Depolymerized holothurian glycosaminoglycan (DHG) is a fucosylated chrondroitin sulfate that possesses antithrombin-independent antithrombotic properties and inhibits factor X activation by the intrinsic tenase complex (factor IXa–factor VIIIa). The mechanism and molecular target for intrinsic tenase inhibition were determined and compared with inhibition by low-molecular-weight heparin (LMWH). DHG inhibited factor X activation in a noncompetitive manner (reduced Vmax(app)), with 50-fold higher apparent affinity than LMWH. DHG did not affect factor VIIIa half-life or chromogenic substrate cleavage by factor IXa–phospholipid but reduced the affinity of factor IXa for factor VIIIa. DHG competed factor IXa binding to immobilized LMWH with an EC50 35-fold lower than soluble LWMH. Analysis of intrinsic tenase inhibition, employing factor IXa with mutations in the heparin-binding exosite, demonstrated that relative affinity (Ki) for DHG was as follows: wild type > K241A > H92A > R170A > > R233A, with partial rather than complete inhibition of the mutants. This rank order for DHG potency correlated with the effect of these mutations on factor IXa–LMWH affinity and the potency of LMWH for intrinsic tenase. DHG also accelerated decay of the intact intrinsic tenase complex. Thus, DHG binds to an exosite on factor IXa that overlaps with the binding sites for LMWH and factor VIIIa, disrupting critical factor IXa–factor VIIIa interactions.
Introduction
Despite being the primary treatment for acute thrombosis since the 1930s, the precise mechanism for heparin's antithrombotic efficacy remains incompletely understood.1 Unfractionated (UH) and low-molecular-weight (LMWH) heparins are heterogeneous mixtures of oligosaccharide chains with several potential mechanisms of action. Antithrombin-dependent mechanisms accelerate the inhibition of coagulation proteases by conformational activation of the serpin or by the binding of antithrombin and protease to the same chain (template mechanism).2 The template mechanism requires a chain length of more than 16 to 18 oligosaccharides, and the therapeutic equivalence of UH and LMWH (fewer than 16 oligosaccharides) in the treatment of venous thromboembolism suggests that this mechanism is not critical to antithrombotic efficacy.3-5 Therefore, because acceleration of thrombin inhibition is almost solely dependent on a template mechanism, thrombin does not appear to be the critical target.6 Instead, therapeutic efficacy is attributed to the high-affinity pentasaccharide present in 15% to 25% of LMWH chains, which accelerates factor Xa and factor IXa inhibition via conformational activation of antithrombin.2,7-9 Nevertheless, the effectiveness of heparin with low affinity for antithrombin in a rabbit model of venous thrombosis raises the possibility that antithrombin-independent mechanisms contribute importantly to antithrombotic efficacy.10
Antithrombin-independent mechanisms of heparin action include inhibition of factor VIII activation by thrombin and of factor X activation by the intrinsic tenase complex.11,12 These activities target the rate-limiting step for thrombin generation, occur at therapeutic heparin concentrations, and appear to be mediated by exosite interactions with vitamin K–dependent proteases.13-16 We recently demonstrated that heparin oligosaccharides inhibit factor X activation by the intrinsic tenase complex through direct binding to a factor IXa exosite, which disrupts critical protease-cofactor interaction(s).16 Mutagenesis of factor IXa demonstrates that LMWH and factor VIIIa bind to overlapping sites within the protease domain, and oligosaccharide binding to this exosite reduces factor IXa–factor VIIIa affinity and accelerates decay of the intrinsic tenase complex.17
Depolymerized holothurian glycosaminoglycan (DHG) is a low-molecular-weight form (MW 12500) of fucosylated chondroitin sulfate isolated from the sea cucumber Stichopus japonicus. DHG demonstrates antithrombotic efficacy in models of thrombin-induced pulmonary thromboembolism in the mouse, thrombin-induced venous thrombosis in the rat, and dialysis during renal failure in the dog.18-21 DHG has multiple potential mechanisms of action, including acceleration of thrombin inhibition by heparin cofactor II (HCII), inhibition of factor VIII activation by thrombin, and inhibition of factor X activation by the intrinsic tenase complex.22,23 In contrast to heparin, DHG lacks the antithrombin-dependent activities that contribute to increased bleeding risk.18,22 Inhibition of intrinsic tenase activity occurs at DHG concentrations that are at least one order of magnitude lower than required for its other reported activities.22,23 DHG demonstrates significant affinity for both factor VIIIa and factor IXa, but the specific mechanism for inhibition of the intrinsic tenase complex is undefined.22 Given the critical role of this enzyme complex in the propagation of blood coagulation, the molecular target and mechanism for DHG inhibition of intrinsic tenase were investigated. In particular, the effect of DHG on the kinetics of factor X activation, cofactor stability, protease-cofactor and protease-heparin binding, intrinsic tenase stability, and the ability to inhibit factor X activation by recombinant factor IXa with mutations in the heparin-binding exosite was evaluated. The results indicate that the heparin-binding exosite of factor IXa is the molecular target for this inhibition and that DHG and LMWH share a common mechanism for inhibition of factor X activation by the intrinsic tenase complex.
Materials and methods
Materials
Human plasma–derived factor X, XIa, and thrombin were purchased from Enzyme Research (South Bend, IN). Recombinant human factor VIII (Kogenate FS) was generously provided by Andreas Mueller-Beckhaus of Bayer (Berkeley, CA). Recombinant hirudin, bovine serum albumin (BSA), and poly-l-lysine were purchased from Sigma (St Louis, MO). Chromogenic substrates were purchased as follows: S-2765 (N-α-benzyloxycarbonyl-d-Arg-Gly-Arg-pNA) from DiaPharma (Franklin, OH) and Pefachrome IXa (CH3SO2-d-CHG-Gly-Arg-pNA) from Centerchem (Stamford, CT). DHG was generously provided by Kazuhisa Minamiguchi of Taiho Pharmaceuticals (Saitama, Japan). LMWH (Dalteparin) was obtained from Pharmacia & UpJohn (Kalamazoo, MI). Biotinylated LMWH (Ardeparin) was purchased from Celsus (Cincinnati, OH), and biotin-X-hydrazide was obtained from Pierce Endogen (Rockford, IL). Strepavidin-coated surface plasmon resonance sensor chips were purchased from BiaCore (Piscataway, NJ). Phosphatidylserine (PS) and phosphatidylcholine (PC) were purchased from Avanti Lipids (Alabaster,AL). Cholesterol was purchased from Calbiochem (San Diego, CA). Phosphatidylcholine-phosphatidylserine-cholesterol (molar ratio, 75:25:1) phospholipid vesicles (PC/PS vesicles) were prepared by extrusion through a 100-nm polycarbonate filter.24 The molar concentration of phospholipid was determined with an elemental phosphorus assay.25
Mutagenesis and expression of recombinant human factor IX
Recombinant human factor IX constructs in the pCMV5 vector were transfected into human embryo kidney (HEK) 293 cells and stable cell lines selected.17 HEK 293 cell lines stably transfected with the cDNA for factor IX wild type (WT) and factor IX R170A were generously provided by Darrel Stafford (University of North Carolina, Chapel Hill).26 Cell lines expressing factor IX WT, H92A, R170A, R233A, and K241A were expanded in cell culture and incubated in serum-free medium supplemented with 10 μg/mL vitamin K and insulin-transferrinselenite (Sigma). The conditioned medium was harvested every 48 hours for 10 days, centrifuged to remove cellular debris, and 5 mM benzamidine added prior to storage at –80°C.17
Purification and activation of recombinant FIX
Recombinant human factor IX was purified from conditioned media by conventional anion exchange chromatography and calcium-dependent elution from a Resource Q fast performance liquid chromatography (FPLC) column (Amersham Biosciences, Piscataway, NJ).17 Protein concentrations were initially determined by absorbance at 280 nm (Abs280) using an extinction coefficient (ϵ0.1%) of 1.43. Purified factor IX was activated with human factor XIa (150:1 substrate-enzyme molar ratio) at 4°C for 2 to 6 hours depending on the mutant protein. Factor XIa was depleted by incubation with polyclonal anti–human factor XI antisera cross-linked to Affi-gel beads (Bio-Rad Laboratories, Hercules, CA) in a spin column. The Abs280 of the purified factor IXa was determined and the protein placed in aliquots at –80°C. An aliquot was later thawed to quantitate factor IXa catalytic sites by titration with antithrombin III as described,27 except that the factor IXa–antithrombin–heparin incubation step was prolonged (60 minutes) to assure the reaction was complete for recombinant factor IXa with decreased heparin affinity. The active protease concentration determined by antithrombin titration was used for enzymatic analysis.
Factor X activation by the intrinsic tenase complex (factor IXa–factor VIIIa–phospholipid)
Factor X activation by the factor IXa–factor VIIIa–phospholipid complex was determined in a reaction containing 0.1 nM human factor IXa, 1.0 nM thrombin-activated factor VIIIa, and 50 μM PC/PS phospholipid vesicles in “tenase buffer” containing 0.15 M NaCl, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% polyethylene glycol with an average of 8000 (PEG-8000), as previously described.16 The reaction was initiated by addition of factor X immediately after factor VIIIa and incubated 15 to 30 seconds for determination of factor X kinetics (or 60 seconds for other assays) at room temperature. The reaction was terminated by addition of EDTA/Sequa-brene (Sigma, Saint Louis, MO), and the amount of factor Xa was determined by comparing the rate of S-2765 substrate hydrolysis to a standard curve.16 Incubation times were restricted to conditions under which less than 10% total substrate cleavage occurred. Kinetic constants for factor X activation were obtained by plotting the rate of factor Xa generation (nanomoles per minute) versus substrate concentration and fitting the data by nonlinear regression to the Michaelis-Menten equation.
Chromogenic substrate hydrolysis by factor IXa–phospholipid
The effect of increasing DHG concentration on the rate of hydrolysis of Pefachrome IXa by factor IXa–phospholipid was examined in reactions containing 40 nM factor IXa, 50 μM PC/PS vesicles, 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 30% ethylene glycol, 1 mg/mL BSA, 0.1% PEG-8000, and 2.5 mM Pefachrome IXa. Initial rates of substrate cleavage were determined by monitoring the absorbance at 405 nm over 10 minutes at room temperature in a kinetic microtiter plate reader (Vmax Reader; Molecular Devices, Sunnyvale, CA).
Determination of the in vitro half-life of factor VIIIa and stability of the intrinsic tenase complex
Recombinant factor VIII (20 nM) was activated with 40 nM thrombin for 30 seconds, neutralized with 60 nM hirudin, and diluted 1:2 into tenase buffer in the absence or presence of 25 to 50 nM DHG. Aliquots were removed from the factor VIIIa incubation mixtures over time and diluted 25-fold into the intrinsic tenase assay containing 1.5 nM factor IXa, 200 nM factor X, and 50 μM PC/PS vesicles to determine residual factor VIIIa activity. Starting activity was normalized to 100%, activity plotted versus time, and the data fit to an exponential function.12 Alternatively, the stability of the intrinsic tenase complex in the absence or presence of 4 to 6 nM DHG was determined in a reaction containing 1.0 nM thrombin-activated factor VIIIa, 0.2 nM factor IXa, and 50 μM PC/PS vesicles in tenase buffer by varying the time of factor X (200 nM) addition between 0 and 15 minutes.
Determination of the affinity (apparent Kd) for factor VIIIa–factor IXa complex formation
Affinity of the factor IXaB–factor VIIIa complex was assessed by monitoring intrinsic tenase complex activity at limiting concentrations of FVIIIa.16 Thrombin-activated factor VIIIa (0.15 nM) was incubated with increasing amounts of factor IXa (0 to 30 nM), in the presence of 50 μM PC/PS, and 200 nM factor X. Factor IXa concentration was plotted versus the rate of factor Xa generation and the data fit by nonlinear regression to a single site–binding model to determine the Kd(app). The factor IXa–factor VIIIa concentration under assay conditions in the absence or presence of DHG was calculated using the experimentally determined Kd(app) to solve the quadratic equation as described.16
Competition of factor IXa–LMWH surface binding by soluble DHG and LMWH using surface plasmon resonance
Reference and immobilized LMWH binding surfaces were prepared on a BiaCore SA chip with biotin-X-hydrazide (Pierce Endogen) and biotin–LMWH (Ardeparin) respectively, in 0.3 M NaCl, 20 mM HEPES (pH 7.4), 0.05% Tween 20, as described previously.16 Binding surfaces were regenerated with 1 M NaCl, 20 mM HEPES (pH 7.4). Direct binding of recombinant proteins to the immobilized LMWH surface was assessed by injection of 100 nM factor IXa at 5 μL/min for 120 seconds (association phase) followed by 2 μM LMWH at 5 μL/min for 180 seconds (dissociation phase). Final sensorgrams were obtained by subtracting the reference surface from the LMWH surface signal, averaging replicate determinations, and subtracting the mean sham injection (buffer only) signal.28
To assess the relative ability of soluble DHG and LMWH to compete with the immobilized LMWH surface for binding to factor IXa, a competition binding assay was performed to determine their respective EC50 values. Factor IXa (100 nM) was incubated with increasing concentrations of DHG or LMWH for 10 minutes at room temperature prior to injection at 5 μL/min for 120 seconds. For each glycosaminoglycan concentration, the final sensorgram response at 90 seconds after injection was plotted as the relative proportion of remaining free factor IXa, with response units for FIXa alone (no DHG or LMWH) normalized to 1. The EC50 was determined by fitting the data to the following equation: B = (EC50)n /(EC50)n + [I]n), where B represents the fractional specific binding, I represents the concentration of glycosaminoglycan used as competitor, EC50 represents the concentration of DHG or LMWH that causes a 50% reduction in the surface plasmon binding response, and n represents the pseudo-Hill coefficient.29
Determination of the Ki for inhibition of factor X activation by DHG
The rate of factor X activation by the intrinsic complex in the presence of DHG was determined over 60 seconds at room temperature in a reaction containing 1.0 nM FVIIIa, 0.2 nM recombinant FIXa, 200 nM FX, and 50 μM PC/PS. The rate of factor X generation was plotted versus DHG concentration, and the data were fit by nonlinear regression to the equation for partial noncompetitive inhibition to determine the values for Vmax(app), Vimax(app), and Ki for DHG.12,30-32 Vmax(app) and Vimax(app) represent the maximal velocity in the absence and presence of inhibitor, and Ki is the inhibition constant for the enzyme-inhibitor complex.
Results
The effect of depolymerized holothurian glycosaminoglycan (DHG) on factor X activation by the intrinsic tenase complex
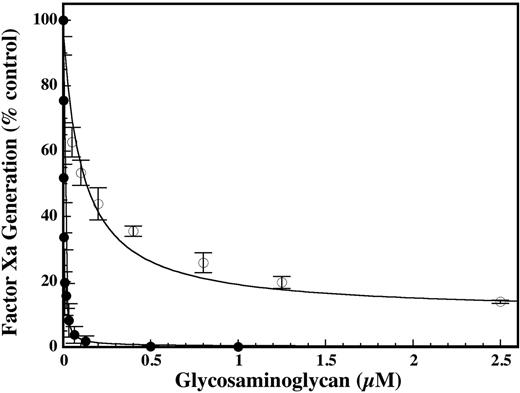
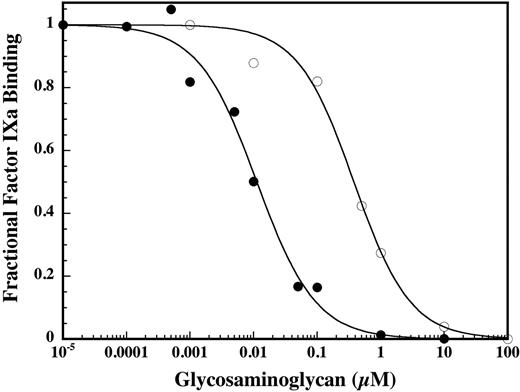
Increasing concentrations of DHG resulted in essentially complete inhibition of factor X activation by the intrinsic tenase complex. Fitting the data to a noncompetitive inhibition model (Figure 2) yielded a Ki value for DHG that was about 50-fold lower than LMWH on a molar basis, demonstrating significantly higher potency (Figure 1). Additionally, DHG demonstrated complete inhibition of factor X activation by the intrinsic tenase complex, in contrast to the partial inhibition observed for LMWH.12,16 To assess the appropriateness of the noncompetitive inhibition model, the effect of DHG on the kinetics of factor X activation was evaluated. The presence of inhibitory concentrations of DHG resulted in a dose-dependent reduction in the Vmax(app) for factor X activation without a consistent effect on the Km(app) (Figure 2), corresponding with a noncompetitive inhibition pattern. Due to the dominant role of exosite interactions in macromolecular substrate recognition by coagulation proteases, active site-directed inhibitors exhibit a noncompetitive inhibition pattern.33,34 To directly assess the effect of DHG on the active site of factor IXa, cleavage of the chromogenic substrate Pefachrome IXa by factor IXa–phospholipid was examined. DHG (0 to 1 μM) did not significantly inhibit Pefachrome IXa cleavage (data not shown), suggesting that DHG does not noncompetitively inhibit factor X activation by direct interaction with the active site or S4/S3-S1 subsites of factor IXa.
Comparison of the effect of DHG and LMWH on factor X activation by the intrinsic tenase complex. Increasing concentrations of DHG (•) or LMWH (○) were added to a reaction containing final concentrations of 1.0 nM factor VIIIa, 0.2 nM factor IXa, 200 nM factor X, and 50 μM PC/PS vesicles (75:25) in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. The rate of factor X activation (% control) by the intrinsic tenase complex was determined as described in “Materials and methods.” Mean values were plotted with error bars representing ± SD (n = 3 to 4). The inhibition constants (Ki) for DHG and LMWH were determined by fitting the data by nonlinear regression to the equation for partial, noncompetitive inhibition. Ki values ± SE for DHG and LMWH were 2.2 ± 0.2 and 112 ± 21 nM, respectively.
Comparison of the effect of DHG and LMWH on factor X activation by the intrinsic tenase complex. Increasing concentrations of DHG (•) or LMWH (○) were added to a reaction containing final concentrations of 1.0 nM factor VIIIa, 0.2 nM factor IXa, 200 nM factor X, and 50 μM PC/PS vesicles (75:25) in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. The rate of factor X activation (% control) by the intrinsic tenase complex was determined as described in “Materials and methods.” Mean values were plotted with error bars representing ± SD (n = 3 to 4). The inhibition constants (Ki) for DHG and LMWH were determined by fitting the data by nonlinear regression to the equation for partial, noncompetitive inhibition. Ki values ± SE for DHG and LMWH were 2.2 ± 0.2 and 112 ± 21 nM, respectively.
Effect of DHG on cofactor stability
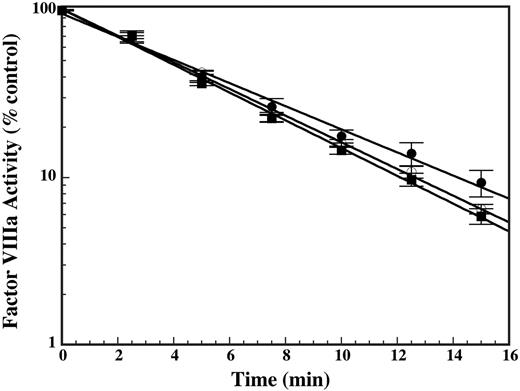
To assess whether the reduction in Vmax(app) for factor X activation was due to a direct effect on cofactor activity, the effect of DHG on cofactor stability was examined. Factor VIIIa activity degrades in a first-order manner due to dissociation of the noncovalently associated A2 domain.35 The effect of DHG on cofactor stability was assessed by examining the in vitro half-life of factor VIIIa in the absence or presence of DHG. Limiting amounts of thrombin-activated factor VIIIa were added to the tenase reaction over time to determine remaining factor VIIIa activity relative to baseline. Despite the presence of maximally inhibitory DHG concentrations (25 or 50 nM), only very modest differences in the rate of reduction in factor VIIIa activity were observed in the presence of DHG (Figure 3). These modest differences cannot account for the magnitude of DHG inhibition observed during the enzymatic assays. The lack of a significant effect on cofactor half-life suggests that DHG does not meaningfully accelerate dissociation of the A2 domain from factor VIIIa nor inhibit factor X activation via direct destabilization of cofactor activity.
Effect of DHG on apparent factor IXa–factor VIIIa affinity
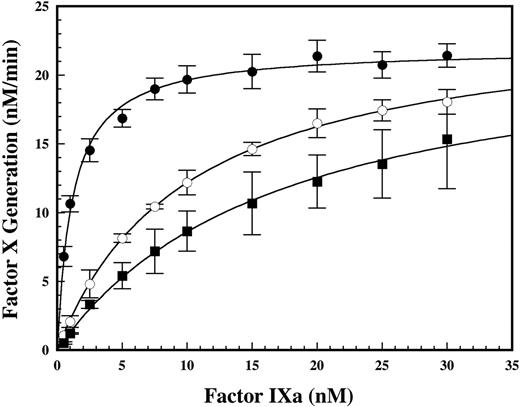
To address whether the decrease in Vmax(obs) for factor X activation was due to effects on enzyme complex assembly (decreased effective enzyme concentration) or reduced catalytic activity of the intact enzyme complex, the effect of DHG on factor IXa–factor VIIIa affinity was assessed in a functional binding assay. Titration of limiting amounts of cofactor with factor IXa in the absence and presence of inhibitory concentrations of DHG demonstrated a dose-dependent reduction in the apparent affinity of the protease-cofactor interaction (Figure 4). In the presence of 4 or 6 nM DHG, an 8- or 14-fold increase was observed in the Kd(app) for the factor IXa–factor VIIIa interaction, respectively. In contrast, the Bmax was similar in the absence or presence of DHG, suggesting that the effect of this glycosaminoglycan on factor IXa–factor VIIIa complex assembly on PC/PS vesicles was overcome by excess factor IXa. Using the experimentally determined Kd(app) to calculate the predicted factor IXa–factor VIIIa complex concentration under assay conditions for DHG concentration-dependent inhibition (Figure 1), the reduction in apparent affinity of the protease-cofactor interaction was sufficient to explain the magnitude of observed inhibition. This result is consistent with the complete inhibition of factor X activation at high DHG concentrations, suggesting that the protease-cofactor interactions relevant for generation of enzymatic activity are completely disrupted.
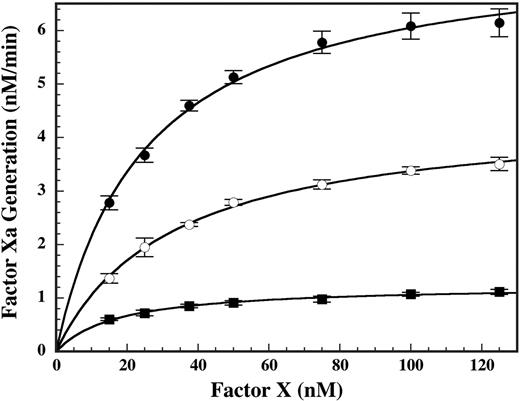
Effect of DHG on the kinetics of factor X activation by the intrinsic tenase complex. The kinetics of factor X activation by the intrinsic tenase complex in the presence of 0 nM (•), 1 nM (○), or 2 nM (▪) DHG was determined in reactions containing 1.0 nM factor VIIIa, 0.1 nM factor IXa, 15 to 125 nM factor X, and 50 μM PC/PS in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. Mean values were plotted with error bars representing ± SD (n = 3 to 4). The Km(app) and Vmax(app) for factor X activation were determined by fitting the data by nonlinear regression to the Michaelis-Menten equation. The Kmax(app) and Vmax(app) values ± SE for factor X activation were 25.1 ± 1.7 nM and 7.6 ± 0.2 nM/min, 32.4 ± 1.6 nM and 4.5 ± 0.1 nM/min, and 17.2 ± 1.4 nM and 1.2 ± 0.03 nM/min in the presence of 0, 1, or 2 nM DHG, respectively.
Effect of DHG on the kinetics of factor X activation by the intrinsic tenase complex. The kinetics of factor X activation by the intrinsic tenase complex in the presence of 0 nM (•), 1 nM (○), or 2 nM (▪) DHG was determined in reactions containing 1.0 nM factor VIIIa, 0.1 nM factor IXa, 15 to 125 nM factor X, and 50 μM PC/PS in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. Mean values were plotted with error bars representing ± SD (n = 3 to 4). The Km(app) and Vmax(app) for factor X activation were determined by fitting the data by nonlinear regression to the Michaelis-Menten equation. The Kmax(app) and Vmax(app) values ± SE for factor X activation were 25.1 ± 1.7 nM and 7.6 ± 0.2 nM/min, 32.4 ± 1.6 nM and 4.5 ± 0.1 nM/min, and 17.2 ± 1.4 nM and 1.2 ± 0.03 nM/min in the presence of 0, 1, or 2 nM DHG, respectively.
Effect of DHG on factor IXa–heparin binding
To assess whether DHG and LMWH interact with overlapping binding sites on factor IXa, the ability of soluble DHG to compete with the binding of factor IXa to immobilized LMWH was examined by a solution competition approach detected by surface plasmon resonance.16 Preincubation of factor IXa with DHG effectively competed the binding of protease to the immobilized LMWH surface, suggesting that DHG interacts with an enzymatic exosite that significantly overlaps the heparin-binding site. The apparent affinity of DHG for factor IXa was significantly higher than LMWH in solution, based on the approximate 35-fold reduction in the EC50 value of DHG relative to LMWH (Figure 5). This difference in apparent affinity between DHG and LMWH for factor IXa parallels their respective relative potency in the inhibition of factor X activation by the intrinsic tenase complex (Figure 1).
Effect of DHG on the in vitro half-life of factor VIIIa. Recombinant factor VIII (20 nM) was activated for 30 seconds with 40 nM thrombin, neutralized with 60 nM hirudin, and immediately diluted 1:2 into the tenase reaction buffer in the absence (•) or presence of 25 (○) or 50 nM (▪) DHG. Residual factor VIIIa activity was determined by sampling into the intrinsic tenase assay as described (see “Materials and methods”) and the data fit to a simple exponential decay. Mean values for factor VIIIa activity were plotted versus time with error bars representing ± SD (n = 3). The kobs for loss of factor VIIIa activity was 0.16 min–1 for cofactor alone and 0.18 min–1 or 0.19 min–1 for cofactor plus 25 or 50 nM nM DHG, respectively.
Effect of DHG on the in vitro half-life of factor VIIIa. Recombinant factor VIII (20 nM) was activated for 30 seconds with 40 nM thrombin, neutralized with 60 nM hirudin, and immediately diluted 1:2 into the tenase reaction buffer in the absence (•) or presence of 25 (○) or 50 nM (▪) DHG. Residual factor VIIIa activity was determined by sampling into the intrinsic tenase assay as described (see “Materials and methods”) and the data fit to a simple exponential decay. Mean values for factor VIIIa activity were plotted versus time with error bars representing ± SD (n = 3). The kobs for loss of factor VIIIa activity was 0.16 min–1 for cofactor alone and 0.18 min–1 or 0.19 min–1 for cofactor plus 25 or 50 nM nM DHG, respectively.
Effect of DHG on the affinity of the factor IXa–factor VIIIa complex on phospholipid vesicles. The apparent affinity (Kd(app)) of the factor IXa–factor VIIIa interaction was determined using enzymatic activity to detect complex formation in the absence (•) and presence of 4 (○) or 6 (▪) nM DHG. The rate of factor X activation by factor IXa–factor VIIIa was determined by adding increasing concentrations of factor IXa (0 to 30 nM) into a reaction containing 0.15 nM factor VIIIa, 200 nM factor X, and 50 μM PC/PS vesicles. Mean values were plotted versus factor IXa concentration with error bars representing ± SD (n = 4 to 6). The Kd(app) was determined by fitting the data to a single site–binding model. The Kd(app) and Bmax values ± SE were 1.2 ± 0.1 nM and 21.9 ± 0.3 nM/min, 10.0 ± 0.3 nM and 24.4 ± 0.3 nM/min, and 16.9 ± 1.4 nM and 23.2 ± 0.9 nM/min in the presence of 0, 4, and 6 nM DHG, respectively.
Effect of DHG on the affinity of the factor IXa–factor VIIIa complex on phospholipid vesicles. The apparent affinity (Kd(app)) of the factor IXa–factor VIIIa interaction was determined using enzymatic activity to detect complex formation in the absence (•) and presence of 4 (○) or 6 (▪) nM DHG. The rate of factor X activation by factor IXa–factor VIIIa was determined by adding increasing concentrations of factor IXa (0 to 30 nM) into a reaction containing 0.15 nM factor VIIIa, 200 nM factor X, and 50 μM PC/PS vesicles. Mean values were plotted versus factor IXa concentration with error bars representing ± SD (n = 4 to 6). The Kd(app) was determined by fitting the data to a single site–binding model. The Kd(app) and Bmax values ± SE were 1.2 ± 0.1 nM and 21.9 ± 0.3 nM/min, 10.0 ± 0.3 nM and 24.4 ± 0.3 nM/min, and 16.9 ± 1.4 nM and 23.2 ± 0.9 nM/min in the presence of 0, 4, and 6 nM DHG, respectively.
Competition of factor IXa binding to immobilized LMWH by soluble DHG and LMWH. Increasing concentrations of DHG (•) or LMWH (○) were preincubated with 100 nM factor IXa for 10 minutes prior to injection over reference and immobilized LMWH surfaces on the BiaCore 2000. Injections were performed and sensograms generated as described in “Materials and methods.” The concentration of free factor IXa was determined by the binding response at 90 seconds after injection. The concentration of glycosaminoglycan was plotted versus the proportion of remaining free factor IXa and fit by nonlinear regression to obtain the EC50 for competition by the glycosaminoglycan (see “Materials and methods”). The fitted values for the EC50 ± SE were 0.011 ± 0.002 μM for DHG and 0.38 ± 0.05 μM for LMWH.
Competition of factor IXa binding to immobilized LMWH by soluble DHG and LMWH. Increasing concentrations of DHG (•) or LMWH (○) were preincubated with 100 nM factor IXa for 10 minutes prior to injection over reference and immobilized LMWH surfaces on the BiaCore 2000. Injections were performed and sensograms generated as described in “Materials and methods.” The concentration of free factor IXa was determined by the binding response at 90 seconds after injection. The concentration of glycosaminoglycan was plotted versus the proportion of remaining free factor IXa and fit by nonlinear regression to obtain the EC50 for competition by the glycosaminoglycan (see “Materials and methods”). The fitted values for the EC50 ± SE were 0.011 ± 0.002 μM for DHG and 0.38 ± 0.05 μM for LMWH.
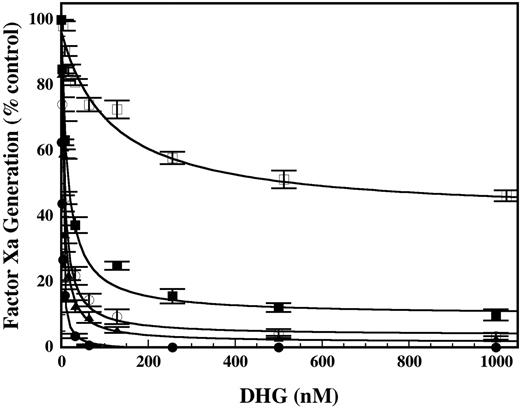
Effect of mutations in the heparin-binding exosite of factor IXa on the ability of DHG to inhibit factor X activation by intrinsic tenase. Increasing concentrations of DHG were added to a reaction mixture containing 0.2 nM factor IXa wild type (•), H92A (○), R170A (▪), R233A (□), or K241A (▴) with 1.0 nM factor VIIIa, 200 nM factor X, and 50 μM PC/PS vesicles in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. The rate of factor X activation (nM/min) by the intrinsic tenase complex was determined as described in “Materials and methods.” The rate of factor X activation (nM/min) by intrinsic tenase was normalized to activity present in the absence of DHG. Mean values (% control; no DHG) were plotted with error bars representing ± SD (n = 3 to 6). The inhibition constant (Ki) for DHG was determined by fitting the data by nonlinear regression to the equation for partial, noncompetitive inhibition. The Ki values ± SE for the recombinant proteins were as follows: WT, 1.6 ± 0.1 nM; H92A, 6.5 ± 0.5 nM; R170A, 13.9 ± 2.1 nM; R233A, 113.9 ± 29.3 nM; and K241A, 4.6 ± 0.4 nM.
Effect of mutations in the heparin-binding exosite of factor IXa on the ability of DHG to inhibit factor X activation by intrinsic tenase. Increasing concentrations of DHG were added to a reaction mixture containing 0.2 nM factor IXa wild type (•), H92A (○), R170A (▪), R233A (□), or K241A (▴) with 1.0 nM factor VIIIa, 200 nM factor X, and 50 μM PC/PS vesicles in 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000. The rate of factor X activation (nM/min) by the intrinsic tenase complex was determined as described in “Materials and methods.” The rate of factor X activation (nM/min) by intrinsic tenase was normalized to activity present in the absence of DHG. Mean values (% control; no DHG) were plotted with error bars representing ± SD (n = 3 to 6). The inhibition constant (Ki) for DHG was determined by fitting the data by nonlinear regression to the equation for partial, noncompetitive inhibition. The Ki values ± SE for the recombinant proteins were as follows: WT, 1.6 ± 0.1 nM; H92A, 6.5 ± 0.5 nM; R170A, 13.9 ± 2.1 nM; R233A, 113.9 ± 29.3 nM; and K241A, 4.6 ± 0.4 nM.
Effect of factor IXa mutations on the ability of DHG to inhibit factor X activation by the intrinsic tenase complex
The effect of mutations in the heparin-binding exosite of factor IXa on the ability of DHG to inhibit factor X activation by the intrinsic tenase complex was assessed with previously characterized recombinant factor IXa mutants.17 Factor X activation by factor IXa wild type (WT) was completely inhibited by DHG, with an apparent inhibitor affinity (Ki) similar to plasma-derived factor IXa (Figure 6). Factor IXa H92A and K241A demonstrated 3- to 4-fold increases in the Ki for DHG relative to factor IXa WT and minimal residual activity. Factor IXa R170A demonstrated significant resistance to inhibition with an 8- to 9-fold increase in the Ki for DHG compared with the factor IXa WT and more than 10% residual activity. Remarkably, factor IXa R233A demonstrated a 70-fold increase in the Ki for DHG relative to factor IXa WT and more than 40% residual activity. Thus, the apparent inhibitor affinity (Ki) of DHG for the intrinsic tenase complex containing each of the recombinant factor IXa's was ranked as follows: WT > H92A > K241A > R170A >> R233A. Disruption of the DHG–factor IXa interaction by these mutations results in partial rather than complete inhibition of intrinsic tenase complex, similar to the pattern of inhibition by LMWH.
The effect of DHG on the rate of intrinsic tenase decay
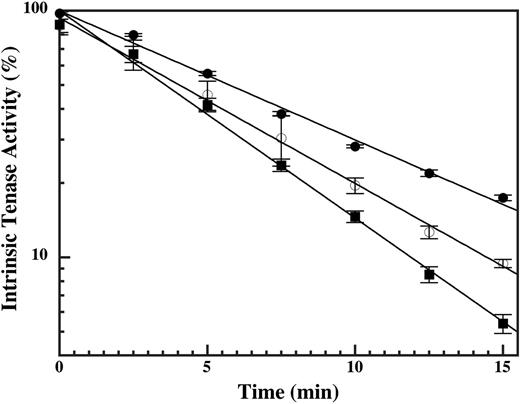
Mutations in the heparin-binding exosite of factor IXa, or the presence of LMWH, disrupt protease interactions with the factor VIIIa A2 domain and accelerate decay of the intrinsic tenase complex.17 The effect of DHG was similarly examined under conditions in which the decay of intrinsic tenase activity depends primarily on the rate of A2 domain dissociation.36 The rate of intrinsic tenase decay was examined by varying the time of factor X addition to a tenase reaction containing 1.0 nM factor VIIIa and 0.2 nM factor IXa in the absence and presence of submaximal inhibitory DHG concentrations. Addition of 4 or 6 nM DHG resulted in a 33% or 58% increase in the rate of intrinsic tenase decay, respectively (Figure 7). These results suggest that DHG binding to factor IXa destabilizes the critical interaction(s) with the factor VIIIa A2 domain within the intrinsic tenase complex.
Discussion
In vitro and ex vivo modeling of coagulation demonstrates that activation of factor X by the intrinsic tenase complex (factor IXa–factor VIIIa) is the rate-limiting step for thrombin generation formation during the propagation phase of coagulation.13,37-40 Given the critical role of this enzyme complex, the mechanism and molecular target for DHG inhibition of the intrinsic tenase complex were examined and compared with the antithrombin-independent inhibition by LMWH. Consistent with previous work, DHG essentially completely inhibited factor X activation by the intrinsic tenase complex (Figure 1). Based on the predominant effect of the inhibitor on the Vmax(app) for factor X activation (Figure 2), the data were fit to the equation for partial, noncompetitive inhibition to allow direct comparison with the inhibitor affinity (Ki) for LMWH. On the basis of relative Ki values, DHG demonstrated a dramatically higher (50-fold) apparent affinity for the intrinsic tenase complex compared with LMWH (Figure 1). Additionally, DHG demonstrated complete inhibition of factor X activation, as opposed to partial inhibition with LMWH. The cause of the marked decrease in the Vmax(app) for factor X activation was determined by evaluating the effects of DHG on the active site, cofactor activity, and factor IXa–factor VIIIa binding. The initial binding of macromolecular substrate (Km(app)) to membrane-bound coagulation enzyme complexes is exosite mediated, followed by intramolecular binding of the substrate to the active site and catalysis of the scissile bond (Vmax(app)). Thus, active site inhibitors competitively inhibit cleavage of peptide substrates and noncompetitively inhibit cleavage of macromolecular substrate.41 DHG did not significantly inhibit chromogenic substrate hydrolysis, suggesting that it does not inhibit factor X activation by direct interaction with the active site (data not shown). Likewise, DHG did not appreciably shorten the in vitro half-life of factor VIIIa, suggesting that it does not inhibit factor X activation via direct destabilization of cofactor activity (Figure 3). However, DHG reduced the apparent affinity of factor IXa–factor VIIIa interaction on phospholipid vesicles in a dose-dependent manner (Figure 4), indicating reduced assembly of the enzyme complex. The predicted reduction in factor IXa–factor VIIIa concentration based on experimentally determined Kd(app) values in the presence of DHG was sufficient to completely account for the magnitude of inhibition. Thus, reduction in the Vmax(app) for factor X activation was due to decreased assembly of the intrinsic tenase complex in the presence of DHG rather than reduced catalytic activity of the intact complex. These results are consistent with the complete inhibition of factor X activation observed at high DHG concentrations.
Effect of DHG on the rate of intrinsic tenase decay. Factor X (200 nM) was added at intervals between 15 seconds (time 0) and 15 minutes after cofactor addition to 0.2 nM factor IXa wild type, 1.0 nM factor VIIIa, 50 μM PC/PS vesicles, 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000 in the absence (•) and presence of 4 (○) or 6 (▪) nM DHG. The rate of factor X activation (nM/min) by the intrinsic tenase complex was determined as described in “Materials and methods.” Mean values (% control) were plotted with error bars representing ± SD (n = 3). The kobs for intrinsic tenase decay was 0.12 min–1, 0.16 min–1, and 0.19 min–1 in the presence of 0, 4, or 6 nM DHG, respectively.
Effect of DHG on the rate of intrinsic tenase decay. Factor X (200 nM) was added at intervals between 15 seconds (time 0) and 15 minutes after cofactor addition to 0.2 nM factor IXa wild type, 1.0 nM factor VIIIa, 50 μM PC/PS vesicles, 0.15 M NaCl, 20 mM HEPES (pH 7.4), 2 mM CaCl2, 1 mg/mL BSA, and 0.1% PEG-8000 in the absence (•) and presence of 4 (○) or 6 (▪) nM DHG. The rate of factor X activation (nM/min) by the intrinsic tenase complex was determined as described in “Materials and methods.” Mean values (% control) were plotted with error bars representing ± SD (n = 3). The kobs for intrinsic tenase decay was 0.12 min–1, 0.16 min–1, and 0.19 min–1 in the presence of 0, 4, or 6 nM DHG, respectively.
The molecular target for inhibition of intrinsic tenase activity by DHG was evaluated by competition of protease binding to immobilized LMWH and analysis of factor IXa mutants. The lack of a significant effect on apparent macromolecular substrate affinity (Figure 2), cofactor stability (Figure 3), and the reduction in factor IXa–factor VIIIa affinity (Figure 4) suggested that DHG interacts directly with the protease. DHG efficiently competed factor IXa binding to immobilized LMWH, consistent with the conclusion that these glycosaminoglycans bind to overlapping sites on the protease (Figure 5). The EC50 for DHG was significantly lower (35-fold) than that observed for LMWH in solution, suggesting that DHG binds to factor IXa with significantly higher affinity than LMWH. The relative affinity of DHG and LMWH for factor IXa based on EC50 values correlated with the 50-fold greater potency for intrinsic tenase inhibition by DHG versus LMWH based on relative Ki values (Figure 1). Likewise, the effect of mutations in the heparin-binding exosite of factor IXa on the relative ability of DHG to inhibit factor X activation by the intrinsic tenase complex closely paralleled their effects on inhibition by LMWH. Based on relative Ki values, the recombinant proteases were inhibited by DHG in decreasing order of potency: WT > K241A > H92A > R170A > > R233A (Figure 6). This rank order correlates with the effect of these mutations on the relative affinity of the factor IXa–LMWH interaction and the apparent inhibitor affinity of LMWH (Ki) for the intrinsic tenase complex.17 The relative (fold) increase in the Ki value compared with wild-type factor IXa for each of these mutations was similar for DHG and LMWH. The overall magnitude of the resistance to inhibition with these mutations (especially R233A) was greater for LMWH, likely reflecting the lower overall affinity of the factor IXa–LMWH interaction. Submaximal inhibitory concentrations of DHG also modestly accelerated decay of the intrinsic tenase complex in a dose-dependent fashion, suggesting destabilization of the factor IXa–A2 domain interaction (Figure 7). These results suggest that DHG binds tightly to the heparin-binding exosite of factor IXa, resulting in reduced protease-cofactor affinity and destabilization of the factor VIIIa A2 domain within the intrinsic tenase complex.
Mutagenesis of factor IXa demonstrates that the binding sites for factor VIIIa and heparin overlap substantially on the protease, and this exosite contributes to the ability of protease to stabilize the half-life of factor VIIIa activity on the phospholipid membrane.17 The present results identify a common molecular target for antithrombin-independent inhibition of the intrinsic tenase complex by the structurally diverse glycosaminoglycans, DHG and LMWH. Differences in the magnitude of maximal inhibition (complete versus partial) between these glycosaminoglycans appear to depend, in part, on relative factor IXa–glycosaminoglycan affinity. DHG binds to factor IXa with higher affinity than LMWH (Figure 5), resulting in more extensive disruption of the factor IXa–factor VIIIa interaction and essentially complete inhibition of factor X activation. This complete inhibition suggests that DHG not only disrupts the interaction with the A2 domain but also affects the interface with the factor VIIIa light chain, because the latter dominates factor IXa–factor VIIIa affinity.42,43 The more extensive disruption of the protease-cofactor interaction by DHG may reflect an extended binding site on the protease relative to LMWH or additional steric interference by this highly branched glycosaminoglycan. Mutations in the factor IXa heparin-binding exosite reduce affinity for DHG, resulting in decreased inhibitor affinity for the tenase complex (Ki) and partial rather than complete inhibition of factor X activation (Figure 6). Submaximal inhibitory concentrations of DHG destabilized the factor VIIIa A2 domain within the intrinsic tenase complex (Figure 7); however, this mechanism appears less important than for inhibition by LMWH. This conclusion is based on the substantial effects of DHG on overall factor IXa–factor VIIIa affinity (Kd(app)) with minimal change in Bmax values, suggesting that the inhibition is reversible at high protease concentrations (Figure 4). In contrast, LMWH demonstrates modest changes in factor IXa–factor VIIIa affinity (Kd(app)) with a significant reduction in the Bmax, demonstrating a lack of reversibility at high protease concentrations.16 Mutagenesis of the 484-510 or 558-565 loops of the factor VIIIa A2 domain demonstrates a similar effect in factor IXa–factor VIIIa functional binding assays.44,45 In contrast to DHG, the effect of LMWH on apparent factor IXa–factor VIIIa affinity is insufficient to explain the magnitude of the inhibition, which includes a significant component of accelerated decay of the intrinsic tenase complex.16
In vitro analysis demonstrates that DHG inhibits factor X activation by the intrinsic tenase complex at significantly lower concentrations (Ki about 2 nM) than required for optimal acceleration of thrombin inhibition by HCII (about 2.4 μM) or inhibition of factor VIII activation by thrombin (more than 80 nM).22,23 While DHG and heparin share a common molecular target and mechanism for inhibition of intrinsic tenase, both the in vitro and in vivo effects of DHG appear independent of antithrombin.18,22 Thus, DHG represents a lead compound for analysis of this antithrombotic mechanism in the absence of confounding antithrombin-dependent activities. Screening and selection of related glycosaminoglycans for the ability to disrupt the factor IXa–A2 domain interaction will identify critical structural determinants and provide additional candidates to explicitly test this mechanism. Selective targeting of the intrinsic tenase complex in animal models of thrombosis is associated with significantly less bleeding risk compared with UH and LMWH at equivalent therapeutic doses.46-50 Likewise, DHG demonstrates significantly less prolongation of the rat tail bleeding time and reduced bleeding time and blood loss from a template incision of the dog ear, compared with equitherapeutic doses of UH or LMWH.19-21 Because the factor IXa–A2 domain interaction is required for factor X activation by the intrinsic tenase complex,51 disruption of this protein-protein interaction is expected to proportionally reduce plasma thrombin generation based on the rate-limiting nature of the intrinsic tenase complex.13,37,40 This novel antithrombotic strategy should allow titration of thrombin generation while limiting the risk of bleeding at supratherapeutic doses.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-07-3043.
Supported by a Grant-in-Aid from the Northland Affiliate of the American Heart Association, a grant from the Wisconsin Alumni Research Foundation, and National Institutes of Health grant HL080452 (J.P.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Darrell Stafford for providing the human factor IX cDNA, factor IX WT, and R170A cell lines; Andreas Mueller-Beckhaus of Bayer for providing recombinant factor VIII (Kogenate FS); Kazuhisa Minamiguchi of Taiho Pharmaceuticals for providing DHG; and Andre Radloff for assistance with cell culture.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal