The generation of pathogen-specific immune responses is dependent on the signaling capabilities of pathogen-recognition receptors. DC-SIGN is a C-type lectin that mediates capture and internalization of viral, bacterial, and fungal pathogens by myeloid dendritic cells. DC-SIGN–interacting pathogens are thought to modulate dendritic cell maturation by interfering with intracellular signaling from Toll-like receptor molecules. We report that engagement of DC-SIGN by specific antibodies does not promote dendritic cell maturation but induces ERK1/2 and Akt phosphorylation without concomitant p38MAPK activation. DC-SIGN ligation also triggers PLCγ phosphorylation and transient increases in intracellular calcium in dendritic cells. In agreement with its signaling capabilities, a fraction of DC-SIGN molecules partitions within lipid raft–enriched membrane fractions both in DC-SIGN–transfected and dendritic cells. Moreover, DC-SIGN in dendritic cells coprecipitates with the tyrosine kinases Lyn and Syk. The relevance of the DC-SIGN–initiated signals was demonstrated in monocyte-derived dendritic cells, as DC-SIGN cross-linking synergizes with TNF-α for IL-10 release and enhances the production of LPS-induced IL-10. These results demonstrate that DC-SIGN–triggered intracellular signals modulate dendritic cell maturation. Since pathogens stimulate Th2 responses via preferential activation of ERK1/2, these results provide a molecular explanation for the ability of DC-SIGN–interacting pathogens to preferentially evoke Th2-type immune responses.
Introduction
The functional consequences of the dendritic cell (DC)–T lymphocyte interactions are critically dependent on the maturation state of the DC.1 In the steady state, immature DCs capture and process antigens and promote either deletion of self-specific T cells or the generation and expansion of regulatory T cells, thus resulting in tolerance and preventing autoimmune responses. By contrast, in the presence of pathogens, DCs acquire the capacity to initiate potent immune responses (DC maturation).2 Pathogen recognition is accomplished by pathogen-associated molecular patterns (PAMPs) receptors, which include members of the Toll-like receptor (TLR) and lectin protein families3 and endow DCs with the ability to respond to exogenous agents and microbes.
The DC maturation program exhibits a huge degree of plasticity, thus allowing the generation of pathogen-tailored immune responses. Indeed, gene expression profile analysis of DCs exposed to pathogens or pathogen-derived products has confirmed that DC maturation is pathogen specific.4 The intracellular signaling pathways that regulate DC maturation are beginning to be unraveled. In the case of TLR ligands, activation of NF-κB is an absolute requirement for DC maturation,5 but the modulation of NF-κB–dependent gene transcription by other signaling routes contributes to the generation of pathogen-specific responses.6 As an example, the differential ability of maturation-inducing agents to promote IL-12p70 release depends on their distinct capacity to activate p38MAPK, which “conditions” the IL-12p35 regulatory region for NF-κB occupancy.7 Unlike p38MAPK, activation of the MEK-ERK signaling axis impairs the acquisition of maturation parameters,8 and PI3K activation also modulates NF-κB–induced dendritic cell maturation.9 Comparison of the intracellular signals from TLR4 and TLR2 has recently suggested that TLR agonists differentially instruct dendritic cells to initiate Th responses via modulation of intracellular signaling pathways10 : TLR4 ligation favors pro-Th1 dendritic cell maturation through the p38MAPK-dependent synthesis of IL-12p70, whereas TLR2 ligands stimulate Th2 responses via preferential activation of ERK1/2 and c-fos.11
DCs display a large array of cell surface lectins and lectinlike molecules whose contribution to the maturation program is not completely understood. DC-SIGN is a C-type lectin implicated in capture and uptake of viral (HIV, hepatitis C virus [HCV], Ebola, dengue), bacterial, fungal, and parasite pathogens by DCs.3,12 Most DC-SIGN–interacting microbes elicit Th2-type responses that result in impaired pathogen clearance and the establishment of chronic infections. This has led to the proposal that pathogens subvert DC-SIGN functions as a means to avoid immunosurveillance and the generation of effective immune responses.3,12 In this regard, the shift of the immune responses toward Th2 caused by Mycobacterium tuberculosis appears to depend on lipoarabinomannan, which increases IL-10 release from DCs by interacting with DC-SIGN.12 Therefore, the determination of DC-SIGN–initiated intracellular signals might facilitate the development of therapeutic strategies against the pathogens recognized by this lectin. We now present evidence that DC-SIGN ligation triggers activation of PI3K and ERK1/2, and a rapid and transient increase in intracellular calcium mobilization, in both dendritic cells and transfected cells, and that DC-SIGN colocalizes with protein tyrosine kinases in specialized membrane microdomains. The functional relevance of these intracellular signals is illustrated by the enhanced release of IL-10 observed in maturing dendritic cells upon DC-SIGN cross-linking. These results demonstrate that DC-SIGN–triggered intracellular signals modulate dendritic cell maturation and provide a molecular explanation for the ability of DC-SIGN–interacting pathogens to preferentially provoke Th2-type immune responses.
Materials and methods
Cell culture
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from healthy donors over a Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient according to standard procedures. Monocytes were purified from PBMCs by a 1-hour adherence step at 37°C in complete medium or by magnetic cell sorting using CD14 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). To generate monocyte-derived dendritic cells (MDDCs), adherent or CD14+ cells (> 95% monocytes) were cultured at 0.5 to 1 × 106 cells/mL in RPMI with 10% fetal calf serum (FCS), 25 mM HEPES, and 2 mM glutamine (complete medium), at 37°C in a humidified atmosphere with 5% CO2. Complete medium was supplemented with 1000 U/mL granulocyte-macrophage–colony-stimulating factor (GM-CSF, Leucomax; Schering-Plough, Kenilworth, NJ) and 1000 U/mL IL-4 (PreProtech, Rocky Hill, NJ) for 5 to 7 days, with cytokine addition every second day. MDDC maturation was induced with either TNF-α (20 ng/mL; Peprotech EC, London, England), ultrapure LPS from Escherichia coli 0111:B4 (10 ng/mL), or Pam3Cys (20 μg/mL; InvivoGen, San Diego, CA). The human cell lines Jurkat (T-cell lymphoma) and THP-1 (monocytic leukemia) were cultured in complete medium. DC-SIGN–expressing Jurkat cells (Jurkat-DC-SIGN) were generated after electroporation of the pCDNA3-DC-SIGN plasmid,13 selection in complete medium with 600 μg/mL G418 (Gibco, Grand Island, NY), and cell sorting with the MR-1 antibody. A similar procedure was done to generate Jurkat-mock cells, which are stably transfected with an empty pCDNA3.1(–) vector. Cells were observed under a Zeiss Axiovert 25 microscope equipped with a 32 ×/0.4 Ph1 lens (Zeiss, Jena, Germany), and were photographed with a RICOH XR-X3000 camera (Ricoh, West Caldwell, NJ). Image acquisition was performed with Dell photographic editor software in the Dell AIO printer (Dell, Round Rock, TX); subsequent processing was done with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Flow cytometry and antibodies
Phenotypic analysis of MDDCs and cell lines was carried out by immunofluorescence. Monoclonal antibodies used for cell surface staining included phycoerythrin-labeled anti-CD83 (BD Biosciences, San Diego, CA), FITC-FA6-152 (anti-CD36; Immunotech, Marseille, France), and MR1 (anti–DC-SIGN, CD209).13 In this case, incubation with the unlabeled primary antibody was followed by incubation with FITC-labeled F(ab′)2 goat anti–mouse IgG. All incubations were done in the presence of 50 μg/mL human IgG to prevent binding through the Fc portion of the antibodies. P3X63 myeloma supernatant was included as negative control, and flow cytometry analysis was performed with an EPICS-CS (Coulter, Madrid, Spain) using log amplifiers.
Determination of IL-10 levels
Immature MDDCs (106/mL complete medium) were treated with maturing agents (LPS at 10 ng/mL, Pam3Cys at 20 μg/mL, or TNF-α at 20 ng/mL) and either in the absence or presence of purified antibody against DC-SIGN (MR-1) or purified mouse IgG as control. After 18 hours, cell supernatants were collected and IL-10 levels determined using the IL-10 ELISA Set (Immunotools, Friesoythe, Germany) following the manufacturer's recommendations. MDDC supernatants were assayed undiluted and diluted 1:3 in complete medium.
DC-SIGN cross-linking experiments
MDDCs or Jurkat cells were washed extensively in RPMI and cultured in RPMI containing 0.5% FCS to reduce the basal level of activation. Preliminary experiments indicated that MDDCs cultured overnight in RPMI 0.5% FCS exhibited a low level of ERK phosphorylation, whereas Jurkat cells required a 24/36-hour culture period. Then cells were transferred to a 37°C water bath and treated with the stimulatory agents for 5 minutes or the indicated period of time. In all cases, 2 × 106 MDDCs or Jurkat cells was used for each experimental condition. Stimulatory antibodies were added at 20 μg/mL, and included purified monoclonal antibodies against DC-SIGN (MR-1),13 CD38 (HB136), CD70 (qa32), CD11c (HC1/1), and c-Myc (9E10). As positive control, cells were treated with either TNF-α (20 ng/mL), PMA (10 ng/mL), or a monoclonal antibody against CD3 at 20 μg/mL (OKT3). In some experiments, negative controls also included purified human IgG (AP3D11). After stimulation, cells were immediately lysed with 2 × lysis buffer (40 mM HEPES [pH 7.6], 300 mM NaCl, 2 mM EGTA, 1% NP-40, 100 μM phenylarsine oxide, 100 mM NaF, 2 mM Na3VO4, 2 mM Pefabloc, 20 mM iodoacetamide, and 2 μg/mL aprotinin, antipain, leupeptin, and pepstatin), and cell lysates subjected to Western blot with antibodies specific for the activated/phosphorylated forms of various signaling pathways.
Western blot
Cell lysates (10 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred onto an Immobilon polyvinylidene difluoride membrane (PVDF; Millipore, Bedford, MA). After blocking with 1% BSA in 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Tween-20, protein detection was performed using the Supersignal West Pico Chemiluminescent system (Pierce, Rockford, IL). For reprobing, membranes were incubated in stripping buffer (62.5 mM Tris-HCl [pH 6.7], 100 mM β-mercaptoethanol, 2% SDS) for 30 minutes at 50°C with occasional agitation. Detection of phosphotyrosine, phospho-p38MAPK, phospho-PI3K, phospho-PLCγ, phospho-ERK, and phospho-ZAP70 was carried out using polyclonal antibodies specific for antiphosphotyrosine (RC20-HRP; BD Biosciences), anti–phospho-p38MAPK (T180/Y182, no. 9211; Cell Signaling Technology, Beverly, MA), anti–phospho-Akt (no. 9271; Cell Signaling Technology), anti–phospho-PLCγ (sc-12943; Santa Cruz Biotechnology, Santa Cruz, CA), anti–phospho-ZAP70 (Y493, no. 9212; Cell Signaling Technology), and a monoclonal antibody anti–phospho-p44/42 MAPK (T202-Y204, no. 9101; Cell Signaling Technology). As a control for protein loading, blots were reprobed with polyclonal antisera against ERK (no. 9102; Cell Signaling Technology), p38MAPK (no. 9212; Cell Signaling Technology), PLCγ (sc-423; Santa Cruz Biotechnology), or tubulin (clone DM 1A; Sigma, Barcelona, Spain).
Isolation of detergent-insoluble and -soluble cell membrane fractions by sucrose gradient ultracentrifugation and membrane distribution of DC-SIGN
Detergent solubilization of cells (7-9 × 107) at 37°C with 1% Brij 98 (Sigma Aldrich, St Louis, MO) was carried out as described.14 Cell lysates were mixed with an equal volume of 80% sucrose and transferred to Sorvall ultracentrifuge tubes (Sorvall, Asheville, NC). The following were overlaid: 2 mL 30% sucrose, followed by 1 mL 5% sucrose in 20 mM HEPES (pH 7.6), 150 mM NaCl, 1 mM EGTA, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 20 μM phenylarsine oxide, 1 mM phenylmethylsulfonyl fluoride, 10 mM iodoacetamide, and a mixture of small peptide protease inhibitors at 1 μg/mL each. All the sucrose solutions were prepared in the same buffer without detergent and in the presence of small peptide protease inhibitors at 1 μg/mL. Samples were centrifuged for 18 to 20 hours at 200 000g in a Sorvall AH-650 rotor. Eight 0.5-mL fractions were collected on ice, from the top to the bottom of the gradients. Aliquots of each fraction (18 μL) of the gradient were diluted with 9 μL3 × Laemmli sample buffer and resolved on 12.5% SDS-PAGE under nonreducing conditions, transferred to PVDF, and immunoblotted with specific antibodies, which included polyclonal antibodies against DC-SIGN,15 Lck (sc-13; Santa Cruz Biotechnology), ZAP-70 (sc-574; Santa Cruz Biotechnology), LAT (06-807; Upstate Biotechnology, Charlottesville, VA), CD3ϵ (A0452; Dako, Glostrup, Denmark), Syk (AW 1373-13, kindly provided by Dr Arthur Weiss), Lyn (sc-15; Santa Cruz Biotechnology), ERK (sc-154; Santa Cruz Biotechnology), cholera toxin–HRP (C4672; Sigma) for ganglioside GM1 detection, or a monoclonal antibody against Ras (RAS10; Upstate Biotechnology). In indicated experiments, pools of the sucrose gradient fractions were collected: the pool of the low-density fractions corresponding to the 5%/30% interface (fractions 2-4) was referred to as rafts; the pool of fractions 5 and 6 was termed intermediate (intrm.); and the pool of the high-density soluble material corresponding to fractions 7 and 8 was referred to as soluble. Cytoskeletal-associated rafts (CARs) were extracted from the pellet by treatment with 60 mM octyl d-glucoside (ODG) and 1% Brij 98, and the supernatant was collected after centrifugation at 13 000g.16,17 In indicated experiments, immunoprecipitation under solubilizing conditions was carried out on pooled rafts, intermediate and soluble. Those pools were diluted with lysis buffer containing 1% Brij 98 + 60 mM ODG to less than 20% sucrose.
Immunoprecipitation of protein assemblies was performed by incubation of these pools with purified monoclonal antibodies against DC-SIGN (MR-1) or anti-Lyn (sc-7274; Santa Cruz Biotechnology) followed by capture of the immune complexes on Protein G superparamagnetic Microbeads (Miltenyi Biotech) as described elsewhere.14 Immunoprecipitates were then subjected to Western blot using polyclonal antibodies against DC-SIGN (DSG-1),15 Syk (AW 1373-13), Lyn (sc-15), or antiphosphotyrosine (RC20-HRP). In some experiments, cells were surface-labeled with biotin. To that end, cells were washed in PBS (pH 8.0) and incubated in 0.5 mg/mL biotinamidohexanoic acid 3-sulfo-N-hydroxysuccinimide ester sodium salt (B1022; Sigma Aldrich) for 30 minutes at room temperature. After labeling, cells were extensively washed in PBS, lysed, and subjected to fractionation by sucrose gradient ultracentrifugation as indicated.14 After separation, lipid raft–containing and soluble fractions were pooled and immunoprecipitated with Streptavidin-agarose (Sigma Aldrich), and immunoprecipitated material was analyzed by SDS-PAGE and Western blot with antibodies specific for DC-SIGN or LAT.
Intracellular calcium determination
MDDCs (2.5 × 106 cells/mL) were resuspended in complete medium and incubated with Fluo-3AM (Calbiochem, San Diego, CA; 300 μM in DMSO, 10 μL/106 cells) for 30 minutes at 37°C. Cells were then washed, resuspended in RPMI containing 2 mM CaCl2, and maintained at 37°C before addition of purified anti–DC-SIGN MR-1 monoclonal antibody (20 μg/mL) or an isotype-matched control. Calcium flux was measured in an EPICS XL flow cytometer at 525 nm (Coulter, Madrid, Spain). Fluo-3AM loading was controlled using ionomycin as ionophore (5 μg/mL). In some experiments SDF-1α (50 mM; Preprotech) was added before addition of the monoclonal antibody.
Results
DC-SIGN engagement leads to ERK and Akt activation but does not induce MDDC maturation
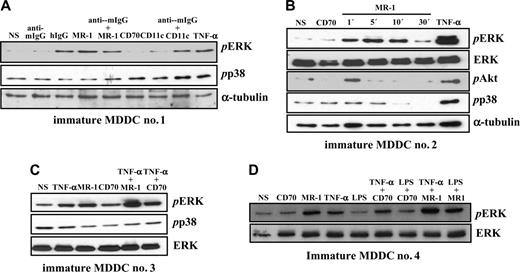
To evaluate the signaling capability of DC-SIGN, we initially assessed the influence of an anti–DC-SIGN antibody on the MDDC maturation state. Whereas LPS induced MDDC maturation parameters (CD83 induction and loss of CD36), purified MR-1 (anti–DC-SIGN) or HB136 (anti-CD38) antibodies at 20 μg/mL (1X) or 60 μg/mL (3X) for 24 hours neither altered cell morphology (data not shown) nor affected the expression of CD83 or CD36 (Figure 1A). Therefore, ligation of DC-SIGN on the cell surface with the MR-1 antibody does not lead to overt MDDC maturation. However, cross-linking of DC-SIGN with MR-1 induced a change in the profile of phosphotyrosine-containing proteins in MDDCs (Figure 1B). The change occurred within 1 minute (data not shown) and was not further amplified upon cross-linking with a secondary antibody, which, per se, did not induce any change in the phosphotyrosine profile (Figure 1B). We reasoned that the observed effect of cross-linking was unlikely to be due to endotoxin contamination of the antibody, because neither primary nor secondary control antibodies alone mimicked the stimulatory effect and MDDCs exposed to the anti–DC-SIGN antibody did not exhibit any maturation-specific parameters (Figure 1A).
DC-SIGN ligation on MDDCs does not induce maturation but alters the profile of phosphotyrosine-containing proteins. (A) Immature MDDCs were isolated and either not stimulated (NS) or treated with lipopolysaccharide (LPS) or monoclonal antibodies against CD38 (HB136) or DC-SIGN (MR-1) at 20 μg/mL (1X) or 60 μg/mL (3X). After 48 hours, cells were collected and the cell surface expression of CD83 and CD36 was determined by flow cytometry, using an isotype-matched anti–c-Myc antibody (9E10) as control. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. Three experiments were performed on MDDCs from independent donors, and a representative experiment is shown. (B) DC-SIGN ligation induces changes in the pattern of tyrosine phosphorylation in MDDCs. Cells were either left untreated (not stimulated, NS) or incubated with monoclonal antibodies against DC-SIGN (MR-1) as ligation agent, or with MR-1 plus a cross-linking secondary antibody (anti–mouse F(ab′)2, anti–mouse IgG). After 5 minutes, cells were lysed, and the lysates were probed for phosphotyrosine residues by Western blot using the RC20-HRP monoclonal antibody. As a control, a monoclonal antibody against c-Myc (anti–c-Myc) was used. Two experiments on MDDCs from independent donors rendered similar results, and one of them is shown.
DC-SIGN ligation on MDDCs does not induce maturation but alters the profile of phosphotyrosine-containing proteins. (A) Immature MDDCs were isolated and either not stimulated (NS) or treated with lipopolysaccharide (LPS) or monoclonal antibodies against CD38 (HB136) or DC-SIGN (MR-1) at 20 μg/mL (1X) or 60 μg/mL (3X). After 48 hours, cells were collected and the cell surface expression of CD83 and CD36 was determined by flow cytometry, using an isotype-matched anti–c-Myc antibody (9E10) as control. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. Three experiments were performed on MDDCs from independent donors, and a representative experiment is shown. (B) DC-SIGN ligation induces changes in the pattern of tyrosine phosphorylation in MDDCs. Cells were either left untreated (not stimulated, NS) or incubated with monoclonal antibodies against DC-SIGN (MR-1) as ligation agent, or with MR-1 plus a cross-linking secondary antibody (anti–mouse F(ab′)2, anti–mouse IgG). After 5 minutes, cells were lysed, and the lysates were probed for phosphotyrosine residues by Western blot using the RC20-HRP monoclonal antibody. As a control, a monoclonal antibody against c-Myc (anti–c-Myc) was used. Two experiments on MDDCs from independent donors rendered similar results, and one of them is shown.
Analysis of the pattern of tyrosine phosphorylation after DC-SIGN cross-linking revealed an increased phosphorylation in the 42 to 50 kDa range, suggesting that ERK1/2 MAP kinases might be candidate substrate proteins. To determine whether this was the case, immature MDDCs were treated with MR-1 and probed for the presence of phosphorylated ERK. DC-SIGN ligation promoted ERK activation after 5 minutes, whereas an antibody against CD70 was without effect and antibodies against CD11c triggered significant ERK phosphorylation only upon cross-linking with a secondary antibody (Figure 2A). Of interest, further cross-linking of DC-SIGN with an anti–mouse IgG resulted in weaker ERK activation (Figure 2A). By contrast, DC-SIGN ligation did not induce p38 phosphorylation, whereas TNF-α promoted both ERK and p38 activation (Figure 2A).
Kinetic experiments on MDDCs from an independent donor indicated that DC-SIGN ligation-induced ERK phosphorylation is a transient event that takes place as early as 1 minute after stimulation and vanishes after 30 minutes, a time point at which p38 activation also appears to be diminished by DC-SIGN ligation (Figure 2B). Moreover, DC-SIGN ligation promoted a weak and transient activation of PI3K, as evidenced by the appearance of phosphorylated Akt only 1 minute after stimulation (Figure 2B). Altogether, these results indicate that antibody ligation of DC-SIGN on the cell surface of MDDCs induces a wave of intracellular signaling that results in transient activation of ERK and PI3K, 2 kinases critically involved in dendritic cell maturation.9,18
DC-SIGN engagement was further evaluated for its capacity to modulate MDDC maturation signals initiated from other cell surface receptors. To this purpose, we initially focused on TNF-α, which induces maturation markers on MDDCs.19 Whereas TNF-α and DC-SIGN ligation induced ERK activation to a similar extent (3.5-fold and 5.8-fold increase, respectively, over background levels), addition of both stimuli to MDDCs resulted in further enhanced phosphorylation of ERK (to 11.5-fold), whereas the combination of TNF-α and a control antibody did not result in any enhancement (Figure 2C). Analysis of MDDCs from an independent donor produced essentially similar results and indicated that DC-SIGN cross-linking is capable of inducing ERK phosphorylation even in the presence of ultrapure LPS, which failed to promote significant ERK activation by itself (Figure 2D). Altogether, these results demonstrate that DC-SIGN is capable of modulating the intracellular signals originated from maturation-inducing factors.
DC-SIGN ligation on MDDCs induces ERK phosphorylation and collaborates with TNF-α–initiated signals for enhanced ERK activation. (A) Activation of ERK by DC-SIGN engagement. DC-SIGN on MDDCs was engaged by the anti–DC-SIGN MR-1 antibody and the cells were incubated at 37°C for 5 minutes. For comparison, cells were treated with the indicated combinations of antibodies or TNF-α (20 ng/mL). Following cell lysis, phosphorylated ERK and phosphorylated p38 were detected using specific polyclonal antisera. Blots were then stripped and probed for α-tubulin levels as a control for protein loading (bottom panel). (B) DC-SIGN on MDDCs was engaged by the anti–DC-SIGN MR-1 antibody, and the cells were incubated at 37°C for the indicated periods of time. For comparison, cells were treated with an antibody against CD70 or TNF-α (20 ng/mL) for 5 minutes. Following cell lysis, phosphorylated ERK, p38, and Akt, and total ERK were detected using specific polyclonal antisera. Blots were then stripped and probed for α-tubulin levels as a control for protein loading. (C-D) Immature MDDCs from 2 independent donors were incubated with an antibody against DC-SIGN (MR-1) or against CD70, either alone or in combination with TNF-α (20 ng/mL) or LPS (10 ng/mL), and the cells were incubated at 37°C for 5 minutes. For comparison, cells were treated with either LPS (10 ng/mL) or TNF-α (20 ng/mL) for 5 minutes. Following cell lysis, phosphorylated ERK (pERK), phosphorylated p38 (pp38), or total ERK content (ERK) was detected using specific polyclonal antisera. Each experiment was done on MDDCs from at least 4 independent donors, and representative experiments are shown. In all panels, NS refers to cells that were not stimulated.
DC-SIGN ligation on MDDCs induces ERK phosphorylation and collaborates with TNF-α–initiated signals for enhanced ERK activation. (A) Activation of ERK by DC-SIGN engagement. DC-SIGN on MDDCs was engaged by the anti–DC-SIGN MR-1 antibody and the cells were incubated at 37°C for 5 minutes. For comparison, cells were treated with the indicated combinations of antibodies or TNF-α (20 ng/mL). Following cell lysis, phosphorylated ERK and phosphorylated p38 were detected using specific polyclonal antisera. Blots were then stripped and probed for α-tubulin levels as a control for protein loading (bottom panel). (B) DC-SIGN on MDDCs was engaged by the anti–DC-SIGN MR-1 antibody, and the cells were incubated at 37°C for the indicated periods of time. For comparison, cells were treated with an antibody against CD70 or TNF-α (20 ng/mL) for 5 minutes. Following cell lysis, phosphorylated ERK, p38, and Akt, and total ERK were detected using specific polyclonal antisera. Blots were then stripped and probed for α-tubulin levels as a control for protein loading. (C-D) Immature MDDCs from 2 independent donors were incubated with an antibody against DC-SIGN (MR-1) or against CD70, either alone or in combination with TNF-α (20 ng/mL) or LPS (10 ng/mL), and the cells were incubated at 37°C for 5 minutes. For comparison, cells were treated with either LPS (10 ng/mL) or TNF-α (20 ng/mL) for 5 minutes. Following cell lysis, phosphorylated ERK (pERK), phosphorylated p38 (pp38), or total ERK content (ERK) was detected using specific polyclonal antisera. Each experiment was done on MDDCs from at least 4 independent donors, and representative experiments are shown. In all panels, NS refers to cells that were not stimulated.
DC-SIGN engagement triggers intracellular signaling in myeloid and lymphoid cell lines
Next, we tested whether the signaling capability of DC-SIGN is restricted to dendritic cells, where it is expressed in very high levels, or can be observed in other cell types. To that end, DC-SIGN was engaged with the MR-1 antibody on the surface of THP-1 cells, which exhibit a weak basal level of the lectin (Figure 3A). Although to a lower extent than in MDDCs, engagement of DC-SIGN on the surface of THP-1 also resulted in enhanced ERK activation, which was comparable with the phosphorylation level induced upon phorbol ester treatment (Figure 3B).
To extend these findings to a different cell lineage, the signaling ability of DC-SIGN was evaluated in Jurkat-DC-SIGN transfectants, which exhibit a high level of DC-SIGN cell surface expression (Figure 3C). Like K562 cells,20 Jurkat cells overexpressing DC-SIGN formed large homotypic aggregates (Figure 3D) and showed rapid lectin down-regulation upon ligation on the cell surface (data not shown), thus confirming that DC-SIGN retains its adhesive and antigen-capture capabilities in Jurkat cells.21 Accordingly, ligation of DC-SIGN in Jurkat cells led to ERK activation but had no effect on the phosphorylation state of ZAP-70 (Figure 3E) or p38MAPK (Figure 6A). Therefore, engagement of DC-SIGN on myeloid (MDDC, THP-1) or lymphoid (Jurkat) results in ERK phosphorylation.
DC-SIGN ligation in myeloid and lymphoid cell lines results in ERK activation. (A) Cell surface expression of DC-SIGN in THP-1 cells, as determined by flow cytometry. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. (B) DC-SIGN on THP-1 cells was engaged by the anti–DC-SIGN MR-1 antibody (20 μg/mL), and the cells were incubated at 37°C for 5 minutes. As a control, cells were either incubated with an anti-CD70 monoclonal antibody or with PMA (10 ng/mL). After cell lysis, phosphorylated ERK (pERK, top panel) and total ERK (ERK, middle panel) were detected using specific polyclonal antisera. The bottom panel illustrates the level of pERK relative to the level of total ERK under each condition, as determined by densitometric analysis. NS indicates not stimulated. (C) Cell surface expression of DC-SIGN in mock-transfected (Jurkat) and DC-SIGN–transfected Jurkat cells (Jurkat-DC-SIGN) as determined by flow cytometry, using an isotype-matched anti–c-Myc antibody (9E10) as control. The mean fluorescence intensity is indicated in each case. (D) Homotypic aggregation of mock-transfected (Jurkat) and DC-SIGN–transfected (Jurkat-DCSIGN) Jurkat cells, as observed by reverse-phase microscopy at 2 different amplifications (5 ×, 10 ×). (E) DC-SIGN on Jurkat-DC-SIGN transfectants was ligated by the anti–DC-SIGN MR-1 antibody alone or in the presence of an anti-CD3 monoclonal antibody as control, and the cells were incubated at 37°C for 1 or 5 minutes. Mock-transfected Jurkat cells were subjected to the same treatments for control purposes. After cell lysis, phosphorylated ERK (pERK), phosphorylated ZAP-70 (pZAP-70), and total content of ERK and ZAP-70 were detected using specific polyclonal antisera. Each experiment was done 3 times with similar results. Representative results are shown.
DC-SIGN ligation in myeloid and lymphoid cell lines results in ERK activation. (A) Cell surface expression of DC-SIGN in THP-1 cells, as determined by flow cytometry. The percentage of marker-positive cells and the mean fluorescence intensity are indicated in each case. (B) DC-SIGN on THP-1 cells was engaged by the anti–DC-SIGN MR-1 antibody (20 μg/mL), and the cells were incubated at 37°C for 5 minutes. As a control, cells were either incubated with an anti-CD70 monoclonal antibody or with PMA (10 ng/mL). After cell lysis, phosphorylated ERK (pERK, top panel) and total ERK (ERK, middle panel) were detected using specific polyclonal antisera. The bottom panel illustrates the level of pERK relative to the level of total ERK under each condition, as determined by densitometric analysis. NS indicates not stimulated. (C) Cell surface expression of DC-SIGN in mock-transfected (Jurkat) and DC-SIGN–transfected Jurkat cells (Jurkat-DC-SIGN) as determined by flow cytometry, using an isotype-matched anti–c-Myc antibody (9E10) as control. The mean fluorescence intensity is indicated in each case. (D) Homotypic aggregation of mock-transfected (Jurkat) and DC-SIGN–transfected (Jurkat-DCSIGN) Jurkat cells, as observed by reverse-phase microscopy at 2 different amplifications (5 ×, 10 ×). (E) DC-SIGN on Jurkat-DC-SIGN transfectants was ligated by the anti–DC-SIGN MR-1 antibody alone or in the presence of an anti-CD3 monoclonal antibody as control, and the cells were incubated at 37°C for 1 or 5 minutes. Mock-transfected Jurkat cells were subjected to the same treatments for control purposes. After cell lysis, phosphorylated ERK (pERK), phosphorylated ZAP-70 (pZAP-70), and total content of ERK and ZAP-70 were detected using specific polyclonal antisera. Each experiment was done 3 times with similar results. Representative results are shown.
DC-SIGN is found in lipid-rich regions on the cell surface of transfected lymphoid cells
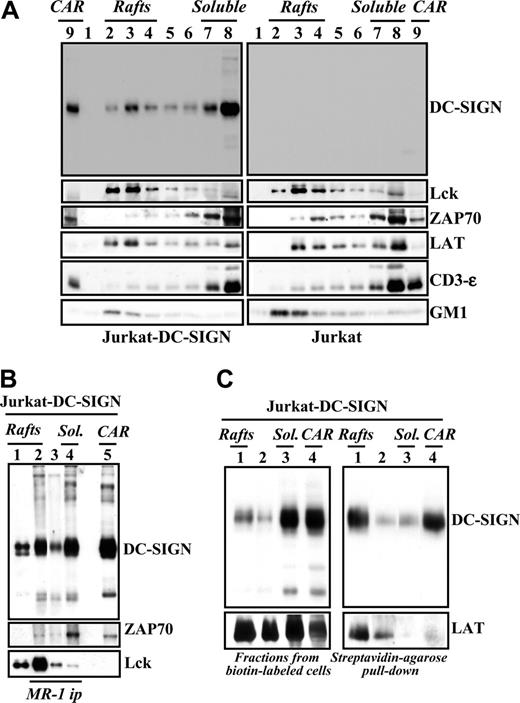
Lipid rafts are specialized membrane regions that facilitate outside-in signaling by generating a physical environment rich in kinases, adaptors, and intracellular effectors.22 The ability of DC-SIGN to trigger intracellular signals prompted us to analyze its membrane distribution in immature MDDCs and Jurkat-DC-SIGN cells. In agreement with a previous report,23 analysis of Jurkat-DC-SIGN transfectants revealed the presence of DC-SIGN in lipid rafts (Figure 4A). Although DC-SIGN was mainly found in the soluble fractions, a percentage of the molecules was detected in GM1-rich lipid rafts, where Lck and LAT, but not ZAP-70, were also detected (Figure 4A). To examine potential interactions of DC-SIGN with signaling proteins, DC-SIGN was immunoprecipitated from the distinct membrane fractions in the presence of 1% Brij 98 + 60 mM octyl d-glucoside (ODG), which efficiently disrupts many lipid raft–protein associations. As shown in Figure 4B (lane 2), Lck tyrosine kinase was coimmunoprecipitated with DC-SIGN from the lipid raft–containing fractions, whereas ZAP-70 was detectable in DC-SIGN immunoprecipitates from the soluble pool (Figure 4B lane 4). These results suggest that DC-SIGN can be found in 2 distinct microdomain localizations that differ in their content of signaling molecules. Next, to determine the microdomain location of cell surface DC-SIGN, lipid raft–containing and soluble membrane fractions were generated from biotin-labeled Jurkat-DC-SIGN cells, and the resulting fractions were immunoprecipitated with Streptavidin-agarose. In agreement with experiments using unlabeled cell lysates, a large proportion of DC-SIGN was found in the soluble fraction pool (Figure 4C left panel). However, pull down of cell surface molecules by immunoprecipitation with Streptavidin-agarose revealed that most biotin-labeled DC-SIGN molecules partitioned within the lipid raft– and cytoskeletal-associated raft-containing fractions (Figure 4C right panel). Densitometric analysis of the results shown in Figure 4C indicated that 80% of the total cell surface expression of DC-SIGN (biotin-labeled DC-SIGN) is found within the lipid raft–containing fractions. These results indicate that most DC-SIGN molecules on the cell surface are included in lipid rafts, whereas DC-SIGN molecules in the soluble fraction pool are not accessible for biotin labeling and might be contained in intracellular compartments.
DC-SIGN is present within lipid rafts in lymphoid cells and coprecipitates with tyrosine kinases. (A) Jurkat-DC-SIGN (left panels) and mock-transfected Jurkat cells (right panels) were lysed in 1% Brij 98 lysis buffer at 37°C, and Brij 98–insoluble fractions 2 to 4 (lanes 2-4, rafts) and the high-density Brij 98–soluble fractions 7 to 8 (lanes 7-8, soluble) were separated by 12.5% SDS-PAGE under nonreducing conditions. Cytoskeletal-associated rafts (CARs), obtained by solubilization of the cell pellet with Brij 98 + octyl d-glucoside in lysis buffer, were analyzed in parallel (lane 9). The distribution of DC-SIGN, Lck, ZAP-70, LAT, CD3ϵ, and ganglioside GM1 in the distinct fractions was determined by immunoblotting with specific antibodies or cholera toxin–HRP (for GM1). (B) Coprecipitation of DC-SIGN and tyrosine kinases. DC-SIGN was immunoprecipitated with the MR-1 antibody (MR1 ip) from lipid raft–containing fractions 2 to 4 (rafts, lane 2), fractions 5 to 6 (between the rafts and the soluble material, lane 3), and the 7 to 8 soluble fractions (sol., lane 4), and the immunoprecipitated material was subjected to SDS-PAGE and immunoblotting with antibodies against DC-SIGN, Lck, or ZAP-70. As a control, fractions containing either rafts (lane 1) or cytoskeletal-associated rafts (CARs, lane 5) were analyzed in parallel. (C) Jurkat-DC-SIGN cells were cell-surface labeled with biotin, lysed in 1% Brij 98 lysis buffer at 37°C, and Brij 98–insoluble fractions (lanes 1, rafts), intermediate fractions (lanes 2), high-density Brij 98–soluble fractions (lanes 3, sol.), and cytoskeletal-associated raft-containing fractions (CARs, lane 4) were obtained. An aliquot from each fraction was removed and analyzed by 12.5% SDS-PAGE under nonreducing conditions and subjected to Western blot with anti–DC-SIGN or anti-LAT polyclonal antisera (left panel). Then, fractions were subjected to pull-down with Streptavidin-agarose, and the immunoprecipitated material was separated by 12.5% SDS-PAGE under nonreducing conditions and subjected to Western blot with anti–DC-SIGN or anti-LAT polyclonal antisera (right panel). Each experiment was performed twice with similar results, and one of the experiments is shown.
DC-SIGN is present within lipid rafts in lymphoid cells and coprecipitates with tyrosine kinases. (A) Jurkat-DC-SIGN (left panels) and mock-transfected Jurkat cells (right panels) were lysed in 1% Brij 98 lysis buffer at 37°C, and Brij 98–insoluble fractions 2 to 4 (lanes 2-4, rafts) and the high-density Brij 98–soluble fractions 7 to 8 (lanes 7-8, soluble) were separated by 12.5% SDS-PAGE under nonreducing conditions. Cytoskeletal-associated rafts (CARs), obtained by solubilization of the cell pellet with Brij 98 + octyl d-glucoside in lysis buffer, were analyzed in parallel (lane 9). The distribution of DC-SIGN, Lck, ZAP-70, LAT, CD3ϵ, and ganglioside GM1 in the distinct fractions was determined by immunoblotting with specific antibodies or cholera toxin–HRP (for GM1). (B) Coprecipitation of DC-SIGN and tyrosine kinases. DC-SIGN was immunoprecipitated with the MR-1 antibody (MR1 ip) from lipid raft–containing fractions 2 to 4 (rafts, lane 2), fractions 5 to 6 (between the rafts and the soluble material, lane 3), and the 7 to 8 soluble fractions (sol., lane 4), and the immunoprecipitated material was subjected to SDS-PAGE and immunoblotting with antibodies against DC-SIGN, Lck, or ZAP-70. As a control, fractions containing either rafts (lane 1) or cytoskeletal-associated rafts (CARs, lane 5) were analyzed in parallel. (C) Jurkat-DC-SIGN cells were cell-surface labeled with biotin, lysed in 1% Brij 98 lysis buffer at 37°C, and Brij 98–insoluble fractions (lanes 1, rafts), intermediate fractions (lanes 2), high-density Brij 98–soluble fractions (lanes 3, sol.), and cytoskeletal-associated raft-containing fractions (CARs, lane 4) were obtained. An aliquot from each fraction was removed and analyzed by 12.5% SDS-PAGE under nonreducing conditions and subjected to Western blot with anti–DC-SIGN or anti-LAT polyclonal antisera (left panel). Then, fractions were subjected to pull-down with Streptavidin-agarose, and the immunoprecipitated material was separated by 12.5% SDS-PAGE under nonreducing conditions and subjected to Western blot with anti–DC-SIGN or anti-LAT polyclonal antisera (right panel). Each experiment was performed twice with similar results, and one of the experiments is shown.
DC-SIGN is found in lipid-rich regions on MDDCs and coprecipitates with tyrosine kinases
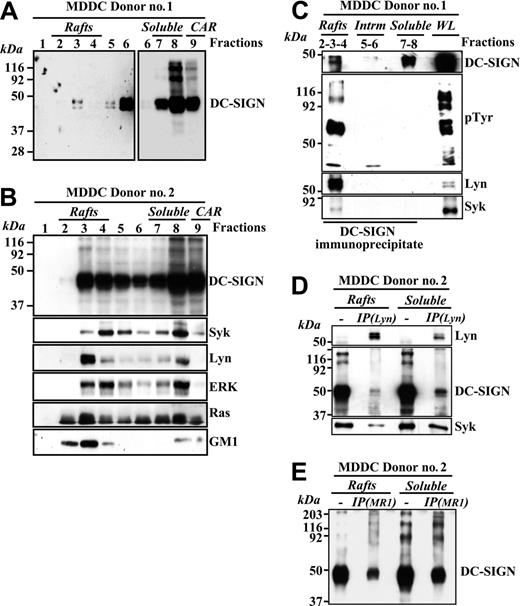
To determine the membrane distribution of DC-SIGN in myeloid cells, detergent-insoluble and -soluble membrane fractions were isolated from MDDCs. Although a high percentage of the molecule appeared in soluble fractions, a fraction of DC-SIGN also partitioned within lipid rafts on MDDCs (Figure 5A). Fractionation of MDDCs from an independent donor further confirmed this DC-SIGN distribution and revealed that most of the intracellular membrane-bound tyrosine kinase Lyn partitioned into low-density fractions 2 to 4, which is consistent with its residency in floating lipid rafts, whereas the signaling molecules Syk, ERK, and Ras were partly localized in the lipid-rich regions on MDDCs (Figure 5B). Since DC-SIGN coprecipitates with ZAP-70 and Lck in lymphoid cells, we next evaluated whether DC-SIGN associated with related molecules in MDDC membranes. As shown in Figure 5C, DC-SIGN brought down phosphotyrosine-containing molecules from lipid raft fractions. Moreover, a significant amount of Lyn and a lesser amount of Syk tyrosine kinases could be detected in the DC-SIGN precipitates from lipid rafts (Figure 5C), indicating that DC-SIGN colocalizes with signaling molecules in glycolipid-enriched membranes from MDDCs. Conversely, neither Syk nor Lyn was detected in DC-SIGN immunoprecipitates from the soluble membrane fractions, in spite of the fact that DC-SIGN is more abundant in this fraction (Figure 5C). In addition, analysis of membrane fractions from an independent MDDC donor demonstrated the presence of DC-SIGN and Syk in Lyn immunoprecipitates (Figure 5D), further confirming the relationship between DC-SIGN and these 2 kinases within lipid rafts. However, not all DC-SIGN molecules are associated to Lyn since DC-SIGN can be easily pulled down with the MR-1 antibody from the post–anti-Lyn immunoprecipitation flow-through from either lipid rafts or soluble membrane fractions (Figure 5E).
DC-SIGN is present within lipid rafts in MDDCs and coprecipitates with Lyn and Syk tyrosine kinases. (A-B) Immature MDDCs from 2 independent donors were lysed in 1% Brij 98 lysis buffer at 37°C and fractionated by sucrose density gradient centrifugation as described in “Materials and methods.”14 The low-density Brij 98–insoluble fractions 2 to 4 (lanes 2-4, rafts) and the high-density Brij 98–soluble fractions 6 to 8 (lanes 6-8, soluble) were separated by 12.5% SDS-PAGE under nonreducing conditions, and the distribution of DC-SIGN was determined by immunoblotting. Cytoskeletal-associated rafts (CARs), obtained by solubilization of the cell pellet with Brij 98 + octyl d-glucoside in lysis buffer, were analyzed in parallel (lane 9). (A) The left panel was intentionally exposed for longer than the blot section shown in the right panel. (B) The distribution of Syk, Lyn, ERK, Ras, and ganglioside GM1 in the distinct fractions was determined by immunoblotting with specific antibodies or cholera toxin–HRP (for GM1). (C) Coprecipitation of DC-SIGN, Lyn, and Syk tyrosine kinases. DC-SIGN was immunoprecipitated with the MR-1 antibody from lipid raft–containing fractions 2 to 4 (rafts), fractions 5 to 6 (intrm. indicates intermediate between the rafts and the soluble material), and the 7 to 8 soluble fractions (soluble), and the precipitated material was subjected to SDS-PAGE and immunoblotting with antibodies specific for DC-SIGN, Lyn, Syk, and phosphotyrosine-containing proteins. As a control, an aliquot from the whole lysate before fractionation (WL indicates whole lysate) was analyzed in parallel. (D) Presence of DC-SIGN and Syk in Lyn immunoprecipitates. Lyn was immunoprecipitated (IP(Lyn)) from the lipid raft–containing fraction pool (rafts) or the soluble material–containing fraction pool (soluble), and the precipitated material was subjected to SDS-PAGE and immunoblotting with antibodies specific for Lyn, DC-SIGN, and Syk. As a control, aliquots from the rafts and soluble fraction pools before immunoprecipitation were analyzed in parallel. (E) Presence of DC-SIGN in the post–anti-Lyn immunoprecipitation flow-through. Post–anti-Lyn immunoprecipitation flow-through from either lipid rafts or soluble membrane fraction pools (those indicated in D) were subjected to a further immunoprecipitation with a monoclonal antibody against DC-SIGN (IP(MR1)), and the precipitated material was subjected to SDS-PAGE and immunoblotting with a polyclonal antibody against DC-SIGN (DSG1). As a control, aliquots from the rafts and soluble fraction pools before immunoprecipitation were analyzed in parallel.
DC-SIGN is present within lipid rafts in MDDCs and coprecipitates with Lyn and Syk tyrosine kinases. (A-B) Immature MDDCs from 2 independent donors were lysed in 1% Brij 98 lysis buffer at 37°C and fractionated by sucrose density gradient centrifugation as described in “Materials and methods.”14 The low-density Brij 98–insoluble fractions 2 to 4 (lanes 2-4, rafts) and the high-density Brij 98–soluble fractions 6 to 8 (lanes 6-8, soluble) were separated by 12.5% SDS-PAGE under nonreducing conditions, and the distribution of DC-SIGN was determined by immunoblotting. Cytoskeletal-associated rafts (CARs), obtained by solubilization of the cell pellet with Brij 98 + octyl d-glucoside in lysis buffer, were analyzed in parallel (lane 9). (A) The left panel was intentionally exposed for longer than the blot section shown in the right panel. (B) The distribution of Syk, Lyn, ERK, Ras, and ganglioside GM1 in the distinct fractions was determined by immunoblotting with specific antibodies or cholera toxin–HRP (for GM1). (C) Coprecipitation of DC-SIGN, Lyn, and Syk tyrosine kinases. DC-SIGN was immunoprecipitated with the MR-1 antibody from lipid raft–containing fractions 2 to 4 (rafts), fractions 5 to 6 (intrm. indicates intermediate between the rafts and the soluble material), and the 7 to 8 soluble fractions (soluble), and the precipitated material was subjected to SDS-PAGE and immunoblotting with antibodies specific for DC-SIGN, Lyn, Syk, and phosphotyrosine-containing proteins. As a control, an aliquot from the whole lysate before fractionation (WL indicates whole lysate) was analyzed in parallel. (D) Presence of DC-SIGN and Syk in Lyn immunoprecipitates. Lyn was immunoprecipitated (IP(Lyn)) from the lipid raft–containing fraction pool (rafts) or the soluble material–containing fraction pool (soluble), and the precipitated material was subjected to SDS-PAGE and immunoblotting with antibodies specific for Lyn, DC-SIGN, and Syk. As a control, aliquots from the rafts and soluble fraction pools before immunoprecipitation were analyzed in parallel. (E) Presence of DC-SIGN in the post–anti-Lyn immunoprecipitation flow-through. Post–anti-Lyn immunoprecipitation flow-through from either lipid rafts or soluble membrane fraction pools (those indicated in D) were subjected to a further immunoprecipitation with a monoclonal antibody against DC-SIGN (IP(MR1)), and the precipitated material was subjected to SDS-PAGE and immunoblotting with a polyclonal antibody against DC-SIGN (DSG1). As a control, aliquots from the rafts and soluble fraction pools before immunoprecipitation were analyzed in parallel.
Additional intracellular signals initiated upon DC-SIGN engagement
The maturation state of dendritic cells is a critical parameter that determines not only whether an immune response is generated but the type of immune response.2 Besides NF-κB activation, other signaling pathways have an impact on dendritic cell maturation, including MEK-ERK8 and PI3K9 activation, and transient calcium increases.24 Given its ability to prompt ERK and Akt phosphorylation, we tested whether DC-SIGN–initiated signals also affected intracellular calcium levels. Engagement of DC-SIGN in Jurkat-DC-SIGN cells triggered PLC-γ phosphorylation, an effect that was not observed with either anti-CD38 or anti-CD3 antibodies (Figure 6A). In accordance with this finding, DC-SIGN ligation on the surface of immature MDDCs promoted a transient calcium mobilization (Figure 6B). Therefore, DC-SIGN engagement on the cell surface promotes phosphorylation of ERK, Akt, and PLC-γ, and leads to transient changes in intracellular calcium concentration.
DC-SIGN ligation results in ERK and PLC-γ activation in transfected Jurkat cells and promotes transient calcium mobilization in MDDCs. (A) DC-SIGN on Jurkat-DC-SIGN transfectants was ligated by the anti–DC-SIGN MR-1 antibody, and the cells were incubated at 37°C for 5 minutes. As a control, cells were incubated with either anti-CD38 or anti-CD3 monoclonal antibodies. Mock-transfected cells were subjected to the same treatments for control purposes. After cell lysis, phosphorylated ERK (pERK), p38 (pp38), and PLC-γ (pPLC-γ), and total levels of ERK and PLC-γ were detected using specific polyclonal antisera. The experiment was performed twice with similar results, and one of the experiments is shown. NS indicates not stimulated. (B-C) Calcium determination in MDDCs after DC-SIGN ligation. Fluo-3AM–loaded MDDCs were left untreated (B) or treated with 50 nM SDF-1α (C), and subsequently incubated with anti–DC-SIGN MR-1 monoclonal antibody (20 μg/mL) (right panel) or an isotype-matched control antibody (left panel). Calcium flux was determined by flow cytometry at the indicated time points. Arrows indicate the time of addition of the MR-1 monoclonal antibody. Similar results were obtained from 3 independent experiments, and 1 of them is shown.
DC-SIGN ligation results in ERK and PLC-γ activation in transfected Jurkat cells and promotes transient calcium mobilization in MDDCs. (A) DC-SIGN on Jurkat-DC-SIGN transfectants was ligated by the anti–DC-SIGN MR-1 antibody, and the cells were incubated at 37°C for 5 minutes. As a control, cells were incubated with either anti-CD38 or anti-CD3 monoclonal antibodies. Mock-transfected cells were subjected to the same treatments for control purposes. After cell lysis, phosphorylated ERK (pERK), p38 (pp38), and PLC-γ (pPLC-γ), and total levels of ERK and PLC-γ were detected using specific polyclonal antisera. The experiment was performed twice with similar results, and one of the experiments is shown. NS indicates not stimulated. (B-C) Calcium determination in MDDCs after DC-SIGN ligation. Fluo-3AM–loaded MDDCs were left untreated (B) or treated with 50 nM SDF-1α (C), and subsequently incubated with anti–DC-SIGN MR-1 monoclonal antibody (20 μg/mL) (right panel) or an isotype-matched control antibody (left panel). Calcium flux was determined by flow cytometry at the indicated time points. Arrows indicate the time of addition of the MR-1 monoclonal antibody. Similar results were obtained from 3 independent experiments, and 1 of them is shown.
DC-SIGN engagement influences cytokine production during MDDC maturation
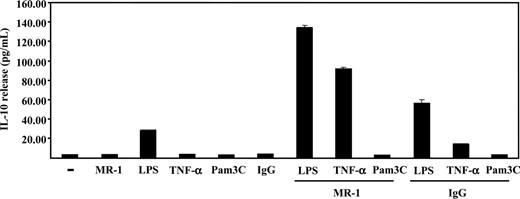
The ability of DC-SIGN to modify the activation state of 3 key signaling molecules (ERK, Akt, and PLC-γ) suggested that DC-SIGN ligation might exert a modulatory effect on MDDC maturation. Since ERK activation has been linked to Th2-type polarization and enhanced IL-10 production,11,25 the release of IL-10 in response to DC-SIGN ligation was determined. Immature MDDCs did not produce detectable IL-10 in response to either TNF-α, Pam3Cys (a synthetic TLR2 ligand), MR-1 antibody, or a control mouse IgG, and only LPS was capable of inducing the production of moderate levels of IL-10 (Figure 7). However, the presence of MR-1 antibody enhanced LPS-induced IL-10 release (Figure 7). Moreover, and although each agent alone had no effect, the simultaneous presence of MR-1 and TNF-α resulted in high levels of IL-10 production (Figure 7). These results are in agreement with the enhanced ERK phosphorylation observed upon MDDC treatment with anti–DC-SIGN and TNF-α, and demonstrate that DC-SIGN engagement on the membrane of immature dendritic cells modulates the maturation program initiated by other cell surface receptors.
DC-SIGN ligation in immature MDDCs enhances maturation-dependent IL-10 production. MDDCs were incubated with LPS, TNF-α, Pam3Cys, MR-1, or an isotype-matched antibody (IgG), either alone or in the indicated combinations, for 18 hours in complete medium. After incubation, supernatants from MDDCs were collected and IL-10 content was determined by enzyme-linked immunosorbent assay (ELISA). The data indicate means (SD of triplicate samples from 1 representative experiment of 3 experiments on MDDCs from independent donors).
DC-SIGN ligation in immature MDDCs enhances maturation-dependent IL-10 production. MDDCs were incubated with LPS, TNF-α, Pam3Cys, MR-1, or an isotype-matched antibody (IgG), either alone or in the indicated combinations, for 18 hours in complete medium. After incubation, supernatants from MDDCs were collected and IL-10 content was determined by enzyme-linked immunosorbent assay (ELISA). The data indicate means (SD of triplicate samples from 1 representative experiment of 3 experiments on MDDCs from independent donors).
Discussion
The generation of pathogen-specific immune responses is based on the plasticity of the dendritic cell maturation process, which results from the integration of all the intracellular signals initiated after recognition of PAMPs by pathogen-recognition receptors. Intracellular signaling from the distinct TLR exhibits the common property of NF-κB activation, but, for DCs, their differential coupling to adapter molecules, their differential activation of MAPKs, and the modulation of their signals by other PAMP receptors contribute to the generation of pathogen-specific dendritic cell maturation and pathogen-tailored immune responses.25 One of the dendritic cell PAMP receptors is the C-type lectin DC-SIGN, which participates in the recognition and capture of numerous viral, bacterial, and fungal pathogens.3 In the present report, we demonstrate that DC-SIGN engagement on the dendritic cell surface induces phosphorylation of ERK and Akt, but not p38MAPK, and promotes a transient calcium flux, which correlates with its ability to trigger PLCγ phosphorylation in transfected lymphoid cells. In agreement with its signaling capability, DC-SIGN in lipid rafts associates with tyrosine kinases of the Src and Syk/ZAP-70 families in both dendritic cells and transfected lymphoid cells. All these signaling events might explain the ability of DC-SIGN–engaging ligands to shift MDDC maturation toward the acquisition of pro-Th2/protolerogenic polarizing capability, which is also exemplified by the fact that DC-SIGN ligation synergizes with TNF-α receptor–initiated signals for enhanced IL-10 release.
The signaling and functional consequences of DC-SIGN ligation here reported are in agreement with the results of Geijtenbeek et al, who showed that Mycobacterium Man-LAM enhanced IL-10 production by MDDCs in a DC-SIGN–dependent manner.12 The comparison of the intracellular signaling pathways activated by TLR4 and TLR2 has demonstrated that preferential activation of p38MAPK leads to IL-12p70–dependent pro-Th1 dendritic cell maturation, while a high ERK/p38MAPK activation ratio promotes pro-Th2 maturation via increased IL-10 release and reduced IL-12p70 synthesis.10,11,25 Therefore, the DC-SIGN–mediated ERK activation fits with the enhanced release of IL-10 seen in maturing MDDCs after DC-SIGN engagement, and is also compatible with the inhibitory action of ERK activation on IL-12p70 release by dendritic cells8 and macrophages.26 Apart from ERK and p38MAPK, other intracellular signals modulate the pro-Th1/Th2 balance during MDDC maturation. In this regard, (1) PI3K activation in dendritic cells has been shown to negatively regulate IL-12 synthesis and, therefore, prevents Th1 polarization27 ; (2) calcium signaling antagonizes IL-12 production by mature dendritic cells, negatively regulates pro-Th1 maturation, and preferentially promotes the acquisition of pro-Th2/Tc2 characteristics24 ; and (3) lysophosphatidic acid–induced increase of intracellular calcium inhibits IL-12 secretion and enhances secretion of IL-10 from mature dendritic cells.28 Therefore, the intracellular signaling triggered upon DC-SIGN engagement (ERK and PI3K activation, transient rise in intracellular calcium concentration) would impair IL-12 and enhance IL-10 release and, consequently, might explain why DC-SIGN ligation favors a pro-Th2/protolerogenic dendritic cell maturation.
The relevance of the DC-SIGN cell surface distribution for pathogen binding has been previously demonstrated.23 In the present report, and in line with recent observations,23 biochemical analysis of MDDC and DC-SIGN transfectants has revealed that a fraction of DC-SIGN molecules is located within lipid raft–enriched membrane fractions in both cell types (Figures 4, 5) and that most DC-SIGN molecules on the plasma membrane reside within lipid raft–enriched microdomains (Figure 4C). Therefore, it is reasonable to assume that the microlocalization of DC-SIGN on the plasma membrane should also contribute to its signaling ability. In this regard, DC-SIGN coprecipitates with Lck or Lyn, 2 Src-family kinases involved in immunoreceptor signaling in lymphoid and myeloid cells.29 The colocalization of DC-SIGN, Lyn, and Syk provides additional structural support for the regulatory role of DC-SIGN during dendritic cell maturation, as both kinases have been found to modulate murine dendritic cell maturation.30-32 In fact, Lyn appears to positively regulate IL-12 production in mice and has been proposed to act as a negative regulator of Th2 immunity.33 Therefore, future experiments are required to determine the functional significance of the DC-SIGN association with Lyn and Syk in dendritic cells, as well as to find out whether the association between DC-SIGN and both kinases reflects direct or indirect interactions. The latter appears as a likely alternative, given the lack of an obvious ITAM motif in the DC-SIGN cytoplasmic tail and the fact that no tyrosine phosphorylation of DC-SIGN has been detected after engagement by either antibodies or pathogenic ligands (data not shown).
Besides DC-SIGN, other lectin receptors expressed on myeloid (Dectin-1) and plasmacytoid (BDCA-2) dendritic cells have been shown to promote intracellular signaling and to modulate TLR signaling. Yeast binding to the β-glucan receptor Dectin-1 synergizes with TLR2 to enhance activation of NF-κB and production of IL-12.34 In addition, Dectin-1 can also promote IL-10 synthesis via recruitment of Syk to its ITAM motif.35 Similarly to DC-SIGN, antibody ligation of the plasmacytoid-specific BDCA2 lectin induces calcium mobilization and protein-tyrosine phosphorylation, resulting in suppression of IFN-α/β induction by plasmacytoid dendritic cells.36 These results, combined with the diversity of their cytoplasmic sequences, suggest that lectins and lectinlike receptors expressed on dendritic cells might exhibit a large degree of variability in the intracellular signaling pathways they activate, providing an additional level of plasticity for the generation of pathogen-specific immune responses. In the case of DC-SIGN, the intracellular signals initiated by this lectin would result in increased pro-Th2/protolerogenic effector functions and might contribute to evasion from immunosurveillance of DC-SIGN–interacting pathogens.3 However, since mice deficient in the DC-SIGN–related molecule SIGNR1 exhibit increased susceptibility to Streptococcus pneumoniae,37 the dissection of the intracellular signals triggered upon DC-SIGN engagement deserves further investigation, as it might clarify the role of the lectin family on the generation of pathogen-specific immune responses.
Prepublished online as Blood First Edition Paper, January 24, 2006; DOI 10.1182/blood-2005-03-1252.
Supported by the Ministerio de Educación y Ciencia (Ministerio de Educación Ciencia [MEC]; grants SAF2005-0021, GEN2003-20649-C06-01/NAC, and AGL2004-02148-ALI), Fundación para la Investigación y Prevención del SIDA en España (FIPSE 36422/03) to A.L.C., and grant SAF2002-00721 to J.S. M.Z. was supported by Instituto Carlos III–Fondo de Investigaciones Sanitarias (FIS), by Ministerio de Sanidad y Consumo (grant FIS03/0389), and by a Ramón y Cajal contract from the Ministerio de Educación y Ciencia. E.C., P.M., and D.S.-G. were supported by Formación de Personal Investigador (FPI) Fellowships from MEC.
E.C. and P.M. contributed equally to this work.
M.Z. and A.L.C. contributed equally to this work.
E.C., P.M., E.S.-F., D.S.-G., A.P.-K., J.L.R.-F., M.M., and M.Z. performed research; M.Z. and A.L.C. designed research; M.Z. and J.S. contributed reagents; and A.L.C. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal