B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived CD5+ B lymphocytes. Several drugs currently used in the therapy of B-CLL act, at least partially, through activation of the p53 pathway. Recently, nongenotoxic small-molecule activators of p53, the nutlins, have been developed that inhibit p53-MDM2 binding. We have investigated the antitumor potential of nutlin-3 in B-CLL and find that it can activate the p53 pathway and effectively induce apoptosis in cells with wild-type p53, including cells with dysfunctional ataxia telangiectasia mutated, but not mutant p53. Nutlin-3 stabilized p53 and induced p53 target genes, including MDM2, p21CIP1, PUMA, BAX, PIG3, and WIG1. Nutlin-3 synergized with the genotoxic drugs doxorubicin, chlorambucil, and fludarabine, but not with acadesine, which induces p53-independent apoptosis. Normal human T cells showed lower sensitivity to nutlin-3 than B-CLL cells and no synergism with the genotoxic drugs. These results suggest that MDM2 antagonists alone or in combination with chemotherapeutic drugs may offer a new treatment option for B-CLL.
Introduction
The tumor suppressor TP53 plays an important role in the control of key genes involved in the regulation of DNA repair, cell cycle, and apoptosis.1,2 p53 is activated in response to DNA damage or other forms of stress, protecting cells from malignant transformation. This is the reason why p53 is frequently inactivated in human cancer. p53 is a short-lived protein, and its cellular level is controlled by the rate at which it is degraded. Although several U3 ubiquitin ligases have been implicated in p53 ubiquitylation and degradation, MDM2 appears to function as a master regulator of p53.3,4 MDM2 not only facilitates p53 degradation, but it also binds p53 and inhibits its transcriptional activity. Therefore, inhibitors of p53-MDM2 binding are expected to stabilize and activate p53. Recently, the first potent and selective small-molecule antagonists of MDM2, the nutlins, have been shown to activate the p53 pathway in cancer cells with wild-type p53 in vitro and in vivo.5
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived CD5+ B lymphocytes.6 TP53 is mutated in only 5% to 10% of B-CLL cases at diagnosis, but in nearly 30% in chemotherapy-resistant tumors. TP53 mutation is associated with poor clinical outcome, shorter survival, and lack of response to therapy with purine nucleoside analogs or alkylating agents.7-11 In fact, alterations in the TP53 gene are among the worst prognostic indicators for B-CLL.12-14 Most of the chemotherapeutic drugs currently used induce cell cycle arrest or apoptosis through activation of p53, and p53 inactivation leads to chemoresistance.1,2 Chemotherapeutic drugs, including purine analogs, topoisomerase inhibitors, and alkylating agents, have been shown to effectively increase p53 levels in B-CLL.15,16 Thus, p53 activation is considered among the critical molecular events in chemotherapy-induced apoptosis in B-CLL cells. Although TP53 is mutated in only 5% to 10% of patients, the p53 pathway could be altered at a higher frequency, thus effectively attenuating p53 function. One of the mechanisms involved in p53 stabilization in response to DNA damage is its phosphorylation by ataxia telangiectasia mutated (ATM) protein.1,2 Interestingly, ATM is inactivated in 10% to 20% of B-CLL cases, thus providing an alternative way to disable p53 function.17-20 Tumors with alterations upstream of p53 would not respond adequately to genotoxic chemotherapeutics that act through the p53 pathway (eg, alkylating agents such as chlorambucil and cyclophosphamide; purine nucleosides such as fludarabine and cladribine; or topoisomerase inhibitors such as doxorubicin and mitoxantrone). Therefore, new therapies that overcome these defects by acting directly on p53 stability may benefit these patients. Nutlins activate p53 by releasing it from MDM2-mediated negative control and thus compensate for defects in the upstream p53 signaling. Here, we investigate the antitumor activity of nutlin-3 in B-CLL cells and show that it activates the p53 pathway, induces apoptosis, and synergizes with several currently used therapeutic agents, suggesting that MDM2 antagonists may provide a novel treatment option for B-CLL patients.
Patients, materials, and methods
Patients with B-CLL and cell isolation
Twenty-six peripheral blood samples from patients with B-CLL were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. Samples were obtained from the Hospital de Bellvitge, Barcelona, Spain. Written informed consent was obtained from all patients in accordance with Hospital de Bellvitge Ethics Committee. Mononuclear cells were isolated from peripheral blood samples by centrifugation on a Ficoll-Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Steinheim, Germany).
Reagents
Nutlin-3a and nutlin-3b were provided by Hoffmann-La Roche.5 Doxorubicin, chlorambucil, and fludarabine were obtained from Sigma-Aldrich (Steinheim, Germany). Acadesine (5-aminoimidazole-4-carboxamide [AICA] riboside) was obtained from Toronto Research Chemicals (North York, ON, Canada). Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) were from Bender MedSystems (Vienna, Austria).
Cell culture
Lymphocytes were cultured immediately after thawing or isolation at a concentration of 2 to 5 × 106 cells/mL in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, and 100 U penicillin and 100 ng/mL streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Analysis of apoptosis by flow cytometry
Apoptosis was measured by surface annexin V staining as previously described.21 To analyze apoptosis in T cells and B cells from the samples, 5 × 105 cells were incubated with the indicated factors. Cells were then washed in phosphate-buffered saline (PBS) and incubated in 50 μL annexin-binding buffer with allophycocyanin (APC)–conjugated anti-CD3 and phycoerythrin (PE)–conjugated anti-CD19 (Becton Dickinson, Franklin Lakes, NJ) for 10 minutes in the dark. Cells were then diluted with annexin-binding buffer to a volume of 150 μL and incubated with 1 μL annexin V–FITC for 15 minutes in the dark. Data were analyzed using Cell Quest software (Becton Dickinson, Mountain View, CA).
Western blot analysis
Cells were lysed with Laemmli sample buffer, and Western blot analysis was performed as described previously22 using antibodies against p53 (Ab-5, Neomarkers, Fremont, CA), MDM2, p21, Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA), Bcl-2 (Dako, A/S, Glostrup, Denmark), Bax (BD-Pharmingen, San Diego, CA), and PUMA (a kind gift from B. Vogelstein, The Johns Hopkins Medical Institutes, Baltimore, MD). Antibody binding was detected using a secondary antibody conjugated to horseradish peroxidase and the enhanced chemiluminescence (ECL) detection system (Amersham, Buckinghamshire, United Kingdom).
Quantitative PCR
Quantitative real-time polymerase chain reaction (PCR) was performed on RNA extracts from 107 cells. RNA was isolated according to the manufacturer's protocol (Ultraspec RNA, Biotecx Laboratories, Houston, TX). Total RNA (5 μg) was reverse transcribed using a Ready-To-Go First-Strand Kit from Amersham Biosciences, using random primers. Ready-to-use primer and probe sets designed by Applied Biosystems (Assay-on-demand Gene Expression Product numbers Hs00355782_m1 (p21CIP1), Hs00248075_m1 (PUMA), Hs00153280_m1 (PIG3), Hs00223860_m1 (WIG1), Hs00263469_m1 (MCG10), Hs00262251_m1 (PRG), and Hs00560402_m1 (NOXA) were used for mRNA detection. β-glucuronidase (GUS) mRNA (Hs99999908_m1) was used for normalization. PCR data were captured using an ABI-PRISM 7700 Sequence Detection System and the Sequence Detector Software (SDS version 1.9; Applied Biosystems, Foster City, CA).
RT-MLPA
RNA was analyzed by reverse transcriptase multiplex ligation–dependent probe amplification (RT-MLPA) using SALSA MLPA KIT R011 Apoptosis mRNA from MRC-Holland (Amsterdam, The Netherlands) for the simultaneous detection of 38 messenger RNA molecules.23 In brief, RNA samples (200 ng total RNA) were first reverse transcribed using a gene-specific probe mix. The resulting cDNA was annealed overnight at 60°C to the MLPA probe mix. Annealed oligonucleotides were ligated by adding Ligase-65 (MRC-Holland, Amsterdam) and incubated at 54°C for 15 minutes. Ligation products were amplified by PCR (33 cycles, 30 seconds at 95°C; 30 seconds at 60°C, and 1 minute at 72°C) with one unlabeled and one FAM labeled primer. The final PCR fragments amplified were separated by capillary electrophoresis on a 48-capillary ABI-Prism 3730 Genetic Analyzer (Applied Biosystems/Hitachi, Foster City, CA). Peak area and height were measured using GeneScan analysis software (Applied Biosystems). The levels of mRNA for each gene were expressed as a normalized ratio of the peak area divided by the peak area of a control gene, resulting in the relative abundance of mRNAs of the genes of interest. The probe set contains probes for mRNAs of 30 apoptosis-related proteins. Areas were normalized to GUS.
Detection of p53 mutations
DNA was isolated according to the manufacturer's protocol (High Pure PCR Template Preparation Kit, Roche Diagnostics GmbH, Mannheim, Germany), and exons 5-8 were sequenced as previously described.24
Statistical analysis
Data were analyzed using SPSS 11.5 software package (Chicago, IL). Results are shown as mean plus or minus standard deviation (SD) of values obtained in independent experiments. The paired Student t test was used to compare the differences between paired samples. Differences were considered significant at P value below .05. Median dose effect analysis was used to assess the interaction between agents.25 The combination index (CI) was calculated for a 2-drug combination using Biosoft CalcuSyn program (Ferguson, MO). A CI of 1 indicates an additive effect; a CI above 1, an antagonistic effect; and a CI below 1, a synergistic effect.
Results
MDM2 antagonists induce apoptosis in B-CLL cells
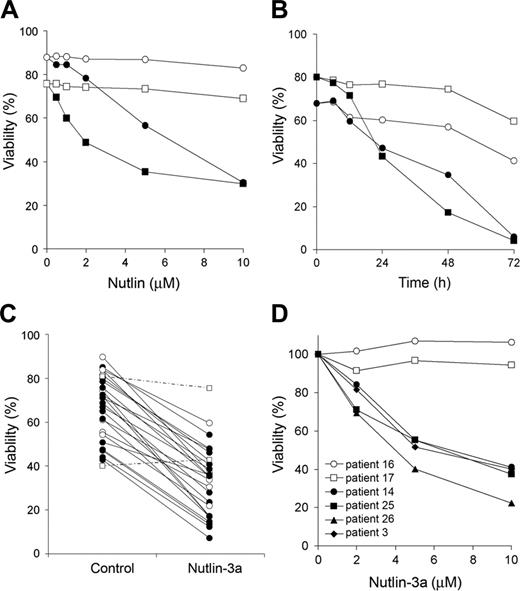
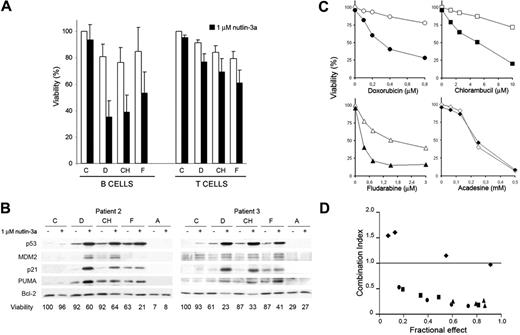
To assess the ability of nutlin-activated p53 to induce apoptosis, we incubated B-CLL cells with a range of nutlin-3 concentration (0.5-10 μM) for 48 hours and measured annexin V positivity. We used both enantiomers of nutlin-3 shown previously to differ significantly in their activity. While the active enantiomer (3a) binds to MDM2 with high affinity, the inactive enantiomer (3b) is approximately 200-fold less potent and thus represents a valuable negative control.5 Nutlin-3a, but not nutlin-3b, induced apoptosis in a dose-dependent manner (Figure 1A), and the half-maximal effective concentration (EC50) was 4.7 ± 1.5 μM(n = 10) ranging from 2.7 to 7.3. Incubation with 5 μM nutlin-3a induced a time-dependent decrease of cell viability (Figure 1B). Most B-CLL samples (24 of 26) were sensitive to nutlin-3a, and cell viability decreased from 67.2% ± 14.2% to 31.6% ± 14.4% (n = 24) after incubation with 5 μM for 48 hours (Figure 1C). Two patients (16 and 17) were resistant to MDM2 inhibitors. Their TP53 status was analyzed by DNA sequencing and fluorescence in situ hybridization (FISH), and we found that TP53 was mutated in both samples. Patient 16 had the mutation M246V, and patient 17 had a frame-shift mutation in one allele (nucleotide deletion in codon 272) and a 17p deletion in the other allele. We also analyzed ATM status in 15 samples by FISH or MLPA (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) and in 5 samples by Western blot (Figure S3). Five samples showed 11q13 deletion (patients 3, 11, 15, 20, and 26), one sample had no expression of ATM protein (patient 25), and one sample had 11q13 deletion and no expression of ATM protein (patient 14). Nutlin-3a induced apoptosis in the samples with 11q deletion or no expression of ATM with similar dose-responses to samples without ATM alterations (Figure 1D). These data confirmed the hypothesis that nutlin-3a induces apoptosis by activation of the p53 pathway irrespective of ATM status.
Cytotoxic effect of MDM2 antagonists on B-CLL cells. (A) Dose response of enantiomers of nutlin-3 on B-CLL cells. Cells from 5 patients were incubated for 48 hours with various doses of the active (nutlin-3a: ▪, •) or the inactive (nutlin-3b: □, ○) enantiomers. The figure shows results from 2 representative patients (patient 1, ▪, □; patient 2, •, ○). (B) Time course of nutlin-3a–induced apoptosis. Cells from patients 11 and 18 were incubated for different times with (▪, •) or without (□, ○)5 μM nutlin-3a (patient 11, ▪, □; patient 18, •, ○). (C) Cells from 26 patients were incubated for 48 hours with or without 5 μM nutlin-3a. ○ represent 6 patients with ATM alterations (patients 3, 11, 15, 20, 26, and 25); □ and discontinuous lines represent 2 resistant patients (patients 16 and 17). Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and is expressed as the percentage of nonapoptotic cells. (D) Dose response of nutlin-3a on B-CLL cells with TP53 (□, ○) or ATM (•, ▪, ▴, ♦) alterations. Cells were incubated for 48 hours with various doses of nutlin-3a. Viability was expressed as the percentage of the viability of untreated cells.
Cytotoxic effect of MDM2 antagonists on B-CLL cells. (A) Dose response of enantiomers of nutlin-3 on B-CLL cells. Cells from 5 patients were incubated for 48 hours with various doses of the active (nutlin-3a: ▪, •) or the inactive (nutlin-3b: □, ○) enantiomers. The figure shows results from 2 representative patients (patient 1, ▪, □; patient 2, •, ○). (B) Time course of nutlin-3a–induced apoptosis. Cells from patients 11 and 18 were incubated for different times with (▪, •) or without (□, ○)5 μM nutlin-3a (patient 11, ▪, □; patient 18, •, ○). (C) Cells from 26 patients were incubated for 48 hours with or without 5 μM nutlin-3a. ○ represent 6 patients with ATM alterations (patients 3, 11, 15, 20, 26, and 25); □ and discontinuous lines represent 2 resistant patients (patients 16 and 17). Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and is expressed as the percentage of nonapoptotic cells. (D) Dose response of nutlin-3a on B-CLL cells with TP53 (□, ○) or ATM (•, ▪, ▴, ♦) alterations. Cells were incubated for 48 hours with various doses of nutlin-3a. Viability was expressed as the percentage of the viability of untreated cells.
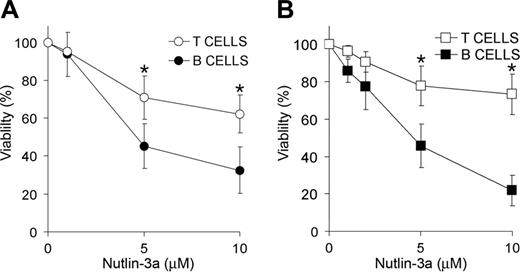
Next, we analyzed the sensitivity of B and T cells to p53-dependent apoptosis induced by nutlin-3a. The number of apoptotic T cells (CD3+ cells) was measured in 6 B-CLL samples (Figure 2A) and 7 blood samples from healthy donors (Figure 2B) exposed to several doses of nutlin-3a for 48 hours. Incubation with 5 μMor 10 μM nutlin-3a reduced the percentage of viable B-CLL cells to 45.1% ± 11.8% and 32.5% ± 12.3%, respectively. In contrast, the percentages of viable T cells were 71.0% ± 11.4% and 62.2% ± 9.9%. Similar results were obtained with cells derived from healthy donors (5 μM nutlin-3a: B cells, 45.6% ± 11.6%; and T cells, 77.7% ± 10.6%; 10 μM nutlin-3a: B cells, 21.8% ± 8.2%; and T cells, 73.3% ± 10.8%). These results indicate that B cells are more sensitive than T cells to nutlin-3a–induced apoptosis.
MDM2 antagonists stabilize p53 and activate p53 target genes
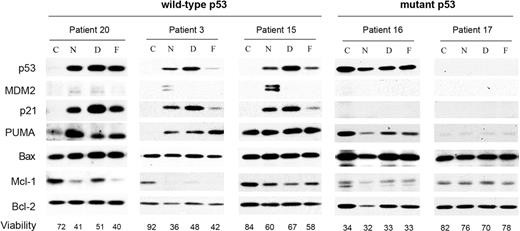
The ability of MDM2 antagonists to stabilize p53 and activate the p53 pathway was analyzed in B-CLL cells isolated from 3 patients with wild-type p53 and 2 patients with mutated p53 (Figure 3). Upon treatment with nutlin-3a, p53 protein accumulated in cells expressing wild-type p53 and led to an elevation of the p53 targets MDM2, p21, and PUMA, but not Bax. Similar changes in these proteins were induced by drugs that activate the p53 pathway through the genotoxic stress they impose (eg, doxorubicin and fludarabine) (Figure 3). Patient 15 showed high basal levels of PUMA, which were not affected by the drugs. As previously described,15 induction of apoptosis in B-CLL reduced the levels of Mcl-1 protein. Levels of Bcl-2 remained constant and were used as loading control. As expected, B-CLL samples with p53 mutations (patients 16 and 17) showed neither stabilization of p53 protein nor induction of p53 targets p21, PUMA, and MDM2. Nutlin-3b did not affect any of the proteins analyzed (data not shown).
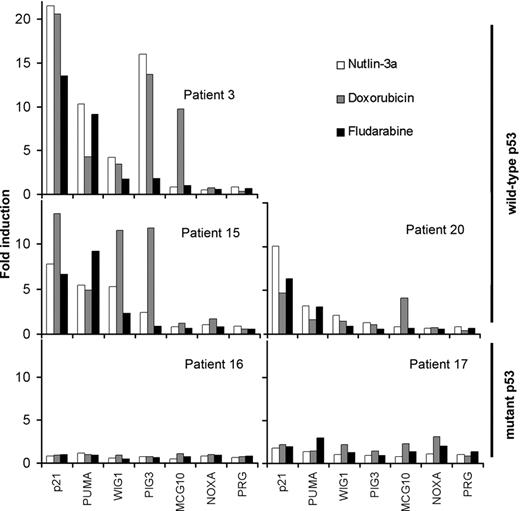
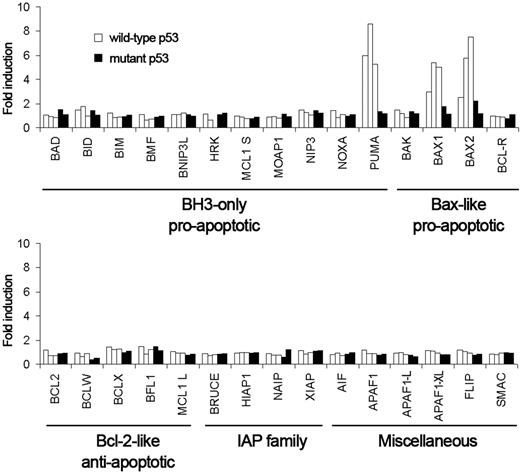
Next, we measured the mRNA levels of the p53 target genes p21CIP1, PUMA, WIG1, PIG3, MCG10, NOXA, and PRG by quantitative real-time PCR (Figure 4). p21CIP1, PUMA, and WIG1 were increased in the B-CLL samples with wild-type p53 after 48 hours of incubation with nutlin-3a, doxorubicin, or fludarabine. The increase of PIG3 and MCG10 depended on the sample and the drug. PIG3 increased in patients 3 and 20 after exposure to nutlin-3a and fludarabine. MGC10 increased in patients 3 and 17 only after doxorubicin treatment. NOXA and PRG mRNA levels did not change in any condition analyzed. None of the p53 targets analyzed was affected by drug treatment in B-CLL samples with mutant p53. Nutlin-3a had the same effect on gene expression when cells were incubated for 24 hours, while nutlin-3b had no effect (data not shown). Additionally, we used RT-MLPA to analyze changes in overall apoptosis expression profile. Nutlin-3a treatment for 48 hours (Figure 5) or 24 hours (Figure S1) increased only PUMA and BAX expression. PUMA and BAX also were induced by doxorubicin and fludarabine treatment (Figure S2). These data demonstrated the ability of nutlin-3a to activate the p53 pathway in B-CLL cells that express wild-type p53.
Differential effect of MDM2 antagonists on B and T cells. Dose response of the cytotoxic effect of MDM2 antagonist nutlin-3a on B and T cells from B-CLL patients (A) or healthy donors (B). Cells were incubated with a range of doses of nutlin-3a (up to 10 μM) for 48 hours. Viability was measured as nonapoptotic CD3+/CD19–T cells (○, □) or CD3–/CD19+ B cells (•, ▪) as described in “Patients, materials, and methods” and expressed as the percentage of the viability of untreated cells. Data are shown as the mean value ± SD of 6 B-CLL patients (A) and 7 healthy donors (B). *P < .05 B cells versus T cells.
Differential effect of MDM2 antagonists on B and T cells. Dose response of the cytotoxic effect of MDM2 antagonist nutlin-3a on B and T cells from B-CLL patients (A) or healthy donors (B). Cells were incubated with a range of doses of nutlin-3a (up to 10 μM) for 48 hours. Viability was measured as nonapoptotic CD3+/CD19–T cells (○, □) or CD3–/CD19+ B cells (•, ▪) as described in “Patients, materials, and methods” and expressed as the percentage of the viability of untreated cells. Data are shown as the mean value ± SD of 6 B-CLL patients (A) and 7 healthy donors (B). *P < .05 B cells versus T cells.
MDM2 antagonists induce p53 stabilization and accumulation of p53 target proteins in B-CLL cells. Cells from B-CLL patients were untreated (C) or treated with 5 μM nutlin-3a (N), 0.8 μM doxorubicin (D), or 3 μM fludarabine (F) for 48 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53, MDM2, p21, PUMA, Bax, Mcl-1, and Bcl-2 were analyzed. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and has been expressed here as the percentage of nonapoptotic cells.
MDM2 antagonists induce p53 stabilization and accumulation of p53 target proteins in B-CLL cells. Cells from B-CLL patients were untreated (C) or treated with 5 μM nutlin-3a (N), 0.8 μM doxorubicin (D), or 3 μM fludarabine (F) for 48 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53, MDM2, p21, PUMA, Bax, Mcl-1, and Bcl-2 were analyzed. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and has been expressed here as the percentage of nonapoptotic cells.
Synergism between MDM2 antagonists and chemotherapeutic drugs
Because both MDM2 antagonists and genotoxic drugs use the p53 pathway, they may synergize in their cytotoxic activity in B-CLL cells that have retained p53 function. To this end, we investigated the effect of combining low doses of nutlin-3a with currently used genotoxic drugs. B-CLL cells isolated from 5 patients were incubated for 48 hours with 1 μM nutlin-3a alone or in combination with suboptimal concentrations of doxorubicin (D; 0.2 μM), chlorambucil (CH; 2.5 μM), or fludarabine (F; 0.4 μM), and their ability to undergo apoptosis was measured in B (CD19+) and T (CD3+) cells. Incubation with 1 μM nutlin-3a alone had no significant effect on either cell type. However, in combination with suboptimal doses of doxorubicin, chlorambucil, or fludarabine, nutlin-3a increased drug-induced apoptosis in B cells but not in T cells (Figure 6A). Interestingly, nutlin-3a synergized with fludarabine, doxorubicin, and chlorambucil in the induction of apoptosis in one sample with 11q deletion (patient 3) (Figure 6B), in one sample with no expression of ATM protein (patient 25), and in one sample with both alterations (patient 14) (Figures S3 and S4).
MDM2 antagonists induce mRNA accumulation of p53 target genes. Cells from B-CLL patients with wild-type p53 (patients 20, 3, and 15) and mutant p53 (patients 16 and 17) were untreated or treated with 5 μM nutlin-3a (□), 0.8 μM doxorubicin (▦), or 3 μM fludarabine (▪) for 48 hours. Cells were lysed, and expression of p53 target genes was analyzed by quantitative PCR as described in “Patients, materials, and methods.” The results are shown as fold induction relative to untreated cells.
MDM2 antagonists induce mRNA accumulation of p53 target genes. Cells from B-CLL patients with wild-type p53 (patients 20, 3, and 15) and mutant p53 (patients 16 and 17) were untreated or treated with 5 μM nutlin-3a (□), 0.8 μM doxorubicin (▦), or 3 μM fludarabine (▪) for 48 hours. Cells were lysed, and expression of p53 target genes was analyzed by quantitative PCR as described in “Patients, materials, and methods.” The results are shown as fold induction relative to untreated cells.
We then analyzed the changes in the levels of p53, MDM2, p21, PUMA, and Bcl-2 proteins induced by drug treatment. p53 levels were very low or undetectable in untreated cells and slightly induced in cells treated with low doses of nutlin-3a or genotoxic drugs, whereas the incubation of the cells with a combination of nutlin-3a and either of the genotoxic drugs potentiated the accumulation of p53. Similarly, p53 targets p21 and PUMA were induced when p53 was increased by the combination of nutlin-3a and doxorubicin or chlorambucil. The combination of nutlin-3a and fludarabine increased PUMA but not p21. In contrast, no change in Bcl-2 or Bax levels was observed (data not shown). MDM2 was undetectable in control cells but became detectable after nutlin-3a treatment. Acadesine, which induces apoptosis of B-CLL cells in a p53-independent manner,26 did not change p53 or p53 targets either alone or in combination with nutlin-3a. Moreover, combination of acadesine with nutlin-3a did not enhance its apoptotic activity. Incubation of B-CLL cells from patient 2 with various concentrations of genotoxic drugs and acadesine, ranging from suboptimal to pharmacological concentrations, in the absence of nutlin for 48 hours produced a dose-dependent loss of viability (Figure 6C). The addition of 1 μM nutlin-3a increased the level of apoptosis induced by genotoxic drugs but not that induced by acadesine. These data were analyzed using the median dose effect analysis, and the combination index (CI) was determined in relation to the fraction of cells affected (Figure 6D). Synergism was observed for doxorubicin, chlorambucil, and fludarabine, as CI values were lower than 1.0, whereas acadesine showed CI values of 1 or higher, indicating absence of synergism.
Apoptosis-related gene expression profile induced by MDM2 antagonists. Cells from B-CLL patients with wild-type p53 (patients 20, 3, and 15; □) and mutant p53 (patients 16 and 17; ▪) were untreated or treated with 5 μM nutlin-3a for 48 hours. Cells were lysed, and expression of apoptosis-related genes was analyzed by RT-MLPA as described in “Patients, materials, and methods.” The results are shown as fold induction relative to untreated cells.
Apoptosis-related gene expression profile induced by MDM2 antagonists. Cells from B-CLL patients with wild-type p53 (patients 20, 3, and 15; □) and mutant p53 (patients 16 and 17; ▪) were untreated or treated with 5 μM nutlin-3a for 48 hours. Cells were lysed, and expression of apoptosis-related genes was analyzed by RT-MLPA as described in “Patients, materials, and methods.” The results are shown as fold induction relative to untreated cells.
Synergism between MDM2 antagonists and chemotherapeutic drugs. (A) Effect on the viability of B cells and T cells in B-CLL. Cells were untreated (C) or treated with 0.2 μM doxorubicin (D), 2.5 μM chlorambucil (CH), or 0.4 μM fludarabine (F) without (□) or with (▪)1 μM nutlin-3a for 48 hours. Viability was measured as nonapoptotic CD3–/CD19+ B cells or CD3+/CD19– T cells as described in “Patients, materials, and methods” and expressed as the percentage of the viability of untreated cells. Data are shown as the mean value ± SD of 5 patients (patients 3, 6, 7, 12, and 13). (B) Effect on the stability and accumulation of p53 targets. B-CLL cells were untreated (C) or treated with 0.2 μM doxorubicin (D), or 2.5 μM chlorambucil (CH), 0.4 μM fludarabine (F), or 0.5 mM acadesine without or with 1 μM nutlin-3a for 48 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53, MDM2, p21, PUMA, and Bcl-2 were determined. (C) Effect of nutlin-3a on chemotherapeutic drugs dose responses. B-CLL cells (patient 2) were cultured with increasing doses of chemotherapeutic drugs without (○, doxorubicin; □, chlorambucil; ▵, fludarabine; ⋄, acadesine) or with (•, doxorubicin; ▪, chlorambucil; ▴, fludarabine; ♦, acadesine) 1 μM nutlin-3a for 48 hours. (D) Combination index (CI) determined in relation to the fraction of cells affected using median dose effect analysis to characterize interactions of nutlin-3a with the chemotherapeutic drugs (•, doxorubicin; ▪, chlorambucil; ▴, fludarabine; ♦, acadesine). CI values < 1.0 correspond to synergistic interactions. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and it is expressed as the percentage of the viability of untreated cells.
Synergism between MDM2 antagonists and chemotherapeutic drugs. (A) Effect on the viability of B cells and T cells in B-CLL. Cells were untreated (C) or treated with 0.2 μM doxorubicin (D), 2.5 μM chlorambucil (CH), or 0.4 μM fludarabine (F) without (□) or with (▪)1 μM nutlin-3a for 48 hours. Viability was measured as nonapoptotic CD3–/CD19+ B cells or CD3+/CD19– T cells as described in “Patients, materials, and methods” and expressed as the percentage of the viability of untreated cells. Data are shown as the mean value ± SD of 5 patients (patients 3, 6, 7, 12, and 13). (B) Effect on the stability and accumulation of p53 targets. B-CLL cells were untreated (C) or treated with 0.2 μM doxorubicin (D), or 2.5 μM chlorambucil (CH), 0.4 μM fludarabine (F), or 0.5 mM acadesine without or with 1 μM nutlin-3a for 48 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53, MDM2, p21, PUMA, and Bcl-2 were determined. (C) Effect of nutlin-3a on chemotherapeutic drugs dose responses. B-CLL cells (patient 2) were cultured with increasing doses of chemotherapeutic drugs without (○, doxorubicin; □, chlorambucil; ▵, fludarabine; ⋄, acadesine) or with (•, doxorubicin; ▪, chlorambucil; ▴, fludarabine; ♦, acadesine) 1 μM nutlin-3a for 48 hours. (D) Combination index (CI) determined in relation to the fraction of cells affected using median dose effect analysis to characterize interactions of nutlin-3a with the chemotherapeutic drugs (•, doxorubicin; ▪, chlorambucil; ▴, fludarabine; ♦, acadesine). CI values < 1.0 correspond to synergistic interactions. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods,” and it is expressed as the percentage of the viability of untreated cells.
Discussion
Using a recently developed inhibitor of the p53-MDM2 interaction, nutlin-3, we show that it stabilizes and activates p53 in B-CLL cells that express wild-type p53, leading to induction of apoptosis. The doses of nutlin-3a showing apoptosis induction in B-CLL samples in vitro are achievable in vivo, as previously demonstrated by its potent antitumor effect in mouse models of human cancer.4 Although nutlin-3 is an effective p53 activator and apoptosis inducer alone, it also synergizes with several currently used genotoxic drugs (eg, fludarabine, chlorambucil, and doxorubicin), which derive their activity, at least in part, from p53 activation. These results suggest that MDM2 antagonists may have therapeutic use in the treatment of B-CLL either as a single agent or in combination with drugs representing the current standard of therapy.
Our experiments indicate that B-CLL cells and normal B cells are more sensitive to nutlin-induced apoptosis than T cells from the same samples. Moreover, nutlin-3a synergizes with chemotherapeutic drugs in B-CLL cells but not in T lymphocytes. Chemotherapeutic drugs, including fludarabine, chlorambucil, and doxorubicin, induce apoptosis equally in both B and T cells,15,27 leading to immunosuppression.28,29 Thus, the differential effect of MDM2 antagonists in B and T lymphocytes is of interest.
In a previous report,16 p53 inactivation was associated with resistance to alkylating agents and fludarabine but not with resistance to anthracyclines (doxorubicin). However, our results show that patients with mutated p53 are more resistant to doxorubicin. B-CLL cells that do not respond to fludarabine or doxorubicin but do respond to nutlin-3a could have alterations upstream of p53 and are good candidates for treatment with MDM2 antagonists. In contrast, patients with mutated p53 are not expected to benefit from nutlin-3a treatment. Furthermore, ex vivo analysis of nutlin-3a–induced apoptosis could be a useful method to detect p53 alterations in B-CLL and other cancer cells.
Nutlin-3a is a specific activator of the p53 pathway, which allows distinction between p53-dependent and p53-independent genes induced by chemotherapy. Our results indicate that p21CIP1, PUMA, BAX, WIG1,30,31 and PIG3,32 induced by nutlin-3a, represent p53 target genes in B-CLL. In contrast, the expression of MCG10 that is induced by doxorubicin but not nutlin-3a could be mediated by a p53-independent mechanism. Such p53-independent mechanisms for doxorubicin and fludarabine in B-CLL have been proposed previously.33,34 Although NOXA and BID are p53 target genes in some cell types,35,36 our results indicate that these genes are not p53 targets in B-CLL cells. In agreement with our results, it was recently reported that fludarabine treatment induces PUMA and Bax expression but not Noxa or Bid.37 PUMA, Bax, and p21 were up-regulated by ionizing radiation in ATM/p53 wild-type tumors but not in p53 mutant or ATM mutant.38
Although p53 is the most frequently altered protein in most solid malignancies, it is rarely mutated in B-CLL at the time of diagnosis. Thus, B-CLL represents a good candidate for treatment with MDM2 antagonists or any other p53 activating agents that require functional p53. From a therapeutic perspective, it is interesting that nutlin synergizes with chemotherapeutic drugs in B-CLL cells but not in normal T cells. This suggests the possibility of lowering the doses of chemotherapeutic drugs used in the treatment of B-CLL and thus reducing cytotoxicity to normal T cells. In conclusion, the results presented here suggest that MDM2 antagonists alone or in combination with chemotherapeutic drugs could offer a new therapeutic option for treatment of B-CLL.
Prepublished online as Blood First Edition Paper, January 26, 2006; DOI 10.1182/blood-2005-08-3273.
Supported by a grant from the Ministerio de Educación y Ciencia and Fondo Europeo de Desarollo Regional (FEDER) (SAF2004-00265) to J.G.; by fellowships from the Ministerio de Educación y Ciencia to L.C.-M., D.I.-S., A.F.S., and A.M.C.; and by a fellowship from the Generalitat de Catalunya to M.d.F.
One of the authors (L.T.V.) is employed by Hoffman La-Roche Inc, whose potential product was studied in the present work.
L.C.-M. performed research and contributed to data analysis and manuscript writing; D.I.-S., A.F.S., A.M.C., and M.d.F. performed research; E.C., C.C., and M.B. contributed with analytical tools; A.F.d.S. and A.D. contributed with patient samples and data; L.T.V. contributed to data analysis and manuscript writing; G.P. designed research and contributed to data analysis; and J.G. designed research and contributed to data analysis and manuscript writing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Dolors Colomer, Dr Eva Domingo, Dr Eva González, Dr Ana Carla Oliveira, Dr Jose Luis Rosa, and Eduard Casas for helpful discussions and suggestions; Robin Rycroft for language assistance; Dr Bert Vogelstein (The Johns Hopkins Medical Institutes, Baltimore) for kindly providing the anti-PUMA antibody; and Dr Miguel A Peinado (IDIBELL-IRO) for kindly providing p53 primers for sequencing. The authors also thank the Unitat de Biologia and the Unitat de Genòmica from the Serveis Cientificotècnics at the Universitat de Barcelona for the technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal