Sham | 3 | 85.37 ± 0.50 | 3.28 ± 0.04 | 9.02 ± 0.31 | ||
| CTL-Ig Burn | 4 | 74.08 ± 2.06 | 9.21 ± 0.53 | 14.91 ± 1.78 | ||
| αPLT-Ig Sham | 4 | 88.67 ± 0.90 | 3.03 ± 0.37 | 6.63 ± 0.48 | ||
| αPLT-Ig Burn | 9 | 66.10 ± 1.48† | 13.19 ± 1.11‡ | 20.35 ± 1.64† | ||
| 48 h after injury | ||||||
| CTL-Ig Sham | 8 | 86.00 ± 1.51 | 4.43 ± 0.22 | 8.21 ± 1.18 | ||
| CTL-Ig Burn | 7 | 70.86 ± 1.69 | 6.18 ± 0.78 | 22.10 ± 1.93 | ||
| αPLT-Ig Sham | 7 | 88.18 ± 1.42 | 4.85 ± 0.36 | 6.26 ± 1.07 | ||
| αPLT-Ig Burn | 5 | 73.24 ± 1.06 | 13.02 ± 0.78§ | 6.11 ± 0.47‡ | ||
Animal group, by time following injury . | . | Percent leukocyte subset, × 109/L, mean ± SEM . | . | . | ||
|---|---|---|---|---|---|---|
| . | No. mice* . | Lymphocytes . | Monocytes . | Neutrophils . | ||
| 24 h after injury | ||||||
| CTL-Ig Sham | 8 | 85.75 ± 1.59 | 4.15 ± 0.31 | 10.38 ± 1.40 | ||
| CTL-Ig Burn | 7 | 68.19 ± 2.48 | 9.68 ± 1.06 | 24.60 ± 3.76 | ||
| αPLT-Ig Sham | 8 | 87.37 ± 1.54 | 3.92 ± 0.30 | 8.00 ± 1.32 | ||
| αPLT-Ig Burn | 10 | 75.05 ± 2.30 | 8.64 ± 0.67 | 16.92 ± 2.03 | ||
| 36 h after injury | ||||||
| CTL-Ig Sham | 3 | 85.37 ± 0.50 | 3.28 ± 0.04 | 9.02 ± 0.31 | ||
| CTL-Ig Burn | 4 | 74.08 ± 2.06 | 9.21 ± 0.53 | 14.91 ± 1.78 | ||
| αPLT-Ig Sham | 4 | 88.67 ± 0.90 | 3.03 ± 0.37 | 6.63 ± 0.48 | ||
| αPLT-Ig Burn | 9 | 66.10 ± 1.48† | 13.19 ± 1.11‡ | 20.35 ± 1.64† | ||
| 48 h after injury | ||||||
| CTL-Ig Sham | 8 | 86.00 ± 1.51 | 4.43 ± 0.22 | 8.21 ± 1.18 | ||
| CTL-Ig Burn | 7 | 70.86 ± 1.69 | 6.18 ± 0.78 | 22.10 ± 1.93 | ||
| αPLT-Ig Sham | 7 | 88.18 ± 1.42 | 4.85 ± 0.36 | 6.26 ± 1.07 | ||
| αPLT-Ig Burn | 5 | 73.24 ± 1.06 | 13.02 ± 0.78§ | 6.11 ± 0.47‡ | ||
Anticoagulated whole blood from experimental or control mice was analyzed for percent lymphocytes, monocytes, and neutrophils at the times listed. Mice per group per time point are listed. Statistical significance of the data (mean ± SEM) was analyzed using the Mann-Whitney test.
Animals per group from 3 independent experiments.
P < .05 for CTL-Ig Burn compared with αPLT-Ig Burn.
P < .005 for CTL-Ig Burn compared with αPLT-Ig Burn.
P < .001 for CTL-Ig Burn compared with αPLT-Ig Burn.
Plasma cytokine levels in mice following thermal injury
As shown in Table 2, we found significant changes in circulating leukocyte subsets in αPLT-Ig–treated thermal-injured mice that were temporally associated with the increased mortality of these mice between 24 and 48 hours after thermal injury. However, the cause of increased mortality in thermal-injured platelet-deficient mice was not evident. Therefore, it was of interest to determine whether changes in leukocyte subsets were associated with changes in plasma cytokine levels in αPLT-Ig–treated mice after thermal injury. Plasma cytokines were determined in these mice between 24 and 48 hours after injury. Plasma levels of TNFα were significantly higher at 24 and 36 hours in αPLT-Ig–treated mice (P < .001) compared with CTL-Ig–treated mice after thermal injury (Figure 5A). TNFα levels, however, were not significantly different between these groups at the later time point. In contrast, plasma levels of IL-6 were significantly elevated at 24 to 36 hours (P < .001) and at 48 hours (P < .005) in αPLT-Ig–treated mice when compared with CTL-Ig–treated mice (Figure 5B). Lastly, plasma levels of MCP-1 were found to be significantly increased at 24 and 36 hours after thermal injury in αPLT-Ig–treated mice when compared with CTL-Ig–treated mice (P < .05; Figure 5C). IL-12p70, IFNγ, and IL-10 were not detected in the plasma at any time point tested.
Platelets are known to express ligands and receptors for costimulatory molecules. It is also known that platelets, once activated, release cytokines including transforming growth factor beta-1 (TGFβ1).25-28 Since platelets are the predominant source of circulating TGFβ1,28 we were interested to determine whether the protective effect of platelets after injury was mediated in part through TGFβ1. Plasma levels of TGFβ1 were determined after thermal injury in CTL-Ig–treated and αPLT-Ig–treated mice. As shown in Figure 5D, plasma levels of TGFβ1 from αPLT-Ig–treated mice were significantly lower between 24 and 36 hours (P < .001) but not at 48 hours after thermal injury when compared with CTL-Ig–treated mice.
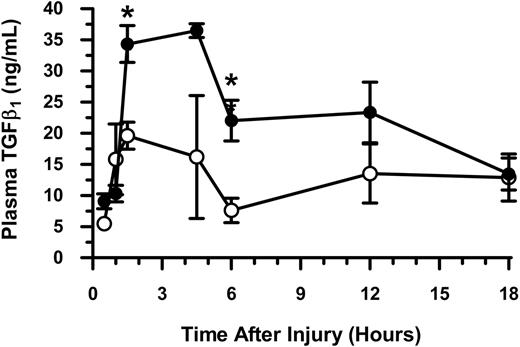
We next wanted to determine more exactly when circulating TGFβ1 levels might be elevated in WT mice after thermal injury. TGFβ1 levels were determined in the plasmas obtained from sham- and thermal-injured WT mice at sequential intervals from 0.5 to 18 hours. As shown in Figure 6, TGFβ1 levels increased significantly by 1.5 hours (P < .05) after thermal injury when compared with sham injury and remained elevated for up to 12 hours after injury. These findings suggest that platelet-derived TGFβ1 may act early to regulate the response to injury. To our surprise, the increase in plasma TGFβ1 levels (Figure 6) directly correlated with the observed decrease in platelet numbers early after injury (Figure 1A), suggesting that the decrease in circulating platelets is reflective of their state of activation (as indicated by the increase in release of TGFβ1).
Plasma cytokine levels following thermal injury in platelet-depleted mice. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TNFα (A), IL-6 (B), MCP-1 (C), and TGFβ1 (D) were determined at the times listed for the groups CTL-Ig Sham (n = 23; open bars), CTL-Ig Burn (n = 29; dark gray bars), αPLT-Ig Sham (n = 23; light gray bars), and PLT-Ig Burn (n = 93; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05; **P < .01; ***P < .005; ****P < .001.
Plasma cytokine levels following thermal injury in platelet-depleted mice. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TNFα (A), IL-6 (B), MCP-1 (C), and TGFβ1 (D) were determined at the times listed for the groups CTL-Ig Sham (n = 23; open bars), CTL-Ig Burn (n = 29; dark gray bars), αPLT-Ig Sham (n = 23; light gray bars), and PLT-Ig Burn (n = 93; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05; **P < .01; ***P < .005; ****P < .001.
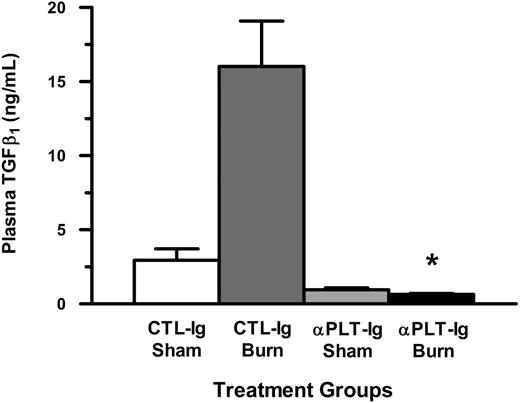
Since TGFβ1 levels peaked at 1.5 hours following injury and correlated with a significant decrease in the number of circulating platelets, we wanted to determine the levels of plasma TGFβ1 at this time point in thermal-injured mice that were platelet deficient. As shown in Figure 7, plasma levels of TGFβ1 were significantly lower in thermal-injured platelet-deficient mice (P < .001) when compared with thermal-injured CTL-Ig–treated mice.
Plasma levels of transforming growth factor beta 1 (TGFβ1) over time in mice following thermal injury. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TGFβ1 were determined at serial times after sham (○) and thermal (•) injury. Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05.
Plasma levels of transforming growth factor beta 1 (TGFβ1) over time in mice following thermal injury. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TGFβ1 were determined at serial times after sham (○) and thermal (•) injury. Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .05.
Discussion
While platelets are traditionally thought to be regulators of hemostasis and coagulation, there is now accumulating evidence that platelets may also be important in the development and progression of inflammatory processes.12,16,17,19,20 Recent studies have demonstrated that platelets participate in the development of both innate and adaptive immune responses.14,15 Thus, it can be perceived that platelets constantly sense and respond to changes in their microenvironment including danger signals such as cytokines or bacterial products released in response to injury or invasion by microorganisms.
The purpose of this study was 2-fold. First, we wanted to determine whether major thermal injury alters circulating platelet abundance. Second, we wanted to determine whether platelets contribute to the host response after injury. Our results show that there is a significant decrease in circulating platelets that leads to a transient thrombocytopenia after thermal injury in the mouse, similar to findings in rats that have undergone sublethal trauma29 and clinical observations in thermal-injured patients.22 Although thermal injury did affect platelet numbers, it was still unclear whether platelets contributed to the host immune response after injury. In addition, it was found that severe injury results in platelet activation and alterations in platelet function. Here, mortality was found to be associated with a decreased platelet function.6 We report here for the first time the use of a novel mouse model of platelet deficiency to more clearly define the role of platelets in the injury response. As demonstrated in our survival studies, thermal injury led to a significantly decreased survival in mice that were made platelet deficient. Our observations of normal hematocrit, no abnormal necropsy findings, and normal histology strongly suggest that mortality in most of the platelet-depleted mice did not occur as a consequence of hemorrhage or hypovolemic shock and that a reduction in circulating platelet numbers significantly influenced survival following thermal injury by other mechanisms. Hence, our findings are consistent with the clinical observations that a deficiency in platelets combined with severe trauma correlates with increased mortality and with the observation that burn patients who undergo episodes of thrombocytopenia have a poor outcome.21,22 Our findings, together with the published clinical observations, suggest that platelets are important in maintaining immune homeostasis after a major injury. Furthermore, the observed thrombocytopenia early after injury may occur as a direct consequence of platelet activation and generation of platelet-leukocyte aggregates and of platelet-derived microparticles. Both of these phenomena have been described in patients after severe trauma and sepsis.21,22,30,31 Our observation of thrombocytopenia in thermal-injured mice may be an important feature of the host response to injury. Our findings, together with published clinical observations, suggest that platelet transfusion and/or immunotherapy with cytokines such as TGFβ1 may be beneficial for the survival of severely injured patients that develop thrombocytopenia. One role for activated platelets may be to tether leukocytes and facilitate their diapedesis across the vasculature. Alternatively, platelet-derived microparticles may shuttle platelet membrane–bound molecules such as cytokines, cytokine receptors, costimulatory molecule ligands, or receptors to the site of injury. Additional experiments will be required to address what mechanisms are responsible for the loss of platelets after injury.
Little information is available on the hematologic and immune alterations that accompany injury-induced thrombocytopenia. In order to investigate these questions, both leukocyte numbers and cytokine levels were evaluated in our platelet-depleted experimental mice. We found that the overall leukocyte and lymphocyte numbers were increased at 24 hours after thermal injury in these mice. Upon closer analysis of the leukocyte subsets, we found that the percentage of circulating monocytes increased significantly at the 36- and 48-hour time points, with only a transient increase in neutrophils in thermal-injured platelet-deficient mice. It is possible that these increased percentages of circulating monocytes and, to a lesser extent, neutrophils may occur as a result of reduced tissue extravasation as a consequence of significantly reduced levels of circulating activated platelets, platelet-derived microparticles, platelet-derived TGFβ1, or reduced platelet CD154 interaction with monocyte CD40. Both TGFβ1 and CD40 activation have been shown to increase monocyte differentiation and maturation; however, in the absence of TGFβ1 alone, as demonstrated in TGFβ1 null mice, there are increased numbers of circulating monocytes and neutrophils.7,32-34 This notion is supported by our findings that circulating TGFβ1 levels are significantly decreased in platelet-deficient mice that had undergone thermal injury. Thus, platelets and platelet-associated cytokines such as TGFβ1 appear to be protective to the host after serious injury and together may function to regulate the tempo and extent of the systemic inflammatory response.
In our studies, we found that thermal injury of platelet-deficient mice led to increased mortality. Although the mechanisms responsible for this outcome were unknown initially, hemorrhage was excluded as cause of death, since necropsy and histology findings were unremarkable and hematocrit values were normal in these mice. Indeed, there have been no reports of platelet depletion in mice with antibodies resulting in mortality due to low platelet counts. Depletion of platelets in mice using polyclonal antibodies or monoclonal antibody cocktails has been used to study the role of platelets in wound healing and the role of platelet caspases in the development of cerebral malaria.3,35,36 However, one study clearly demonstrated that low platelet counts after treatment with low doses of polyclonal antibodies did not cause hemorrhage.37
Plasma levels of transforming growth factor beta 1 (TGFβ1) early following thermal injury in platelet-deficient mice. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TGFβ1 were determined at 1.5 hours following thermal injury for the groups CTL-Ig Sham (n = 8; open bars), CTL-Ig Burn (n = 12; dark gray bars), αPLT-Ig Sham (n = 8; light gray bars), and PLT-Ig Burn (n = 12; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .001.
Plasma levels of transforming growth factor beta 1 (TGFβ1) early following thermal injury in platelet-deficient mice. Anticoagulated whole blood was collected by cardiac puncture and plasma was prepared. Circulating levels of plasma TGFβ1 were determined at 1.5 hours following thermal injury for the groups CTL-Ig Sham (n = 8; open bars), CTL-Ig Burn (n = 12; dark gray bars), αPLT-Ig Sham (n = 8; light gray bars), and PLT-Ig Burn (n = 12; filled bars). Data (mean ± SEM) were analyzed using the Mann-Whitney test. *P < .001.
Thermal injury of platelet-deficient mice led to an exaggerated systemic inflammatory response culminating with increased mortality and was characterized by excessive and prolonged release of IL-6 and MCP-1 and by a marked increase in TNFα. These increases in plasma cytokines may have occurred as a result of increased production by both immune and nonimmune tissues possibly as a consequence of reduced levels of platelet-derived TGFβ1. This idea is supported by the reported evidence that proinflammatory cytokines including IL-1, TNFα, IL-6, and MCP-1 production are regulated by TGFβ1. Moreover, IL-6 has been demonstrated to upregulate MCP-1 production.38-42 Indeed, we clearly show that TGFβ1 levels are significantly decreased for up to 36 hours after thermal injury of platelet-deficient compared with control mice. Our present findings correlate well with those of Yeh et al,43 who demonstrated that there was an increase in mortality due to sepsis in burn patients that had deficient levels of serum TGFβ1 and enhanced levels of IL-6. In contrasting our finding of drastically decreased TGFβ1 after thermal injury in platelet-deficient mice, we also showed that TGFβ1 levels were significantly increased early, from 1.5 to 12 hours after injury in WT mice. This sustained increase in TGFβ1 levels during this early time period may be critical in defining the extent of the systemic response to injury. This is further supported by clinical findings in burn patients.43 Curiously, we also found that this early increase in TGFβ1 parallels the observed transient injury-induced thrombocytopenia. Thus, release of platelet-derived proteins including TGFβ1 and the development of a transient thrombocytopenia may be reflective of platelet activation. Together, these findings further support published results indicating that platelets are a significant source of this cytokine. Indeed, Grainger et al28 have shown that while human platelets possess only about 4000 TGFβ1 molecules/cell, they account for the majority of the circulating TGFβ1 (45 ng/mL). This contrasts with mononuclear cells and granulocytes, which contain 12 000 and 6000 molecules/cell, respectively, and yet only account for 2.7 ng/mL of the circulating TGFβ1.28 Thus, our observed injury-induced thrombocytopenia may be a reflection of platelet activation and TGFβ1 release. Our group and others have reported that injury induces an early state of immune suppression that remits over time.24,44-52 Our findings suggest that this may relate to the early systemic increase in TGFβ1. It is also plausible that increased TGFβ1 may trigger the activation or function of regulatory T cells.44 Thus, in the absence of adequate levels of circulating TGFβ1, injury-induced immune activation may become exacerbated, resulting in increased mortality. Future studies will be necessary to further understand the role of platelets and platelet-derived products in modulating the host response to injury.
A role for platelets in the host response to injury may occur initially through platelet activation, which is followed by release of TGFβ1 and other immunologically important molecules into the circulation. In addition, platelet activation may lead to the generation of platelet-derived microparticles and platelet-derived molecules. The combination of these factors may be protective to the host by regulating the degree and extent of the host inflammatory response. It is clear that future studies are needed to address the expression of these molecules in addition to conventional platelet products in the postinjury period.
In summary, we describe a novel mouse model of platelet deficiency to study the host immune response to injury. We also demonstrate that platelets may be a critical component of this response. Finally, we clearly demonstrate that TGFβ1 is one important molecule that platelets may employ to modulate the injury response. However, the mechanisms by which platelets or their products mediate an immune-modulating function require further characterization.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-09-3776.
Supported by grants from the National Institutes of Health (GM062119-03, J.A.M.; and GM05766406, J.A.L.), the Julian and Eunice Cohen Laboratory for Surgical Research, and the Surgical Infection Society Junior Faculty Research Fellowship (P.H.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Adam Delisle for his excellent technical support.

![Figure 1. Platelet numbers decrease following thermal injury. Groups of mice were either sham treated (n = 4, ○) or thermal injured (n = 8, •). At each time point tested, mice were killed, whole blood was removed by cardiac puncture, and platelet numbers were determined (A). Normal range for platelet numbers is 592 × 109/L (592 × 103/μL) to 2972 × 109/L (2972 × 103/μL), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Values below the dotted line represent thrombocytopenia. Hematocrits were determined from the same blood sample at each time point (B). The gray vertical bar represents the normal limits for hematocrit (.35-.45 [35.1%-45.4%]), as outlined by the data analysis printout for the Hemavet 850 from Drew Scientific. Data are representative of 2 independent experiments (mean ± SEM). Data were analyzed using the Mann-Whitney test. Differences were considered significant when *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/11/10.1182_blood-2005-09-3776/4/m_zh80110696410001.jpeg?Expires=1765052361&Signature=HbzLPJDDCpFGzx5zPKkgLvgRTjIseK244E9RG0i0-g9YstByOcUPsBbYDwiS6f6AMg0BYBh~ILTC6Kw7ugpjXtimx7Yw1bFHjlNBm4Jnx-M3hRZXmS5VQ~j9JNw8TcjeRzaHIYD2gMiPA2lKA336zlY3ips97AAA-C~R9~-6q6shM~ayCplxk2pdtc1jfVIp-ZOpFGex-0NZSqnneiSCQjOaTT033pEhPfHLBh~PGPCRx6Zt2rVzvd3cZeezqPeJTM2d2P6-ZriGWTTwsYW1Of5CU~lDN2Py2nyaCZvSvkGHIFnRaNKPUX7FN1SGjXoVuu7u0KGl96qbwwjVzZafqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal