It has recently been shown that the ultralarge platelet–recruiting von Willebrand factor (VWF) strings formed immediately at exocytosis from endothelial cells may be anchored to the cell surface by interaction with the integral membrane protein P-selectin. This finding of a new binding partner for VWF immediately prompts the question which domains of VWF bind to P-selectin. We have exploited the fact that VWF expression in HEK293 cells triggers the formation of Weibel-Palade body–like structures that can recruit P-selectin. A suitably modified version of this assay using coexpressed truncations of VWF, together with P-selectin variants in HEK293 cells, allowed us to determine which domains of VWF would recruit P-selectin within a physiologically appropriate intracellular environment. Confirming the results of such a cellular assay by conventional coimmunoprecipitation, we concluded that the lumenal domain of P-selectin interacts with the D′-D3 domains of VWF.
Introduction
The hyperactive ultralarge von Willebrand factor (VWF) strings released by endothelial cells are subsequently cleaved into smaller, less active forms by the VWF-cleaving protease ADAMTS13.1 This important antithrombotic process is greatly facilitated by anchoring of the VWF strings to the surfaces of endothelial cells, allowing their extension by flow, presumably exposing the cleavage site to the protease.2 A key step in characterizing this interaction is to establish which domain(s) of VWF bind P-selectin. In endothelial cells, VWF is costored in Weibel-Palade bodies3 (WPBs; for a review, see Michaux and Cutler4 ) with the leukocyte receptor P-selectin.5,6 We recently found that the P-selectin lumenal domain (which becomes extracellular after secretion) is recruited to WPBs in endothelial cells,19 most likely by a direct interaction. By coexpressing P-selectin and VWF constructs in HEK293 cells that form functional WPBs,7 we can monitor which VWF domains can drive P-selectin recruitment. We now demonstrate that the D′-D3 domains of VWF are sufficient to bind the P-selectin lumenal domain and to trigger P-selectin recruitment to WPB.
Study design
HEK293 cells were grown, transfected, and prepared for immunofluorescence as described.7 VWF and P-selectin constructs, the monoclonal anti–VWF-propeptide 239.1, and a rabbit polyclonal anticytoplasmic tail of P-selectin have all been described.8-11,19 Sheep polyclonal anti–human VWF and monoclonal anti–P-selectin AK6 were from Serotec (Oxford, United Kingdom). Images were collected 48 hours after nucleofection, and images shown are projections of confocal sections throughout the cells. Secondary antibodies conjugated with Texas Red and FITC were from Jackson ImmunoResearch Laboratories (West Grove, PA). All coverslips were mounted in ProLong antifade (Molecular Probes, Paisley, United Kingdom). Confocal images were obtained using an MRC 10224 laser scanner (Bio-Rad, Hercules, CA) attached to an OptiPhot 2 microscope with a PlanApo 60 ×/1.40 oil-immersion objective lens (Nikon, Garden City, NY). LaserSharp 2000 software (Bio-Rad) was used to acquire images; Adobe Photoshop 6.0 and Illustrator 10 software (Adobe Systems, Mountain View, CA) were used to process them. In coimmunoprecipitations, nucleofected cells were washed twice with ice-cold PBS, then scraped into lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% vol/vol Triton X-100, 0.5% vol/vol Nonidet P-40, 1 mM Na3VO4, 1 mM NaF, pH 7.4) containing a protease inhibitor cocktail (Sigma-Aldrich, Poole, United Kingdom). The lysate was passed 5 times at moderate hand pressure through a 25-gauge needle, then centrifuged at 12 000g for 10 minutes, and the supernatant was mixed with anti–P-selectin antibody AC1.2 (BD Biosciences PharMingen, San Diego, CA) bound to sheep anti–mouse IgG–coupled paramagnetic beads (Dynal, Oslo, Norway), rotated at 4°C for 1 hour, then washed 5 times in lysis buffer. The beads were boiled in SDS sample buffer, and proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membrane, and visualized using a rabbit anti-GFP antibody (Abcam, Cambridge, United Kingdom) detected with HRP-conjugated goat antirabbit antibodies (Jackson ImmunoResearch, Bar Harbor, ME).
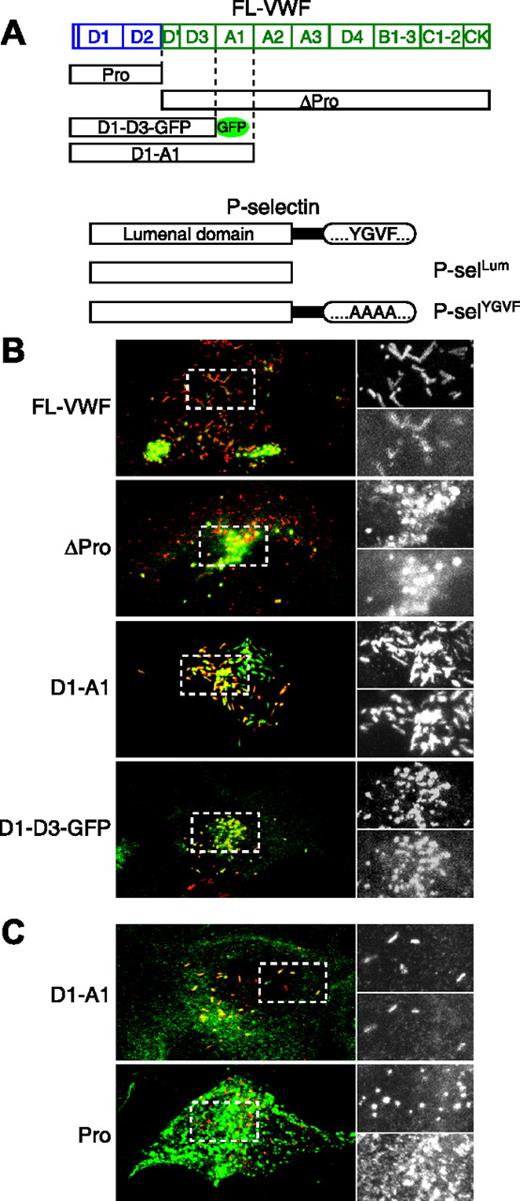
The D′-D3 domain of VWF is sufficient to recruit the P-selectin lumenal domain. HEK293 cells were nucleofected with various VWF constructs (A) and either a soluble lumenal domain of P-selectin (A-B) or a mutated P-selectin (A,C). VWF is stained red, and P-selectin is stained green (note that for D1-D3-GFP, the red and green layers have been interchanged to be consistent with the rest of the figure). Panels on the right are enlargements of the regions boxed with dashed lines on the left. The enlarged top panel is VWF, and the bottom panel is P-selectin. Note that the P-selectin pattern is very different when coexpressed with the propeptide because it is not recruited to the VWF-positive puncta.
The D′-D3 domain of VWF is sufficient to recruit the P-selectin lumenal domain. HEK293 cells were nucleofected with various VWF constructs (A) and either a soluble lumenal domain of P-selectin (A-B) or a mutated P-selectin (A,C). VWF is stained red, and P-selectin is stained green (note that for D1-D3-GFP, the red and green layers have been interchanged to be consistent with the rest of the figure). Panels on the right are enlargements of the regions boxed with dashed lines on the left. The enlarged top panel is VWF, and the bottom panel is P-selectin. Note that the P-selectin pattern is very different when coexpressed with the propeptide because it is not recruited to the VWF-positive puncta.
Results and discussion
D′-D3 domains of VWF drive P-selectin recruitment to WPBs
To determine which VWF domains are required for P-selectin binding, VWF-encoding constructs and a construct encoding a soluble lumenal domain of P-selectin (Figure 1A) were coexpressed in HEK293 cells (Figure 1B). Use of the P-selectin lumenal domain alone ensures that recruitment must be by direct interaction between the 2 proteins because this variant has lost its alternative cytoplasmic signals for targeting to WPBs (K.J. Harrison-Lavoie, manuscript submitted). Expression of full-length VWF (FL-VWF) leads to the formation of WPBs that recruit the lumenal domain of P-selectin (Figure 1B), confirming our data from human umbilical vein endothelial cells (HUVECs) in which the P-selectin lumenal domain is recruited to WPBs.19 However, it also rules out heterodimerization of the lumenal domain with endogenous P-selectin or indeed a necessary role for other endothelial factors in recruitment. Given that the recruitment of P-selectin to HEK293 WPB is highly specific,7 these data strongly argue that this recruitment of soluble P-selectin by VWF reflects a direct interaction between the 2 proteins. We then expressed a mature VWF construct (ΔPro) that does not contain the propeptide (Pro). Although this protein is not stored as efficiently as WT-VWF and has been reported not to be stored in other systems,12 we were able to detect VWF-positive structures that were also positive for P-selectin (Figure 1B, ΔPro). Subsequent expression of VWF constructs with C-terminal deletions led to the conclusion that domains A1 to CK were not essential for P-selectin recruitment because a GFP-tagged construct composed of the first 4 N-terminal domains D1-D2-D′-D3 (D1-D3-GFP; Figure 1A), was able to drive P-selectin recruitment (Figure 1B, D1-D3-GFP). We concluded that the VWF domains sufficient for P-selectin recruitment lie within the D′-D3 domains of mature VWF.
Propeptide is rapidly diluted after secretion13 ; hence, it is an unlikely candidate for binding P-selectin. However, because it might also provide a negative control for binding, we performed an assay for interaction of the propeptide with P-selectin. In this case, we used a mutated full-length P-selectin (P-selYGVF) rather than the lumenal domain alone. This strategy arose from limited antibody availability given that the full-length P-selectin allowed use of an antibody that recognizes the cytoplasmic tail of this protein. Furthermore, it provides a control to eliminate potential artefacts from the expression of the lumenal domain on its own. In the construct used, P-selYGVF, the granule-targeting motif (YGVF) within its cytoplasmic tail,14 is replaced by tetra-alanine (Figure 1A) so that recruitment of this variant to WPBs in HUVECs is dependent on its lumenal domain.19 In HEK293 cells, P-selYGVF is targeted to VWF-positive structures with an efficiency similar to that seen with the P-selectin lumenal domain (compare Figure 1B, D1-A1, with Figure 1C, D1-A1). When P-selYGVF was coexpressed with the VWF propeptide (Pro), however, we found no colocalization. P-selectin staining was dispersed throughout the cell, presumably in endocytic/lysosomal compartments, rather than restricted to VWF-positive puncta, as with the expression of other VWF constructs. This result, together with the recruitment of P-selectin after expression of the D1-D3-GFP construct, led us to conclude that the D′-D3 domains of VWF are sufficient to drive P-selectin recruitment to WPBs, presumably by direct interaction between the 2 proteins.
D1-D3 domains of VWF bind to the lumenal domain of P-selectin
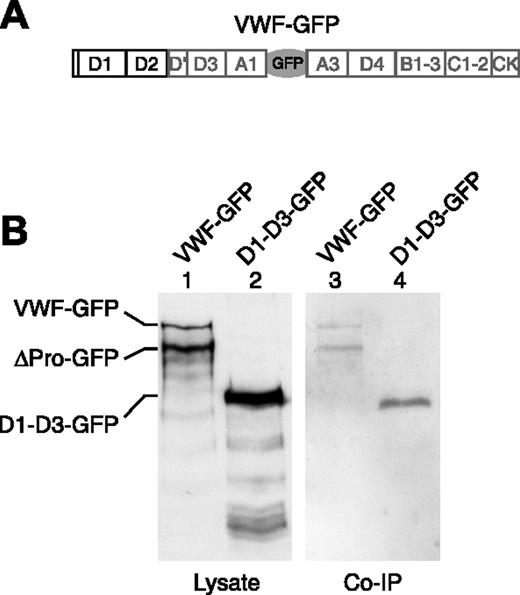
To confirm the likely interaction of P-selectin and VWF, we performed coimmunoprecipitation of these 2 proteins by following a method developed to allow precipitation of VWF by endogenous P-selectin in HUVECs (not shown) in confirmation of the data of Padilla et al.2 Lysates from HEK293 cells cotransfected with GFP-tagged VWF constructs (Figures 1A, 2A) and the P-selectin lumenal domain (Figure 1A) were immunoprecipitated by anti–P-selectin, followed by Western blotting of electrophoresed immunoprecipitates to identify VWF (Figure 2B-C). We found that the full-length VWF-GFP (FL-VWF-GFP) and the cleaved mature VWF (ΔPro-GFP) could indeed interact with the lumenal domain of P-selectin (Figure 2B, lane 3), as could D1-D3-GFP (Figure 2B, lane 4). The appearance of VWF on the blot after coimmunoprecipitation was dependent on the presence of P-selectin in the lysate (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article), and the propeptide alone cannot interact with P-selectin (Figures S1B, S2). These experiments show that VWF can interact with the lumenal domain of P-selectin through its D′-D3 domains.
Coimmunoprecipitation of D1-D3 VWF with an anti–P-selectin antibody. HEK293 cells were nucleofected with the P-selectin lumenal domain, seen in Figure 1A, and either VWF-GFP (A) or D1-D3-GFP in Figure 1A. Cells were lysed, and P-selectin was immunoprecipitated from the lysate by the monoclonal anti–P-selectin antibody AC1.2. (B) Coimmunoprecipitation of VWF with P-selectin was determined by Western blot using a rabbit polyclonal anti-GFP antibody.
Coimmunoprecipitation of D1-D3 VWF with an anti–P-selectin antibody. HEK293 cells were nucleofected with the P-selectin lumenal domain, seen in Figure 1A, and either VWF-GFP (A) or D1-D3-GFP in Figure 1A. Cells were lysed, and P-selectin was immunoprecipitated from the lysate by the monoclonal anti–P-selectin antibody AC1.2. (B) Coimmunoprecipitation of VWF with P-selectin was determined by Western blot using a rabbit polyclonal anti-GFP antibody.
Interaction between P-selectin and VWF has been reported to occur in stimulated but not in resting HUVECs, implying no intracellular interaction or implying, as suggested by the authors, a weak intracellular interaction not detected in their system.2 However, we find that the P-selectin lumenal domain alone is recruited in HUVECs19 and in HEK293 (this study) and that it can immunoprecipitate on VWF. Indeed, it seems unlikely that an interaction between P-selectin and VWF can be satisfactorily established under flow but not within WPBs. Therefore, we suggest that technical differences may explain the discrepancies between these 2 sets of results. The interaction, as monitored by coimmunoprecipitation, may be sensitive to factors relating to the cell type, the antibodies used, or the context in which the D′-D3 domains are presented to P-selectin. In this study, the levels of expressed P-selectin and VWF may be greater than those found in HUVECs and may increase the sensitivity of our assay.
Significantly, this study reveals a novel and crucial role for the D′-D3 domains of VWF. These domains have already been implicated in VWF storage,15 multimerization,16 and binding and stabilization of coagulation factor VIII.17,18 We now show that it is also crucial for P-selectin recruitment. Many mutations in these domains have been identified in patients with von Willebrand disease. Whether some of the clinical VWF variants within this domain affect P-selectin binding is not yet determined. The effect of dysfunctional VWF/P-selectin interaction on the normal progression of thrombus formation at the site of vascular injury is also not defined. Were such mutations to reduce the attachment of VWF to the endothelium after secretion, thus contributing to a reduction in the efficiency of ADAMTS13 cleavage, the data from Padilla et al2 would argue that plasma VWF would contain higher levels of ultralarge multimers of VWF, perhaps such as those seen in thrombotic thrombocytopenic purpura (TTP).
Prepublished online as Blood First Edition Paper, January 17, 2006; DOI 10.1182/blood-2005-09-3635.
Supported by the UK Medical Research Council, a European Union Marie Curie fellowship (G.M.), the American Heart Association (SDG 0435466N), a Hemophilia Association of New York research grant, and the Children's Hospital Research Foundation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank members of the Cutler laboratory for critical reading of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal