Abstract
Fibrillar collagens are among the most potent activators of platelets and play an important role in the initiation of thrombosis. The glycoprotein VI (GPVI)/FcRγ-chain complex is a central collagen receptor and inhibitors of GPVI produce a major defect in arterial thrombogenesis. In this study we have examined arterial thrombus formation in mice lacking the GPVI/FcRγ-chain complex (FcRγ–/–). Using 3 distinct arterial thrombosis models involving deep vascular injury, we demonstrate that deficiency of GPVI/FcRγ is not associated with a major defect in arterial thrombus formation. In contrast, with milder vascular injury deficiency of GPVI/FcRγ was associated with a 30% reduction in thrombus growth. Analysis of FcRγ–/– platelets in vitro, using thrombin-dependent and -independent thrombosis models, demonstrated a major role for thrombin in overcoming the thrombosis defect associated with GPVI/FcRγ deficiency. Inhibition of thrombin in vivo produced a much greater defect in thrombus formation in mice lacking GPVI/FcRγ compared with normal controls. Similarly, thrombin inhibition produced a marked prolongation in bleeding time in FcRγ–/– mice relative to wild-type mice. Our studies define an important role for thrombin in overcoming the hemostatic and thrombotic defect associated with GPVI/FcRγ deficiency. Moreover, they raise the interesting possibility that the full antithrombotic potential of GPVI receptor antagonists may only be realized through the concurrent administration of anticoagulant agents.
Introduction
Platelet adhesion and aggregation at sites of vascular injury is essential for the cessation of bleeding; however, excessive accumulation of platelets at sites of atherosclerotic plaque rupture can result in arterial thrombotic occlusion, leading to acute myocardial infarction and ischemic stroke.1 A major factor contributing to the heightened thrombogenic potential of atherosclerotic lesions is the high content of type I and III fibrillar collagens in advanced plaques.2 These substrates are among the most potent activators of platelets and are thought to play a major role in promoting platelet thrombus formation in vivo. Recently, there has been increased focus on collagen receptors as potential antithrombotic targets, due in part to the recognized importance of collagens in atherothrombosis,3 the demonstration that inhibitors targeting collagen receptors can produce an antithrombotic effect in vivo,4-7 and the finding that collagen receptor deficiency leads to a relatively mild bleeding tendency.8
Considerable progress has been made over the last few years in elucidating the relative roles of 2 major collagen receptors, glycoprotein VI (GPVI) and integrin α2β1, in promoting platelet adhesion and thrombus growth.9 There is compelling evidence that GPVI is of central importance for collagen activation of platelets, with deficiency of GPVI leading to defective platelet adhesion and thrombus growth.4,10 The importance of integrin α2β1 remains controversial, although recent studies have suggested that it also plays a key role in promoting primary platelet adhesion on collagen, but is less important for thrombus growth.11 GPVI is a member of the immunoglobulin superfamily of cell-surface receptors and is noncovalently complexed with the FcRγ-chain. The FcRγ-chain is the critical signaling element of the GPVI/FcRγ complex and is essential for GPVI expression on the surface of human and murine platelets.12-14 Several recent studies have demonstrated an indispensable role for the GPVI/FcRγ complex in promoting thrombus formation in vivo, with defects in thrombus development of similar magnitude to that reported for inhibitors against GPIb/V/IX and integrin αIIbβ3.5-7 This, combined with the demonstration that inhibitors of GPVI produce a relative minor defect in hemostasis, has raised the possibility that GPVI receptor antagonists may have a relatively wide therapeutic window relative to other antiadhesive agents.4,10
To investigate the importance of the GPVI/FcRγ-chain complex in arterial thrombogenesis we have examined thrombus formation in 3 distinct in vivo thrombosis models using FcRγ-deficient (FcRγ–/–) mice. The results of these experiments were compared against integrin αIIbβ3 and P2Y12 receptor antagonists and demonstrated an unexpected minor role for GPVI/FcRγ in promoting arterial thrombosis. Our studies demonstrate that the hemostatic and thrombosis defect associated with GPVI/FcRγ-chain is primarily manifest under experimental conditions limiting thrombin generation. These studies demonstrate an important role for thrombin in overcoming the thrombosis defect associated with GPVI/FcRγ deficiency and raise the possibility that the full antithrombotic potential of GPVI receptor antagonists may only be realized with coadministration of anticoagulant agents.
Materials and methods
Mouse strains
FcRγ-deficient mice were provided by Prof Takashi Saito (Department of Molecular Genetics, Graduate School of Medicine, Chiba, Japan).15
Static calcium assays and flow-based adhesion assays
Washed platelets16 loaded with calcium indicator dyes17 and 2.5 mM Probenecid (Sigma-Aldrich, Castle Hill, Australia) were applied to fibrillar type I collagen–coated coverslips (2.5 mg/mL) for up to 30 minutes at 37°C. Real-time platelet calcium flux was monitored using confocal microscopy and analyzed offline to obtain single-cell calcium tracings, while the number of platelets exhibiting oscillatory calcium flux was calculated as a percentage of total adherent platelets. Adherent platelets mounted on coverslips were then fixed with 2% glutaraldehyde in 100 mM Na2HPO4/NaH2PO4, pH 7.4, for 60 minutes, then incubated with 1% OsO4 in 100 mM Na2HPO4/NaH2PO4, pH 7.4 for 30 minutes. The fixed platelets were dehydrated by successive immersions in increasing concentrations of ethanol followed by critical-point drying. The coverslips on which the fixed platelets were attached were mounted on scanning electron microscopy18 stubs and coated with gold prior to imaging using a Hitachi 5570 scanning electron microscope. The number of spread platelets was quantified as a percentage of total adherent platelets. For flow-based assays,17 anticoagulated whole blood (100 μg/mL lepirudin) or blood collected into 40 μg/mL Factor XIIa (FXIIa) inhibitor (corn trypsin inhibitor; Merck, Kilsyth, Australia) was labeled with DiOC6 (1 μM) and perfused through fibrillar type I collagen–coated microcapillary tubes (2.5 mg/mL) at 500 s–1 for 3 minutes. Platelets and thrombi were observed using an inverted Leica DMIRB microscope (Leica Microsystems, Wetzlar, Germany) and a 40 × 0.55 numeric aperture (NA) water objective. Images were acquired using a Dage-MTI charge-coupled device (CCD) camera 300 ETRCX (Dage-MTI, Michigan City, IN) and analyzed using MCID-M4 software version 3.0 (Imaging Research, St Catharines, ON, Canada). The total number of platelets tethering to the matrix for every 1 × 107 platelets perfused was quantified, and the number of platelets exhibiting stable platelet adhesion (movement no greater than 1 cell diameter within 10 seconds) were quantified and expressed as a percentage of total adherent platelets. Following the experiment, the formed thrombi were fixed with 2% PFA for 15 minutes. The thrombi were imaged with a Zeiss confocal microscope Meta 510 (Zeiss, Oberkochen, Germany). The thrombus volumes per field were calculated in number of pixels with the Software Image J 1.33u (National Institutes of Health, Bethesda, MD).
In vivo thrombosis models
Electrolytic model. A modified version of an electrolytic injury model19 was performed on 5 wild-type (C57Bl/6) and 5 FcRγ-chain–deficient (FcRγ–/–) mice.20 Animals were anesthetized with an intraperitoneal injection of sodium pentobarbitone (30 mg/kg; Sigma-Aldrich) and body temperature maintained at 37°C throughout the experiment via a rectal temperature probe connected to a thermoblanket (Harvard Apparatus, Kent, United Kingdom). A tracheotomy was performed and mice were mechanically ventilated with room air supplemented with O2 via a respiratory pump (MiniVent Type 845; Harvard Apparatus, Hugstetten, Germany). A Doppler flow probe (0.8-mm internal diameter [id]; selected for its smaller overall size compared with the flow probe used for the Folts model) attached to a directional pulsed Doppler flow meter (model 545C-3; Bioengineering, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City) was placed around both isolated carotid arteries to measure blood flow in units of kilohertz Doppler shift. The blood flow signal from the right carotid artery was used to calculate the heart rate (beats per minute), while a platinum hook electrode placed distal to the flow probe on the left carotid artery was used to induce vascular injury. Blood stasis was created by gently clamping the left artery, then vascular injury was induced by delivering an electrical current of 4 mA for 1.25 minutes using a constant current unit (Model CCU1; Grass, Quincy, MA) connected to a Grass SD9 stimulator. Blood flow was allowed to resume immediately after injury and the formation of occlusive thrombi in the carotid artery was indicated by a decrease in blood flow to zero. Changes in blood flow were monitored for 30 minutes and recorded for offline analysis (PowerLab Chart 4.2 software; ADInstruments, Sydney, Australia). The total amount of blood flowing through the injured artery following vascular injury was determined by calculating the area under the blood flow curve.
Folts-like stenosis-injury model. A modified version of the Folts model21 was performed on 9 wild-type (C57Bl/6) and 8 FcRγ–/– mice.20 Animals were anesthetized with an intraperitoneal injection of sodium pentobarbitone (30 mg/kg; Sigma-Aldrich) and placed on a heating pad for the duration of the experiment. A flow probe (0.5-mm id; Transonic Systems, Ithaca NY), connected to a blood flowmeter (T206 Flowmeter; Transonic Systems), was gently placed around the isolated left carotid artery prior to placing a silk suture distal to the flow probe. The suture was tightened gradually until blood flow was decreased by 50% and vascular injury was created by crushing the carotid artery with artery forceps directly over the suture. The formation of occlusive thrombi in the carotid artery was indicated by a decrease in blood flow to zero. This model of arterial thrombosis characteristically produced occlusive thrombi within 2 to 3 minutes of vascular injury. We typically found that 1 set of injuries (5 crushes) would produce sufficient injury to induce occlusive thrombus formation. However, on some occasions several sets of repetitive injuries were required to induce occlusive thrombus formation, presumably due to the cushioning effects of the suture on the vessel wall. Further characterization of this model demonstrated that occlusive thrombus formation was unable to occur in nonstenosed carotid arteries or arteries stenosed by less than 70% (data not shown).
Laser-injury model. A laser-induced injury model was performed on 13 wild-type (C57Bl/6) and 14 FcRγ–/– mice aged between 3 and 5 weeks of age. Animals were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg), and administered a fluorescent dye, DiOC6 (5 μL of a 100 μM solution/g of body weight), to assist thrombus visualization. The mesenteric bowel was gently exteriorized and localized injury of the luminal surface of arterioles induced using a pulsed nitrogen dye laser (440 nm, Micropoint Laser System; Photonics Instruments, St Charles, IL22 ). Severe and moderate injuries have been induced by 2 different laser intensity as previously described.23 Thrombi were observed using an inverted Leica DMIRB microscope (Leica Microsystems). Images acquired by a Cooke SensiCam (Auburn Hills, MI) charge-coupled device (CCD) camera (2 × 2 binning) and analyzed using Slidebook software (Intelligent Imaging Innovations, Denver, CO).
Histology
Carotid arteries were harvested and fixed with 4% paraformaldehyde for more than 48 hours prior to alcohol and xylene processing, then embedded in paraffin. Serial sections (5 μm thick) were cut and stained using Carstair stain24,25 to produce differential staining of platelets (gray-blue to navy), fibrin (bright red), collagen (bright blue), and red blood cells (yellow to orange). Images were visualized using an Olympus BH2-RFCA microscope (Olympus, Tokyo, Japan) and a 20 × /0.46 NA objective. Images were captured using an Olympus DP50-CU 5.0V/2.0A camera and Viewfinder Lite software version 1.0.
Tail bleeding times
Template bleeding time21 (1-mm deep × 5-mm long) was performed on the tails of mice. Tail bleeding time was measured before (control) and 5 minutes after drug administration. The left jugular vein was exposed via blunt dissection to allow for direct injection of drugs using a 30-gauge needle (bolus size approximately 50 μL). An incision 5-mm long and 1-mm deep was made 1 cm from the tail tip using a scalpel blade (taped off 1 mm from the end). The edge of the incision was blotted every 30 seconds until blood flow had ceased. The second incision was made 5 mm distal to the first incision.
Statistical analysis
Data are presented as means ± SEM. Average SEM for carotid blood flow over time was calculated from repeated measures analysis of variance (ANOVA) using the pooled estimate of error from the residual mean square as (error mean square/number of animals) (0.5) after subtracting the sums of the squares for each time. This error bar is shown in Figure 2A. When comparing matched values within or between 2 treatment groups, paired or unpaired Student t tests were used, respectively. The statistical significance of differences between thrombus surface area means was evaluated using a nonparametric Mann-Whitney test and P values of less than .05 were considered to be significant. All tests were performed using Prism software (GraphPad Software, San Diego, CA).
Results
FcRγ/GPVI deficiency does not prevent occlusive thrombus formation in a “Folts-type” arterial thrombosis model
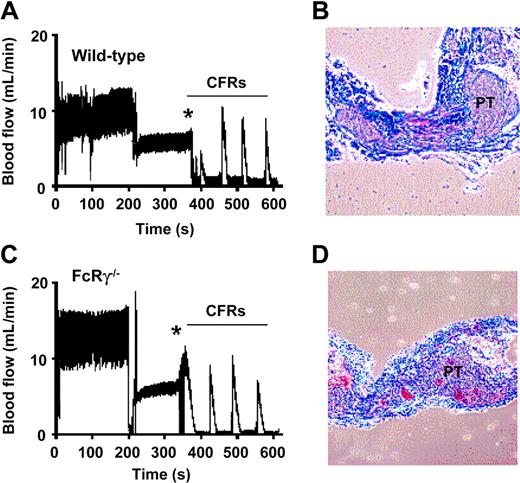
To investigate the importance of the GPVI/FcRγ-chain complex in promoting occlusive thrombus formation in stenosed-injured arteries, we examined FcRγ–/– mice in a modified “Folts-type” carotid artery thrombosis model.19 The Folts model has been extensively used in larger animals and involves repetitive crush injury to areas of arterial stenosis, resulting in platelet exposure to subendothelial thrombogenic components and high shear stress, 2 key factors promoting thrombus growth.20 Development of occlusive thrombi in mice typically occurred within 2 to 3 minutes of vascular injury, and mechanical agitation of the vessel resulted in the embolisation of thrombi and restoration of normal blood flow. Constant formation and dislodgement of thrombi resulted in cyclic flow reductions (CFRs), a characteristic feature of the Folts model. Analysis of wild-type mice revealed that 89% (n = 9) developed continuous CFRs following vascular injury (Figure 1A). Histologic analysis of occluded arteries revealed that the thrombi were principally composed of platelets (Figure 1B) and administration of a mouse integrin αIIbβ3 antagonist GPI562 (4 mg/kg intravenously) completely eliminated CFRs in all treated mice (n = 4, data not shown). Histologic analysis of carotid arteries from GPI562-treated mice confirmed complete absence of thrombi at the site of vessel injury (data not shown). Unexpectedly, vascular injury to the carotid artery in FcRγ–/– mice led to the formation of CFRs in 87% of mice (n = 8) (Figure 1C) and the rate and extent of vessel occlusion in FcRγ–/– mice was no different from matched wild-type controls (data not shown). Histologic analysis also confirmed that there was no difference in thrombus composition between the 2 groups (Figure 1B,D). These findings suggest that the GPVI/FcRγ-chain complex is not essential for arterial thrombotic occlusion following injury to stenosed arteries.
Blockade of integrin αIIbβ3 and P2Y12, but not FcRγ deficiency, prevented occlusive arterial thrombus formation using an electrolytic injury model
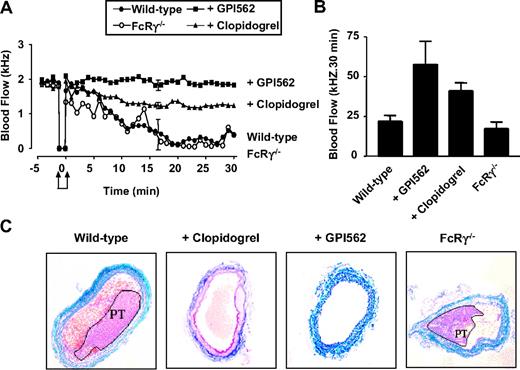
Our findings with the “Folts-type” model contrasted with previous studies demonstrating a central role for GPVI in promoting arterial thrombus formation.4-7 These previous studies have used a variety of experimental mouse thrombosis models, none of which involved fixed arterial stenosis and deep arterial injury, raising the possibility that our findings were specific to the “Folts-type” model. To investigate this possibility we used a second mouse carotid artery thrombosis model that involved electrolytic injury to nonstenosed carotid arteries.19 Electrolytic injury leads to full thickness vascular injury, triggering the formation of platelet-rich thrombi (confirmed by histology) that ultimately occlude the artery within 15 to 20 minutes. The thrombi that formed in this model were typically unstable and prone to spontaneous embolization, leading to variable periods of arterial occlusion. The importance of platelets in this model was confirmed by the ability of the integrin αIIbβ3 inhibitor, GPI562 (4 mg/kg intravenously), to preserve normal carotid artery blood flow following electrical injury (Figure 2A-B). Analysis of FcRγ–/– mice in this model revealed no difference in the rate of occlusive thrombus formation than that observed in matched FcRγ+/+ mice (Figure 2A-B). Analysis of total carotid artery blood flow over a 30-minute period following electrical injury revealed no significant differences between wild-type and FcRγ–/– mice (Figure 2B; 22.0 ± 3.6 kHz and 19.9 ± 4.8 kHz, n = 6). Furthermore, examination of occluded arteries from FcRγ–/– mice demonstrated no difference in thrombus composition compared with wild-type matched controls (Figure 2C). In contrast, pretreating mice with the P2Y12 receptor antagonist clopidogrel (50 mg/kg) maintained carotid blood flow throughout the 30-minute observation period (Figure 2A-B). Histologic analysis revealed that clopidogrel prevented formation of occlusive thrombi, although less effectively as the αIIbβ3 antagonist (Figure 2C). These findings are consistent with the results from the “Folts-type” model, demonstrating a dispensable role for the GPVI/FcRγ-chain complex in arterial thrombogenesis. Moreover, they suggest that the defect in thrombus formation in GPVI/FcRγ-chain–deficient platelets is significantly less than that observed with integrin αIIbβ3 and P2Y12 receptor antagonists.
Effect of FcRγ deficiency on occlusive thrombus formation in a “Folts-type” arterial thrombosis model. (A,C) Repetitive crush injury to stenosed carotid arteries was used to induce occlusive thrombus formation in wild-type and FcRγ–/– mice as described in “Materials and methods.” Vascular injury (indicated by *) led to the formation of repetitive cyclic flow reductions (CFRs) in both the wild-type and FcRγ–/– mice. The blood flow traces are from single-mouse experiments representative of 9 and 8 independent wild-type and FcRγ–/– experiments, respectively. (B,D) Histologic analysis of longitudinally sectioned carotid arteries from wild-type mice and FcRγ–/– mice demonstrating the presence of vaso-occlusive platelet-rich thrombi (PT) in the arterial lumen following the development of CFRs.
Effect of FcRγ deficiency on occlusive thrombus formation in a “Folts-type” arterial thrombosis model. (A,C) Repetitive crush injury to stenosed carotid arteries was used to induce occlusive thrombus formation in wild-type and FcRγ–/– mice as described in “Materials and methods.” Vascular injury (indicated by *) led to the formation of repetitive cyclic flow reductions (CFRs) in both the wild-type and FcRγ–/– mice. The blood flow traces are from single-mouse experiments representative of 9 and 8 independent wild-type and FcRγ–/– experiments, respectively. (B,D) Histologic analysis of longitudinally sectioned carotid arteries from wild-type mice and FcRγ–/– mice demonstrating the presence of vaso-occlusive platelet-rich thrombi (PT) in the arterial lumen following the development of CFRs.
Effect of FcRγ deficiency and integrin αIIbβ3 and P2Y12 inhibition on occlusive arterial thrombus formation following electrolytic injury. (A) Electrolytic injury was induced in the carotid artery of FcRγ-deficient mice (FcRγ–/–, n = 5) or wild-type mice treated with vehicle alone (wild-type, n = 5), GPI562 (5 mg/kg) (+ GPI562, n = 5) or clopidogrel (50 mg/kg) (+ clopidogrel, n = 4) as described in “Materials and methods.” Carotid artery blood flow was monitored over a 30-minute time period following arterial injury (indicated by arrows). Error bars are average SEM from repeated measures ANOVA. (B) Total blood flow over a 30-minute period. Error bars are ± 1 SEM. (C) Histologic analysis of carotid arteries from FcRγ–/– or wild-type mice treated with vehicle alone, GPI562, or clopidogrel. Tissue samples were stained with Castair stain in which platelets stain purple, fibrin stain red, red blood cells stain yellow, and collagen stains blue. Platelet-rich thrombi (PT) are highlighted in wild-type control and FcRγ–/– mice.
Effect of FcRγ deficiency and integrin αIIbβ3 and P2Y12 inhibition on occlusive arterial thrombus formation following electrolytic injury. (A) Electrolytic injury was induced in the carotid artery of FcRγ-deficient mice (FcRγ–/–, n = 5) or wild-type mice treated with vehicle alone (wild-type, n = 5), GPI562 (5 mg/kg) (+ GPI562, n = 5) or clopidogrel (50 mg/kg) (+ clopidogrel, n = 4) as described in “Materials and methods.” Carotid artery blood flow was monitored over a 30-minute time period following arterial injury (indicated by arrows). Error bars are average SEM from repeated measures ANOVA. (B) Total blood flow over a 30-minute period. Error bars are ± 1 SEM. (C) Histologic analysis of carotid arteries from FcRγ–/– or wild-type mice treated with vehicle alone, GPI562, or clopidogrel. Tissue samples were stained with Castair stain in which platelets stain purple, fibrin stain red, red blood cells stain yellow, and collagen stains blue. Platelet-rich thrombi (PT) are highlighted in wild-type control and FcRγ–/– mice.
FcRγ/GPVI is involved in thrombosis using a laser-injury model
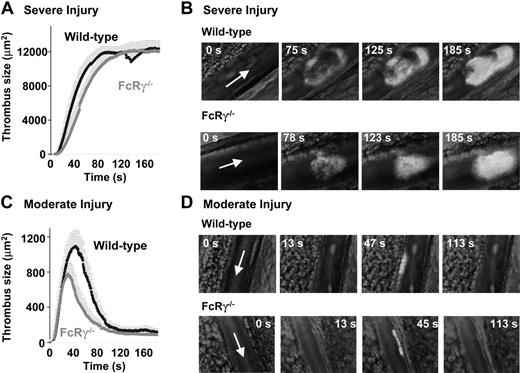
A limitation of the “Folts-type” and electrolytic models was the inability to directly visualize the kinetics of thrombus growth. It is possible that significant differences in the initial rate of thrombus formation, due to defects in the activation of primary adherent platelets, would not be detected by gross alterations in blood flow. To investigate in more detail the kinetics of thrombus formation in wild-type and FcRγ–/– mice we used intravital microscopy to visualize thrombus development in mouse mesenteric arterioles following laser-induced vascular injury.22 As demonstrated in Figure 3A-B, severe vessel injury resulted in the formation of stable, occlusive thrombi (maximum mean surface area of 12 007.5 μm2) over a 90-second period in wild-type mice. Pretreating mice with the αIIbβ3 antagonist integrilin (10 mg/kg intravenously) or clopidogrel (50 mg/kg) prevented vascular occlusion in this model, confirming the central role of platelets in this process.22 Unexpectedly, laser injury of FcRγ–/– arterioles resulted in a similar rate and extent of thrombus formation as the wild-type controls (Figure 3A-B: FcRγ–/–; mean surface area of 12 220.8 μm2, P = .402), and the thrombi that formed under these experimental conditions remained stable over a 3-minute observation period. To investigate whether the GPVI/FcRγ-chain complex played a more important role in promoting formation in nonocclusive thrombi, we examined the kinetics of thrombus development under conditions of less severe vascular injury. As demonstrated in Figure 3C-D, weaker laser intensities induced arteriole injuries, resulting in the formation of transient nonocclusive arterial thrombi. These thrombi were principally composed of platelets, as they were abolished by pretreating mice with the integrin αIIbβ3 antagonist or with clopidogrel.22 Comparative analysis of wild-type and FcRγ–/– mice revealed a 28.7% reduction in the size of thrombi formed in FcRγ–/– mice (maximum thrombus size of 1096 μm2 and 782 μm2 for wild-type and FcRγ–/– mice, respectively, nonsignificant P = .578). Overall, our studies using 3 distinct thrombosis models do not support an essential role for the GPVI/FcRγ-chain complex in arterial thrombogenesis.
Effect of FcRγ deficiency on thrombus formation in a laser-induced arterial thrombosis model. Severe (A-B) or moderate (C-D) laser-induced lesions were generated in the mesenteric arteries of wild-type and FcRγ–/– mice and the surface area of developing thrombi assessed as described previously.22 (A,C) Traces represent the mean surface area (± SEM) of the thrombi developing over 150 seconds (WT: n = 12 vessels in 8 mice; FcRγ–/–: n = 12 vessels in 7 mice). Analysis of mean surface area of thrombi formed in severely injured vessels indicate no significant difference in thrombus formation between wild-type and FcRγ–/– mice (P > .05). In contrast, following moderate injury there was a 30% reduction in the size of thrombi formed in FcRγ–/– mice relative to wild-type mice (WT: n = 19 vessels in 7 mice; FcRγ–/–: n = 29 vessels in 9 mice). Time sequence images of platelet thrombi forming in wild-type and FcRγ–/– mice subjected to severe (B) or moderate (D) injury. Arrow indicates blood flow direction.
Effect of FcRγ deficiency on thrombus formation in a laser-induced arterial thrombosis model. Severe (A-B) or moderate (C-D) laser-induced lesions were generated in the mesenteric arteries of wild-type and FcRγ–/– mice and the surface area of developing thrombi assessed as described previously.22 (A,C) Traces represent the mean surface area (± SEM) of the thrombi developing over 150 seconds (WT: n = 12 vessels in 8 mice; FcRγ–/–: n = 12 vessels in 7 mice). Analysis of mean surface area of thrombi formed in severely injured vessels indicate no significant difference in thrombus formation between wild-type and FcRγ–/– mice (P > .05). In contrast, following moderate injury there was a 30% reduction in the size of thrombi formed in FcRγ–/– mice relative to wild-type mice (WT: n = 19 vessels in 7 mice; FcRγ–/–: n = 29 vessels in 9 mice). Time sequence images of platelet thrombi forming in wild-type and FcRγ–/– mice subjected to severe (B) or moderate (D) injury. Arrow indicates blood flow direction.
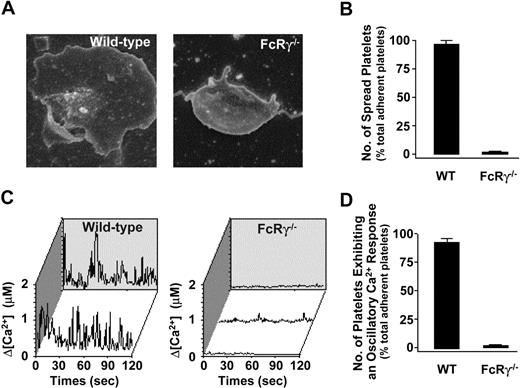
Spreading and calcium flux of FcRγ–/– mouse platelets on a collagen matrix. (A) Representative SEM images of collagen-adherent platelets from wild-type and FcRγ–/– mice. (B) The percentage of adherent platelets (mean ± SEM) that spread on the collagen matrix was quantified as described in “Materials and methods.” (C) Calcium dye-loaded washed platelets were applied to a type 1 fibrillar collagen matrix under static conditions for 30 minutes at 37°C. Individual wild-type platelets underwent an oscillatory Ca2+ response following adhesion to the collagen matrix, whereas FcRγ–/– platelets did not exhibit an oscillatory Ca2+ response. Calcium profiles are from representative single platelets obtained from wild-type or FcRγ–/– mice. (D) The percentage of adherent platelets (mean ± SEM) exhibiting oscillatory calcium flux was quantitated as described in “Materials and methods.”
Spreading and calcium flux of FcRγ–/– mouse platelets on a collagen matrix. (A) Representative SEM images of collagen-adherent platelets from wild-type and FcRγ–/– mice. (B) The percentage of adherent platelets (mean ± SEM) that spread on the collagen matrix was quantified as described in “Materials and methods.” (C) Calcium dye-loaded washed platelets were applied to a type 1 fibrillar collagen matrix under static conditions for 30 minutes at 37°C. Individual wild-type platelets underwent an oscillatory Ca2+ response following adhesion to the collagen matrix, whereas FcRγ–/– platelets did not exhibit an oscillatory Ca2+ response. Calcium profiles are from representative single platelets obtained from wild-type or FcRγ–/– mice. (D) The percentage of adherent platelets (mean ± SEM) exhibiting oscillatory calcium flux was quantitated as described in “Materials and methods.”
FcRγ/GPVI deficiency prevents platelet activation under flow and static conditions
Our results were unexpected given the known importance of collagen fibers in promoting arterial thrombosis and the central role played by the GPVI/FcRγ-chain complex in collagen activation of platelets. To investigate the possibility that our FcRγ–/– mice may have retained some residual responsiveness to collagen, potentially through integrin α2β1, we performed a series of static and flow-based adhesion assays on a type I fibrillar collagen matrix. In contrast to wild-type platelets, FcRγ-deficient platelets were unable to undergo shape change or spread on the collagen substrate under static conditions (Figure 4A-B). Moreover, these platelets did not exhibit a detectable cytosolic calcium response (Figure 4C-D). Similarly, under flow conditions, FcRγ–/– platelets in anticoagulated whole blood were unable to form thrombi under a variety of different flow conditions (500-1800 s–1) (Figure 5A). Notably, these platelets tethered normally to the collagen surface and underwent surface translocation; however, the major defect in these platelets was their inability to form sustained adhesion contacts (Figure 5A-B). These studies confirm previous findings that the platelets from FcRγ–/– mice are unable to become activated on a collagen substrate, independent of the addition of exogenous stimuli.4,26
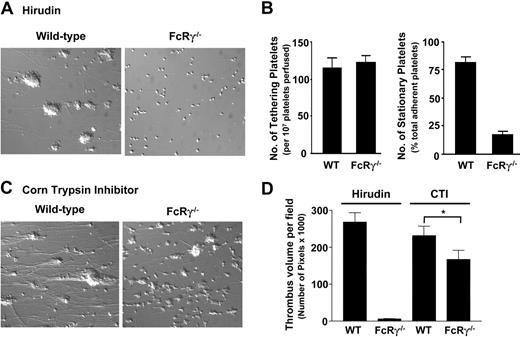
Effect of FcRγ deficiency on platelet tethering, stable adhesion, and thrombus formation on a collagen substrate. DiOC6-loaded (1 μM) whole blood collected into hirudin (100 μg/mL) (A) or into CTI (40 μg/mL) (C) from wild-type or FcRγ–/– mice was perfused through collagen-coated (2.5 mg/mL) microcapillary tubes for 3 minutes at 500 s–1. The images are representative of single platelets or thrombi formed after 3 minutes of blood perfusion. (B) The number of platelets (mean ± SEM) tethering to the matrix and subsequently forming stationary adhesion contacts was quantitated as described in “Materials and methods.” (D) Following blood perfusion over the collagen matrix, the thrombi were fixed with 4% paraformaldehyde, then imaged by confocal microscopy (3 to 6 fields per microslide, n = 3 for each condition). The total volume of thrombi formed per field (mean ± SEM) was determined as described in “Materials and methods.” Thrombi formed from FcRγ–/– mice were reduced in size (*P < .025; n = 3) compared with thrombi formed from wild-type controls.
Effect of FcRγ deficiency on platelet tethering, stable adhesion, and thrombus formation on a collagen substrate. DiOC6-loaded (1 μM) whole blood collected into hirudin (100 μg/mL) (A) or into CTI (40 μg/mL) (C) from wild-type or FcRγ–/– mice was perfused through collagen-coated (2.5 mg/mL) microcapillary tubes for 3 minutes at 500 s–1. The images are representative of single platelets or thrombi formed after 3 minutes of blood perfusion. (B) The number of platelets (mean ± SEM) tethering to the matrix and subsequently forming stationary adhesion contacts was quantitated as described in “Materials and methods.” (D) Following blood perfusion over the collagen matrix, the thrombi were fixed with 4% paraformaldehyde, then imaged by confocal microscopy (3 to 6 fields per microslide, n = 3 for each condition). The total volume of thrombi formed per field (mean ± SEM) was determined as described in “Materials and methods.” Thrombi formed from FcRγ–/– mice were reduced in size (*P < .025; n = 3) compared with thrombi formed from wild-type controls.
Thrombin generation leads to thrombus formation in FcRγ–/– blood after perfusion over a collagen surface
An important activator of platelets is the serine protease thrombin.27 Thrombin generation in vivo is principally dependent on the surface expression of tissue factor (TF); however, in vitro, a large proportion of thrombin generation is initiated by the contact phase of blood coagulation, principally involving Factor XII. This thrombin is rapidly neutralized by the anticoagulants used in our blood collection process, precluding analysis of the importance of this protease in regulating platelet thrombus growth in vitro. To investigate whether thrombin can overcome the thrombosis defect associated with GPVI/FcRγ-chain deficiency, we established an in vitro flow-based thrombosis assay that enables concomitant platelet deposition and thrombin generation on immobilized collagen matrices. This assay was dependent on collecting whole blood in the presence of the FXII inhibitor, corn trypsin inhibitor (CTI), which markedly slows thrombin generation in vitro (“Materials and methods”). In preliminary studies we confirmed that CTI inhibited fibrin formation ex vivo for up to 10 to 15 minutes following blood collection (data not shown). However, thrombin generation and fibrin formation were readily apparent following perfusion of wild-type mouse whole blood over a type I fibrillar collagen matrix. Fibrin formation was apparent within the first 60 to 90 seconds of perfusion and progressive build-up of fibrin after approximately 160 seconds of perfusion, ultimately leading to microcapillary occlusion. The initiator of blood coagulation under these conditions was not identified but presumably reflects the presence of microvesicle-derived tissue factor in whole blood or the presence of a FXIa-like activity on the surface of activated platelets. As demonstrated in Figure 5C, both wild-type and FcRγ–/– platelets were able to form thrombi when CTI-treated whole blood was perfused over a type I collagen matrix at 500 s–1. The individual thrombi formed with FcRγ–/– blood were typically smaller than wild-type thrombi, although analysis of total thrombus volume on the matrix surface revealed a 25% difference between FcRγ–/– and wild-type mice (Figure 4D). These studies demonstrate that thrombin generation on an immobilized thrombogenic substrate is sufficient to restore thrombus development in FcRγ–/– platelets.
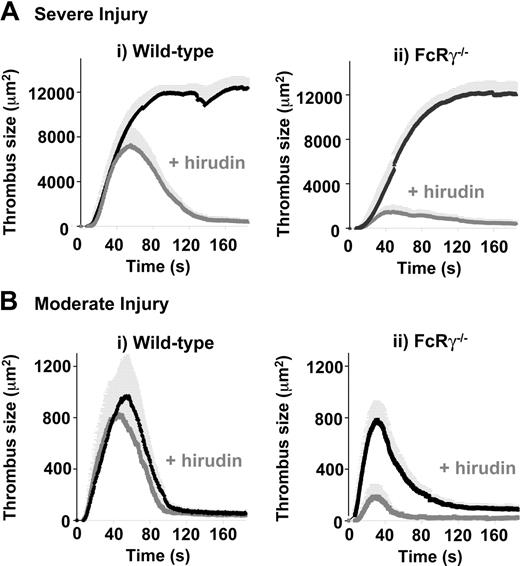
Effect of hirudin on laser-induced thrombus formation in wild-type and FcRγ-deficient mice. Severe (A) or moderate (B) laser-induced lesions were generated in the mesenteric arteries of wild-type (i) and FcRγ–/– (ii) mice treated with vehicle alone or hirudin (10 mg/kg subcutaneously). Traces represent the mean surface area (± SEM) of thrombi developing over 150 seconds (severe injury: (i) WT, n = 12 vessels in 8 mice; WT + Hir, n = 5 vessels in 3 mice; (ii) FcRγ–/–, n = 12 vessels in 7 mice; FcRγ–/– + Hir, n = 5 vessels in 4 mice; moderate injury (i) WT, n = 19 vessels in 7 mice; WT + Hir, n = 11 vessels in 4 mice; (ii) FcRγ–/–, n = 29 vessels in 9 mice; FcRγ–/– + Hir, n = 7 vessels in 4 mice).
Effect of hirudin on laser-induced thrombus formation in wild-type and FcRγ-deficient mice. Severe (A) or moderate (B) laser-induced lesions were generated in the mesenteric arteries of wild-type (i) and FcRγ–/– (ii) mice treated with vehicle alone or hirudin (10 mg/kg subcutaneously). Traces represent the mean surface area (± SEM) of thrombi developing over 150 seconds (severe injury: (i) WT, n = 12 vessels in 8 mice; WT + Hir, n = 5 vessels in 3 mice; (ii) FcRγ–/–, n = 12 vessels in 7 mice; FcRγ–/– + Hir, n = 5 vessels in 4 mice; moderate injury (i) WT, n = 19 vessels in 7 mice; WT + Hir, n = 11 vessels in 4 mice; (ii) FcRγ–/–, n = 29 vessels in 9 mice; FcRγ–/– + Hir, n = 7 vessels in 4 mice).
Hirudin further reduced thrombus formation in FcRγ-deficient mice following laser-injury
To investigate whether thrombin generation was responsible for the near-normal thrombotic response of FcRγ–/– mice in vivo, we examined the effects of high concentrations of the thrombin inhibitor hirudin (10 mg/kg) on laser-induced thrombus formation in mesenteric arterioles. Consistent with previous findings, hirudin had no effect on initial thrombus formation following severe vascular injury; however, thrombi formed under these conditions were invariably unstable, leading to progressive thrombus dissolution from the point of maximum growth (Figure 6Ai). In contrast, initial thrombus formation was markedly impaired in hirudin-treated FcRγ–/– mice, with 10.9% maximal thrombus size of that observed in untreated mice (Figure 6Aii). To investigate whether thrombin was also contributing to the thrombotic response of FcRγ–/– mice following moderate vascular injury, the effects of hirudin on platelet thrombus growth was examined in wild-type and FcRγ–/– mice. Distinct from the severe vascular injury model, hirudin had minimal effect on thrombus kinetics in wild-type mice following milder vascular injury (Figure 6Bi). In contrast, hirudin had a marked effect on thrombus development in FcRγ–/– mice under the same experimental conditions, with 21% maximal thrombus size of that observed in untreated mice (Figure 6Bii). Taken together, these studies define a major role for thrombin in maintaining a robust thrombotic response in mice lacking the GPVI/FcRγ-chain.
Hirudin prolongs FcRγ–/– mouse tail bleeding time
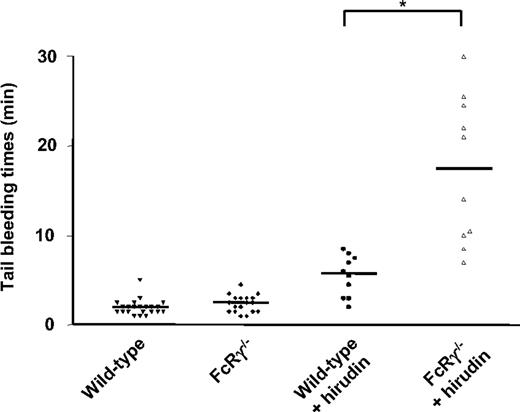
To examine whether these findings are also relevant to hemostasis, we examined the effect of thrombin inhibition on the tail bleeding time of wild-type and FcRγ–/– mice. As demonstrated in Figure 7, there was no significant prolongation in tail bleeding time in FcRγ–/– mice relative to wild-type matched controls, whereas hirudin prolonged the bleeding time approximately 3-fold in wild-type mice. However, in FcRγ –/– mice, hirudin prolonged the bleeding time more than 7-fold. These findings are consistent with our thrombosis studies and confirm an important role for thrombin in maintaining a near-normal hemostatic response following loss of the GPVI/FcRγ-chain complex.
Effect of hirudin on the tail bleeding time of wild-type and FcRγ-deficient mice. Tail bleeding time was measured before and 5 minutes after hirudin (10 mg/kg) administration in FcRγ–/– mice (2.5 ± 0.2 minutes before and 17.3 ± 2.6 minutes after, n = 10; P < .001, paired Student t test) or wild-type mice (2.0 ± 0.2 minutes before and 5.5 ± 0.7 minutes after, n = 10; P = .001, paired Student t test), as described in “Materials and methods.” Baseline tail bleeding times of a further 12 wild-type mice (total, n = 22) and 8 FcRγ–/– mice (total, n = 18) were examined and demonstrated not to be significantly different from each other (P > .05). Horizontal bars represent the mean bleeding time values for each group of animals. *P < .05, Student t test.
Effect of hirudin on the tail bleeding time of wild-type and FcRγ-deficient mice. Tail bleeding time was measured before and 5 minutes after hirudin (10 mg/kg) administration in FcRγ–/– mice (2.5 ± 0.2 minutes before and 17.3 ± 2.6 minutes after, n = 10; P < .001, paired Student t test) or wild-type mice (2.0 ± 0.2 minutes before and 5.5 ± 0.7 minutes after, n = 10; P = .001, paired Student t test), as described in “Materials and methods.” Baseline tail bleeding times of a further 12 wild-type mice (total, n = 22) and 8 FcRγ–/– mice (total, n = 18) were examined and demonstrated not to be significantly different from each other (P > .05). Horizontal bars represent the mean bleeding time values for each group of animals. *P < .05, Student t test.
Discussion
The studies reported here provide new insight into the role of the GPVI/FcRγ receptor in arterial thrombogenesis. Using 3 distinct models of arterial thrombosis we have demonstrated that deficiency of GPVI/FcRγ leads to a relatively modest defect in arterial thrombosis compared to αIIbβ3 or P2Y12 receptor blockade. In fact, the only experimental condition in which FcRγ–/– mice exhibited a marked defect in hemostasis and thrombosis was when these mice were treated with thrombin inhibitors. These in vivo studies have demonstrated a functional overlap between thrombin and GPVI/FcRγ in which reduction in the platelet activating properties of one mechanism can be partially compensated by the other. Overall these studies suggest a greater functional redundancy for GPVI in arterial thrombogenesis than previously recognized. Furthermore, they provide a mechanism to partially explain the varied thrombotic response reported in GPVI-deficient mice. Finally, these studies raise the interesting possibility that the full antithrombotic potential of GPVI inhibitors in vivo may require concurrent administration of anticoagulant agents.
Our initial findings for a near-normal arterial thrombotic response in FcRγ-null mice was unexpected in light of previous findings demonstrating a crucial role for the GPVI/FcRγ receptor complex in arterial thrombogenesis.4-7 These differences are unlikely to be simply explained by differences in the degree of GPVI/FcRγ receptor inhibition in different studies, as a similar defect in collagen responsiveness has been demonstrated in mice treated with GPVI receptor antagonists, with antibodies that down-regulate GPVI surface expression, and in mice with targeted deletion of the FcRγ-chain. A more likely explanation is that the importance of GPVI in thrombosis is influenced by the nature of the thrombogenic injury operating in different experimental models. For example, mice with a targeted deletion of the Gpvi gene formed occlusive thrombi in FeCl3–injured carotid arteries, with the major defect in thrombosis related to thrombus stability, rather than initial thrombus growth (Z. M. Ruggeri, personal oral communication, September 2005). Similarly, studies from the Furie laboratory recently published in abstract form28 have demonstrated that the defect in thrombus formation in FcRγ-null mice is critically dependent on the type of arterial lesion. Similar to our findings, normal occlusion was observed following laser injury of mesenteric arterioles; however, following FeCl3 injury, a major thrombosis defect was observed. The reasons for these differences is currently under investigation and may relate in part to the nature of the thrombogenic elements exposed following different forms of arterial injury. In this context it is of interest that the ratio of tissue factor to platelets present in the laser injury model was 5-fold greater than after FeCl3 treatment,28 suggesting a more prominent role for thrombin generation in the former model. Consistent with this, we have provided 3 independent lines of evidence demonstrating that thrombin is a key variable influencing the thrombotic response in FcRγ-null mice. First, in our in vitro studies, complete inhibition of thrombin abolished thrombus formation on a collagen substrate, whereas thrombin generation partially restored thrombus growth. Second, we have demonstrated a direct relationship between the extent of laser injury and the thrombin-dependency for thrombus growth,22 such that with milder forms of injury there is progressive decrease in the importance of thrombin for thrombosis formation and a concomitant greater role for GPVI. Finally, inhibition of thrombin in vivo produced a profound defect in thrombus growth in FcRγ-null mice. Of note, following moderate vessel injury, deficiency of GPVI/FcRγ receptor or inhibition of thrombin alone produced only minor defect in the kinetics of thrombus formation, whereas concurrent thrombin inhibition and GPVI/FcRγ receptor deficiency almost completely eliminated thrombus formation altogether. These latter findings provide an example of the potential redundant platelet-activating mechanisms in vivo and suggest caution when attempting to exclude the contribution of a specific activating stimulus to the thrombotic process.
Based on findings from in vitro perfusion assays and in vivo thrombosis models, GPVI has recently been proposed to play a potentially important role in promoting the initial recruitment of platelets from flowing blood onto thrombogenic surfaces.4-6 However, in our flow assays we have observed no significant defect in the ability of FcRγ–/– platelets to tether to immobilized collagen, but rather a primary defect in the ability of these platelets to form firm adhesion contacts. This defect is consistent with the role of the GPVI/FcRγ receptor in promoting platelet activation, as a necessary step for up-regulation of the affinity state of surface integrins necessary for firm adhesion. The demonstration that the primary defect in GPVI/FcRγ platelets is linked to defective stable adhesion, rather than reduced tethering is potentially important, as it provides an explanation for the ability of thrombin to induce platelet activation and thrombus growth. Notably, such compensatory mechanisms are unable to overcome the adhesive defects associated with GPIb and αIIbβ3 deficiency, providing further evidence that the major defect in GPVI/FcRγ-null platelets relates to their failure to promote platelet activation rather than a fundamental defect in adhesive capacity.
Our studies highlight the importance of validating potential antithrombotic targets in a broad range of experimental animal models and for the need for detailed characterization of these models using well-defined antithrombotic approaches. Two of the thrombosis models developed in this study were based on models that have been extensively investigated in larger animals. The “Folts-type” stenosis-injury model was originally developed in the dog,29,30 but has been adapted for use in pigs,31 monkeys,32,33 and rabbits,34 and is considered one of the standard models to assess the antithrombotic potential of antiplatelet agents. This model has not been widely used in mice, presumably due to the technical challenges associated with forming reproducible stenoses and injury in such small vessels. However, an advantage of this model is that thrombus formation represents the combined effects of deep-vessel injury and high-shear stress, thereby mimicking 2 of the key elements relevant to thrombus formation in diseased atherosclerotic human arteries. Similarly, the electrolytic model used in this study has also been used extensively in larger animals,35-37 and is generally considered to represent a highly reproducible and reliable model for analysis of acute thrombus formation in larger arteries. The laser injury model in the microcirculation of mice is an increasingly popular method to analyze thrombus formation in vivo due to the ability to cause precise, graded, and localized vascular injury and also because it enables real-time monitoring of the thrombotic process. However, there is increasing recognition that interlaboratory variability with this model may be considerable, necessitating the need for more detailed characterization of such models before firm conclusions can be drawn on the likely importance of individual platelet components to thrombus formation.
Finally, the results presented here have potentially important implications for GPVI as an antithrombotic target. The enthusiasm for GPVI as a thrombosis therapeutic target has stemmed from a number of factors, including the recognition that collagens represent an important thrombogenic element of atherosclerotic lesions, the demonstration that GPVI antagonists produce a potent antithrombotic effect in vivo, and importantly, that mice and humans with GPVI deficiency have a relatively mild bleeding tendency. These latter observations suggest that GPVI antagonists may have a considerably wider therapeutic window than conventional antiplatelet therapies. However, the findings presented here begin to question this hypothesis, as we have demonstrated that the only experimental conditions leading to a major thrombosis defect (ie, concurrent thrombin inhibition in FcRγ–/– mice) also resulted in a significantly prolonged bleeding time. It remains to be established whether lesser degrees of thrombin inhibition in FcRγ–/– mice will preserve normal hemostasis while maintaining an antithrombotic effect. Nonetheless, our studies raise the possibility that inhibition of GPVI per se may not afford the same level of antithrombotic protection as P2Y12 and αIIbβ3 receptor antagonists.
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-10-4244.
Supported by INSERM, Association de Recherche et de Développement en Médecine et en Santé Publique (ARMESA), and grants from the National Health and Medical Research Council (NH&MRC).
P.M., C.N., and C.L.Y. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Christian Gachet for his constant support of the project and fruitful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal