Comment on Prabhala et al, page 300
Multiple myeloma is associated with a deranged immune system, which is thought to be attributed to myeloma infiltration and interference of the bone marrow. Now, Prabhala and colleagues show that dysfunctional Treg cells might also contribute to the abnormalities.
Both T- and B-cell abnormalities have been demonstrated in patients with multiple myeloma (MM).1,2 Consistent findings include suppressed uninvolved immunoglobulin, low CD4+/CD8+ T-cell ratio, and T-cell expansion in both CD4+ and CD8+ T-cell subsets. In addition, a lower expression of T-cell receptor/CD3-associated signaling molecules, such as PKC-α, the presence of activated (HLA-DR+) T cells, and a bias towards induction of a type-2 T-cell response were reported in myeloma patients. Thus, T-cell abnormalities in MM involve changes in the number and activation status of T-cell subsets, as well as in their functions. Although the mechanisms underlying these abnormalities are unknown, it was thought that the presence of myeloma cells in the bone marrow, where human hematopoiesis takes place, was partly responsible.
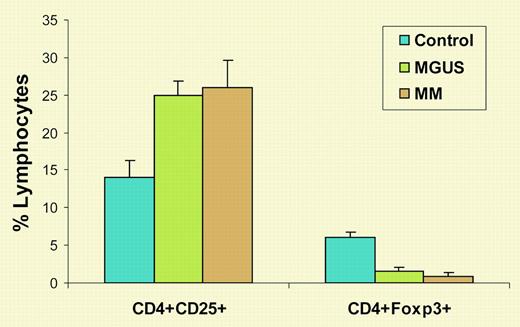
The existence of suppressive or regulatory T cells was proposed in the early 1970s, disputed for many years, and abandoned thereafter. Finally, in 1995, Sakaguchi and colleagues reported that they had identified suppressor T cells as CD4+CD25+ T cells.3 Eight years later, it was discovered that Foxp3 is specifically expressed in suppressive or regulatory CD4+CD25+ T cells.4 T regulatory (Treg) cells play an important role in the maintenance of self-tolerance and control of autoimmunity, regulation of T-cell homeostasis, and modulation of overall immune responses against infection and tumors. In this issue of Blood, Prabhala and colleagues show that, although the percentage of CD4+CD25+ T cells was increased in patients with MM or monoclonal gammopathy of undetermined significance (MGUS) compared with healthy donors, the numbers of CD4+Foxp3+ Treg cells were significantly decreased in these patients (see figure). Moreover, purified CD25+ T cells from MM and MGUS patients were unable to suppress in vitro anti-CD3 antibody-induced T-cell proliferation, even when added in high proportions. Therefore, the investigators concluded that the number and function of Treg cells were decreased in MGUS and MM, which might, at least in part, account for the nonspecific increase in CD4+CD25+ T cells and dysfunctional T-cell responses seen in these patients.
This study is important because it identifies Treg cells as one of the potentially important mediators of immune dysfunction in MM. Furthermore, the same phenomenon was also found in patients with MGUS, suggesting that these T cells may play a role in an early stage of the disease, and monitoring Treg cells at different stages of the disease may provide insight into the immune mechanisms associated with disease development and progress. Moreover, a better understanding of the function of Treg cells will also help develop effective immunotherapy for MM.
This study raises several questions for future studies. For example, what causes the decreased number and dysfunction of Treg cells in patients? One possibility is myeloma cells, which not only physically occupy the bone marrow and damage hematopoiesis, but also secrete cytokines that can impair the number and function of Treg cells.5 Another question is why are there no hyperactive myeloma-specific T cells in patients, and why is it still difficult to immunize patients against myeloma antigens when suppressive Treg cells are deficient? Finally, will purified Treg, instead of CD25+ T cells from patients also be unable to suppress T-cell responses in vitro? We anxiously await answers to these questions. ▪
Numbers of CD4+CD25+ and CD4+Foxp3+ T cells in MM and MGUS. Shown are the percentages of lymphocytes expressing these molecules from healthy blood donors and patients with MGUS or MM.
Numbers of CD4+CD25+ and CD4+Foxp3+ T cells in MM and MGUS. Shown are the percentages of lymphocytes expressing these molecules from healthy blood donors and patients with MGUS or MM.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal