Abstract
Human mesenchymal stem cells (hMSCs) suppress T-cell and dendritic-cell function and represent a promising strategy for cell therapy of autoimmune diseases. Nevertheless, no information is currently available on the effects of hMSCs on B cells, which may have a large impact on the clinical use of these cells. hMSCs isolated from the bone marrow and B cells purified from the peripheral blood of healthy donors were cocultured with different B-cell tropic stimuli. B-cell proliferation was inhibited by hMSCs through an arrest in the G0/G1 phase of the cell cycle and not through the induction of apoptosis. A major mechanism of B-cell suppression was hMSC production of soluble factors, as indicated by transwell experiments. hMSCs inhibited B-cell differentiation because IgM, IgG, and IgA production was significantly impaired. CXCR4, CXCR5, and CCR7 B-cell expression, as well as chemotaxis to CXCL12, the CXCR4 ligand, and CXCL13, the CXCR5 ligand, were significantly down-regulated by hMSCs, suggesting that these cells affect chemotactic properties of B cells. B-cell costimulatory molecule expression and cytokine production were unaffected by hMSCs. These results further support the potential therapeutic use of hMSCs in immune-mediated disorders, including those in which B cells play a major role.
Introduction
Adult bone marrow stromal cells supply the appropriate scaffold for hematopoiesis1 and hematopoietic-cell homeostasis,2 but can also differentiate in vitro in most, if not all, somatic cell types.3,4 Due to their specific capability of generating multiple mesenchymal lineages, bone marrow-derived stromal progenitors have been designated mesenchymal stem cells (MSCs).5 MSCs are clonogenic because they can be isolated from bone marrow and expanded ex vivo without any apparent modification in phenotype or loss of function. Based on these features, MSCs are considered a promising strategy for tissue engineering, repair of damaged tissues, and gene therapy, but their capacity to trans-differentiate also in vivo is still unresolved.
Recently, another unforeseen feature of MSCs has been reported, namely, that MSCs can modulate many T-cell functions including cell activation.6,7 Based on this, human MSCs (hMSCs) have been administered in vivo to improve the outcome of allogeneic transplantation by promoting hematopoietic engraftment8 and to hamper graft-versus-host disease.9 More recently, we have shown that systemic administration of MSCs to mice affected by experimental autoimmune encephalomyelitis (EAE), a prototypical disease mediated by self-reactive T cells, results in striking disease amelioration mediated by the induction of peripheral tolerance.10 In addition, it has been shown that tolerance induction by MSCs may occur also through the inhibition of dendritic-cell maturation and function,11-13 thus suggesting that activated T cells are not the only targets of MSCs. In contrast, the effects of hMSCs on B cells are unknown.
B-cell development occurs in the bone marrow and is strictly dependent on close interaction of B-cell progenitors with stromal cells that produce cytokines capable of supporting B-cell survival and proliferation.14,15 Thus, we investigated whether MSCs, which derive from the marrow stroma, affect mature B-cell functions. Here, we demonstrate that hMSCs significantly affect proliferation, differentiation, and chemotactic behavior of normal mature B cells, thus further supporting the possibility that administration of MSCs may represent a novel therapeutic strategy for immune-mediated disorders.
Materials and methods
Aliquots of bone marrow aspirates were obtained from healthy adult bone marrow donors and peripheral-blood (PB) samples were obtained from healthy adult blood donors after informed consent was obtained, per the Declaration of Helsinki. The ethical board of the G. Gaslini Institute approved the study.
B-cell isolation and culture
Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque density gradient (Sigma Chemical, St Louis, MO) from the PB of 9 healthy donors. Cell suspensions were first depleted of lymphocytes forming rosettes with sheep red blood cells (T cells) and subsequently treated with magnetic activated cell sorting (MACS) CD19-conjugated microbeads, according to the instructions of the manufacturer (Miltenyi Biotech, Auburn, CA). Positively selected cells contained on average 99% B cells, as assessed by flow cytometric analysis with a CD20 monoclonal antibody (mAb). All cell cultures were performed in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma Chemical), in the absence or presence of the following stimuli: the CpG 2006 synthetic oligonucleotide (2.5 μg/mL),16 recombinant CD40L (rCD40L; 100 ng/mL; Immunotech, Marseille, France), anti-human immunoglobulin goat antibodies (2 μg/mL; Immunotech), interleukin 2 (IL-2; 50 U/mL; Proleukin, Chiron Italia, Milan, Italy), IL-4 (10 ng/mL, R&D Systems, Minneapolis, MN), and IL-10 (10 ng/mL; Boehringer Mannheim, Marburg, Germany).
Flow cytometry
Anti-CD19-phycoerythrin (PE) and PE-cyanin 5 (Cy5), CD20-PE were from Caltag Laboratories (Burlingame, CA). Anti-CD80-fluorescein isothiocyanate (FITC), CD86-PE, CD40-PE, CD73-PE, CD44-FITC, CD34-FITC, CD14-PE, CD45-FITC, CD45-PE-Cy5, and anti-HLA-DR-FITC mAbs were from BD PharMingen (San Diego, CA). FITC-conjugated anti-Ki67 mAb was from Dako (Glostrup, Denmark). Unconjugated anti-STRO-1 and anti-Sca-1, anti-CC chemokine receptor (CCR) 7-PE mAbs were from R&D Systems. Anti-CD45-PE, CXC chemokine receptor (CXCR) 4, and CXCR5 mAbs (Serotec, Raleigh, NC) were also used. Cells were stained with fluorochrome-conjugated mAbs or with unconjugated mAbs followed by fluorochrome-conjugated secondary reagents, or with isotype and fluorochrome-matched control antibodies. Cells were then run on a fluorescence-activated cell sorter (FACSscan; Becton Dickinson, San Jose, CA). On average, 104 events were acquired and analyzed using the CellQuest software.
For intracellular staining experiments, purified B cells were cocultured for 24 hours without or with hMSC suspensions at the indicated ratio in the presence of CpG 2006, CD40L, anti-immunoglobulin, IL-2, and IL-4. IL-4 was omitted from the cultures in which endogenous production of the cytokine was tested. After culture, cells were washed in phosphate-buffered saline (PBS; Sigma Chemical) with 1% FBS (staining buffer) and surface stained with CD19-PE mAb for 30 minutes at 4°C in the dark. Cells were washed again in staining buffer and fixed in 4% paraformaldehyde for 20 minutes at 4°C in the dark. Afterward, cells were permeabilized with a buffer containing PBS, 1% FBS, and 0.1% saponin and stained with FITC-conjugated mAbs to human tissue necrosis factor (TNF), interferon γ (IFN-γ), IL-4, IL-10, and IL-12 (p70; Caltag) or FITC-conjugated anti-Ki67 mAb for 30 minutes at 4°C in the dark. Cells were then washed in staining buffer and analyzed by flow cytometry. Isotype-matched, PE- and FITC-conjugated mAbs of irrelevant specificity were tested as negative controls.
The threshold line for calculation of percent positive cells was based on the maximum staining obtained with irrelevant isotype-matched mAbs used at the same concentration as test mAbs. Negative cells were defined such fewer than 1% of cells stained positive with control mAbs.
MSC culture and expansion
MSCs were generated in vitro from normal bone marrow samples from healthy human donors. MNCs were isolated by gradient centrifugation at 900g for 30 minutes on Lympholyte of 1.073 g/mL density (Cedarlane Laboratories, Hornby, ON, Canada), were washed twice with PBS, counted, and plated at a concentration of 20 to 30 × 106 MNCs/75-cm2 flasks (Sardtedt, Numbrecht, Germany). The hMSC medium consisted of Mesencult basal medium supplemented with Mesenchymal Stem Cell Stimulatory Supplement (StemCell Technologies, Vancouver, BC, Canada). Medium containing nonadherent cells was then replaced every 4 days of culture. When cultures reached 90% confluence, cells were trypsinized and replated at the density of 0.7 × 106 cells/75-cm2 flasks. Under such conditions, we selectively expanded cells negative for hematopoietic markers such as CD34, CD45, and CD14, but positive for the adhesion molecule CD44 and other MSC typical markers such as stem-cell antigen 1 (Sca-1), stromal-derived factor 1 (STRO-1), and CD73, as assessed by flow cytometry.17
Proliferation assay
Purified B cells were cocultured in 96-well flat-bottom plates (Costar, Cambridge, MA) with hMSC suspensions from allogeneic donors at the indicated ratios in the presence of CpG 2006, CD40L, anti-immunoglobulin, IL-2, and IL-4 in a total volume of 0.2 mL RPMI 1640 medium per well in triplicate. After 3 days, cultures were pulsed for 16 hours with 0.5 μCi (0.0185 MBq) [3H]-thymidine (MP Biomedicals, Aurora, OH). For transwell experiments, hMSCs and B cells were seeded in 24-well plates on the opposite sides of a 5-μm pore-size polycarbonate membrane (Costar) and cultured for 3 days before being subjected to [3H]-thymidine incorporation. B cells were subsequently harvested onto glass-fiber filter paper (Skatron Instruments, Tranby, Norway), dried, and the incorporated [3H]-thymidine analyzed with liquid scintillation counter (MP Biomedicals). Data were expressed as median counts per minute of triplicate samples. In some experiments, B cells were centrifuged, resuspended in supernatants from hMSCs grown at confluence, and cultured for 3 days as described.
Detection of immunoglobulin production
Purified B cells were cocultured in 96-well round-bottom plates (Costar) with hMSC suspensions from unrelated donors at the indicated ratios in the presence of CpG 2006, CD40L, anti-immunoglobulin, IL-2, IL-4, and IL-10 in a total volume of 0.2 mL RPMI 1640 medium per well in quadruplicate. After 7 days, supernatants were collected and tested by enzyme-linked immunosorbent assay (ELISA). In brief, plates (PBI, Milan, Italy) were coated with isotype-specific goat antimouse mAbs (IgM, IgG, IgA; Dako) diluted in PBS and 2% bovine serum albumin (BSA) and incubated overnight at 4°C. Thereafter, plates were extensively washed and supernatants from cell cultures were added in quadruplicate and incubated at 37°C for 2 hours in a humidified atmosphere. After 3 washings with PBS containing 0.001% Tween 20, horseradish peroxidase-conjugated anti-mouse IgM, or IgG or IgA mAbs (Dako) were added and incubated at 37°C for 2 hours. The reaction was developed with o-phenylenediamine dihydrochloride. For standard immunoglobulin determination, normal serum was diluted in PBS/BSA 1:1000 for IgM, 1:32 000 for IgG, and 1:10 000 for IgA, and ELISAs were performed as described. Immunoglobulin production at the single-cell level was also investigated by enzyme-linked immunospot assay (ELISPOT). After cell stimulation, locally produced immunoglobluins were captured by specific anti-IgM, anti-IgG, and anti-IgA mAbs (5 μg/mL, Southern Biotechnology, Burlingame, CA). After cell lysis, trapped immunoglobulin molecules were revealed by biotinylated goat anti-human immunoglobulin antibodies (Southern Biotechnology), which, in turn, were recognized by streptavidin conjugated to alkaline phosphatase. The reaction was developed with 3-amino-9-ethylcarbazole. Red spots indicated immunoglobulin production by individual cells. For titration curves, 7 serial dilutions of B cells starting from 105 were performed.
Migration assay
Chemotaxis of B-cell suspensions was investigated using 24-transwell plates with a 5-μm pore-size polycarbonate membrane (Costar), as reported.18,19 Purified B cells were cocultured for 24 hours without or with hMSC suspensions at the indicated ratios in the presence of CpG 2006, CD40L, anti-immunoglobulin, IL-2, and IL-4. Thereafter, 5 × 105 B cells were dispensed in the upper chamber, whereas chemokines or medium alone were added to the lower chamber. CXCL12, CXCL13, and CCL19 were tested at 300 ng/mL, a concentration chosen on the grounds of dose-response experiments carried out with normal tonsil B cells.19,20 Plates were incubated for 2 hours at 37°C. Cells that migrated into the lower chambers were harvested, stained with CD19 mAb, and counted. Results were expressed as percent input calculated as the percent ratio between the number of B cells dispensed in the upper chamber and that of B cells recovered from the lower chamber after migration. Net percent input, that is, the difference between input obtained following chemokine stimulation and that obtained on incubation with medium alone, was used for statistical analysis of the results.
Statistical analysis
Data were reported in terms of medians, minimum and maximum values, or first and third quartiles for quantitative data. A nonparametric analysis of variance (Kruskal-Wallis test) has been performed to compare quantitative data among groups; the Mann-Whitney U test with the Bonferroni correction has been used as a post-hoc test after the analysis of variance. P < .05 was considered statistically significant. Statistical analyses were performed using Statistica software (StatSoft, Tulsa, OK).
Results
Effects of hMSCs on B-cell proliferation
We first investigated the effects of hMSC on B-cell proliferation on stimulation with CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. Maximum inhibition of B-cell proliferation was observed at the B-cell/hMSC ratio of 1:1 (P = .008; Figure 1A); this effect was still detectable at a 1:2 ratio (P = .036; Figure 1A) and disappeared at 1:5 and 1:10 ratios (not shown). hMSCs cultured in the absence of B cells and stimulated as described did not proliferate (Figure 1A).
We previously demonstrated that inhibition of T-cell proliferation by hMSCs was unrelated to induction of apoptosis.10 Thus, to investigate the potential involvement of apoptosis in hMSC-mediated inhibition of B-cell proliferation, activated B cells that had been cocultured without or with hMSCs at a 1:1 ratio for 3 days were double-stained with CD19 and annexin V. Similar proportions of annexin V+ apoptotic B cells were detected in cultures performed with or without MSCs (Figure 1B).
hMSCs inhibit B-cell proliferation. (A) B cells purified from PB of healthy donors were cocultured for 3 days with allogeneic hMSC suspensions at 1:1 and 1:2 ratios in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. Cell proliferation was assessed by [3H]-thymidine incorporation. Median counts per minute, maximum and minimum values, from 9 independent experiments are shown. **P = .008; *P = .036. (B) B-cell-enriched populations cultured for 3 days with or without hMSCs at a 1:1 ratio in the presence of stimuli were double-stained with CD19 mAb and annexin V and analyzed by flow cytometry. Results are median percent CD19, annexin V+ cells, and maximum and minimum values from 3 independent experiments. (C) B-cell-enriched populations cocultured with (right panel) or without (left panel) hMSCs at a 1:1 ratio in the presence of stimuli were fixed, permeabilized, and stained with CD19 and anti-Ki67 mAb. A representative experiment is shown. Gated CD19+ B cells were stained with anti-Ki67 mAb (black histograms) or its isotypic control (white histograms). Results are shown as percentage of positive cells. The differences between the histograms of left and right panels are likely related to changes in B-cell shape caused by their coculture with hMSCs.
hMSCs inhibit B-cell proliferation. (A) B cells purified from PB of healthy donors were cocultured for 3 days with allogeneic hMSC suspensions at 1:1 and 1:2 ratios in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. Cell proliferation was assessed by [3H]-thymidine incorporation. Median counts per minute, maximum and minimum values, from 9 independent experiments are shown. **P = .008; *P = .036. (B) B-cell-enriched populations cultured for 3 days with or without hMSCs at a 1:1 ratio in the presence of stimuli were double-stained with CD19 mAb and annexin V and analyzed by flow cytometry. Results are median percent CD19, annexin V+ cells, and maximum and minimum values from 3 independent experiments. (C) B-cell-enriched populations cocultured with (right panel) or without (left panel) hMSCs at a 1:1 ratio in the presence of stimuli were fixed, permeabilized, and stained with CD19 and anti-Ki67 mAb. A representative experiment is shown. Gated CD19+ B cells were stained with anti-Ki67 mAb (black histograms) or its isotypic control (white histograms). Results are shown as percentage of positive cells. The differences between the histograms of left and right panels are likely related to changes in B-cell shape caused by their coculture with hMSCs.
hMSCs inhibit B-cell proliferation through the release of soluble factors. hMSCs and B cells at a 1: 1 ratio were seeded in 24-well plates on the opposite sides of a 5-μm pore-size polycarbonate membrane (transwell) and cultured for 3 days. Aliquots of the same hMSC and B-cell suspensions were cocultured at the same ratio for the same time. Control B cells were cultured alone. Stimuli were CpG 2006, rCD40L, anti-immunoglobulin, IL-2, and IL-4. B-cell proliferation was assessed by [3H]-thymidine incorporation. Results are expressed as median counts per minute, maximum and minimum values from 5 different experiments. **P = .009.
hMSCs inhibit B-cell proliferation through the release of soluble factors. hMSCs and B cells at a 1: 1 ratio were seeded in 24-well plates on the opposite sides of a 5-μm pore-size polycarbonate membrane (transwell) and cultured for 3 days. Aliquots of the same hMSC and B-cell suspensions were cocultured at the same ratio for the same time. Control B cells were cultured alone. Stimuli were CpG 2006, rCD40L, anti-immunoglobulin, IL-2, and IL-4. B-cell proliferation was assessed by [3H]-thymidine incorporation. Results are expressed as median counts per minute, maximum and minimum values from 5 different experiments. **P = .009.
Because hMSCs did not affect B-cell viability, we investigated whether inhibition of B-cell proliferation by hMSCs was related to the arrest of the cell cycle. To this end, activated B cells that had been cocultured with or without hMSCs for 3 days were double-stained with CD19 and Ki67 mAb, which detects cells in the late G1/G2/S/M phases of the cell cycle.21 As shown in Figure 1C, most B cells cultured alone expressed Ki67 (left panel), whereas such expression strongly declined on coculture with hMSCs at a 1:1 ratio (right panel). These results demonstrated that inhibition of B-cell proliferation was attributable to a block in G0/G1 phases of the cell cycle.
hMSC-dependent inhibition of B-cell proliferation is mediated by soluble factors
Because previous reports suggested that MSC inhibition of T cells was mediated both by cell-to-cell contact and soluble factors, we next investigated B-cell proliferation in a transwell system in which B cells, plated in the lower chamber, were physically separated from hMSCs, tested at a 1:1 ratio, and dispensed in the upper chamber. Paired cultures in which B cells were admixed with hMSCs at the same ratio were also set up. Statistically significant inhibition of B-cell proliferation was observed irrespective of the presence or absence of the filter separating B cells from hMSCs (P = .009; Figure 2). These results demonstrate that soluble factors released by hMSCs cultured together with B cells but separated from them are sufficient to provide maximal inhibition of B-cell proliferation. In contrast, supernatants from hMSCs grown at confluence did not inhibit B-cell proliferation (not shown), suggesting that release of inhibitory soluble factors by hMSCs likely required paracrine signals from B cells.
Effects of hMSCs on immunoglobulin production
Purified B cells were next incubated without or with hMSCs at 1:1, 1:2, 1:5, and 1:10 ratios in the presence of CpG, CD40L, anti-immunoglobulin, IL-2, IL-4, and IL-10, and B-cell differentiation was investigated.
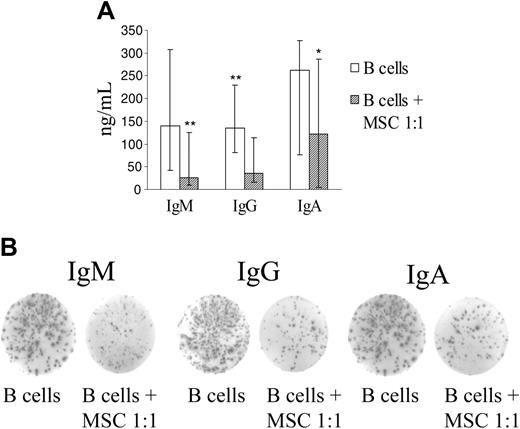
hMSCs inhibited production of IgM, IgG, and IgA at a B-cell/MSC ratio of 1:1 (P = .003 for IgM, P = .001 for IgG, P = .018 for IgA), as assessed by ELISA (Figure 3A). No inhibition of immunoglobulin production was observed at the other B cell/MSC ratios tested (not shown).
hMSCs inhibit immunoglobulin production. Purified B cells were incubated for 7 days without or with hMSC at a 1:1 ratio in the presence of CpG, rCD40L, anti-immunoglobulin antibodies, IL-2, IL-4, and IL-10. B-cell differentiation was evaluated using 2 assays, namely, quantitation of IgM, IgG, and IgA in culture supernatants by ELISA and enumeration of immunoglobulin-secreting cells by ELISPOT. (A) Median immunoglobulin concentrations (ng/mL), maximum and minimum values from 9 experiments performed by ELISA are shown. **P = .003 for IgM and .001 for IgG; *P = .018 for IgA. (B) One representative experiment of the 3 carried out by ELISPOT is shown. Spots indicate immunoglobulin production by individual cells.
hMSCs inhibit immunoglobulin production. Purified B cells were incubated for 7 days without or with hMSC at a 1:1 ratio in the presence of CpG, rCD40L, anti-immunoglobulin antibodies, IL-2, IL-4, and IL-10. B-cell differentiation was evaluated using 2 assays, namely, quantitation of IgM, IgG, and IgA in culture supernatants by ELISA and enumeration of immunoglobulin-secreting cells by ELISPOT. (A) Median immunoglobulin concentrations (ng/mL), maximum and minimum values from 9 experiments performed by ELISA are shown. **P = .003 for IgM and .001 for IgG; *P = .018 for IgA. (B) One representative experiment of the 3 carried out by ELISPOT is shown. Spots indicate immunoglobulin production by individual cells.
Next we investigated whether the reduced amounts of immunoglobulin detected by ELISA was paralleled by a decreased number of antibody-secreting cells. When ELISPOT was used, the number of cells producing IgM, IgG, and IgA was similarly inhibited by hMSCs (Figure 3B).
Effects of hMSCs on B-cell chemokine receptor expression and function
The effects of hMSCs on B-cell chemotaxis were subsequently addressed focusing on chemokine receptors expressed constitutively by B cells homing to secondary lymphoid organs, namely CXCR4, CXCR5, and CCR7.22-24 Purified B cells were cultured for 24 hours without or with hMSCs at a 1:1 or 1:10 ratio in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4.
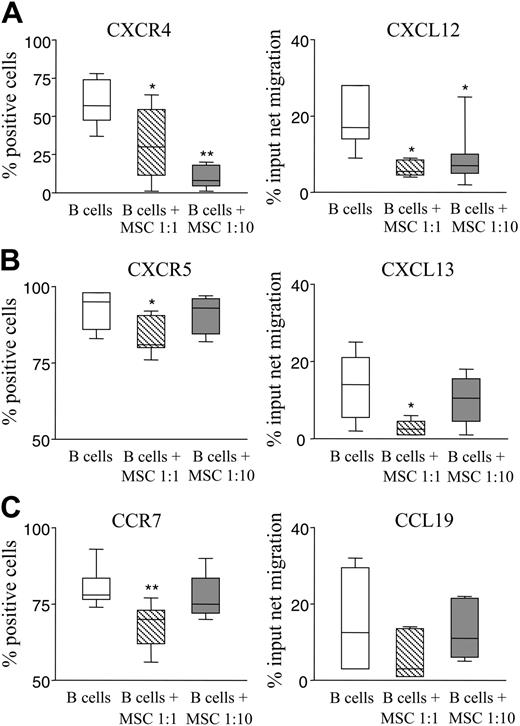
A significant decrease in CXCR4, CXCR5, and CCR7 B-cell expression was observed on culture with hMSCs at a 1:1 ratio (P = .036, .035, and .002, respectively; Figure 4A-C left panels). At the lower hMSC/B-cell ratio, only CXCR4 was significantly down-regulated (P = .003), whereas expression of CCR7 and CXCR5 was unaffected (Figure 4 A-C left panels).
As consequence of the reduced expression of these receptors, we investigated whether hMSCs could affect chemotaxis of activated B cells in response to CXCR4, CXCR5, and CCR7 ligands, namely, CXCL12, CXCL13, and CCL19, respectively. CXCL12-driven B-cell chemotaxis was significantly inhibited on culture with hMSCs at both ratios tested (P = .021 for 1:1 and = .013 for 1:10 ratio; Figure 4A right panel). CXCL13-driven B-cell chemotaxis was significantly reduced following culture with hMSCs at the 1:1 (P = .032), but not the 1:10, ratio (Figure 4B right panel). Finally, CCL19-driven B-cell chemotaxis was also decreased but such reduction did not reach statistical significance with hMSCs at any ratio (Figure 4C right panel). These results demonstrate that hMSCs inhibit CXCR4- and CXCR5-mediated B-cell chemotaxis.
Effects of hMSCs on B-cell expression of costimulatory molecules and cytokine production
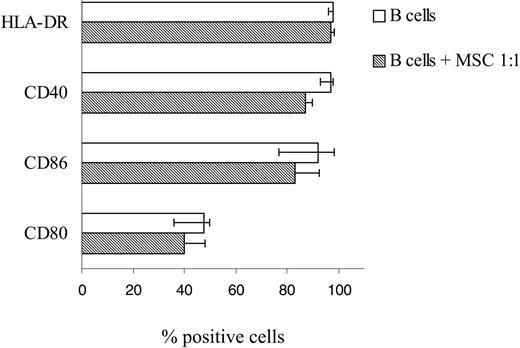
Previous studies have demonstrated that hMSCs may interfere with the adaptive immune response by affecting antigen presentation through down-regulation of costimulatory molecules and HLA-DR on dendritic cells.12 To verify whether hMSCs affected the expression of such molecules also on B cells, purified B cells were cultured for 24 hours with or without hMSCs at a 1:1 ratio, in the presence of CPG2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. Most B cells cultured in the absence of hMSCs were found to express HLA-DR and the large majority of them expressed CD40 and CD86. CD80 was detected in approximately a half the cells. Superimposable results were obtained with B cells that had been cocultured with hMSCs at a 1:1 ratio (Figure 5). These findings suggest that hMSCs did not affect the expression of molecules involved in antigen presentation on activated B cells.
Because B-cell effector functions are paralleled by the production of various cytokines,25 we investigated by intracellular staining the production of TNF, IFN-γ, IL-4, IL-10, and IL-12p70 by B lymphocytes, stimulated as described in the presence or absence of hMSCs at a 1:1 ratio. In the absence of hMSCs, stimulated B cells produced TNF (median, 19%; range, 6%-32% from 8 experiments), IFN-γ (median, 36%; range, 15%-80% from 10 experiments), IL-4 (median, 80%; range, 34%-96% from 10 experiments), and IL-10 (median, 13%; range, 7%-21% from 6 experiments), whereas IL-12p70 was virtually undetectable. Following culture of B cells with hMSCs, a limited decrease in TNF (median, 9%; range, 6%-25% range) and IFN-γ (median, 22%; range, 6%-72%) production, not reaching statistical significance, was observed. No changes for the other cytokines tested were observed.
hMSCs down-regulate B-cell expression of CXCR4, CXCR5, and CCR7 and chemotaxis to CXCL12 and CXCL13. (A-C left panels) Purified B cells were cultured 24 hours without or with hMSCs at 1:1 or 1:10 ratios in the presence of CpG, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. After culture, B cells were stained with anti-CXCR4, anti-CXCR5, or anti-CCR7 mAbs in combination with CD19 mAb and analyzed by flow cytometry. Results are expressed as median percent positive cells, first and third quartiles, obtained from 7 different experiments. *P = .036 for CXCR4 and .035 for CXCR5 at a B-cell/hMSC 1:1 ratio; **P = 0.003 for CXCR4 at a 1:10 ratio and .002 for CCR7 at a 1:1 ratio. (A-C right panels) Chemotaxis of activated B cells cultured with or without hMSCs at a 1:1 or 1:10 ratio in response to CXCL12, CXCL13, and CCL19, that is, the ligands of CXCR4, CXCR5, and CCR7, respectively, or medium alone. Migrated B cells were identified by CD19 staining. Results from 5 different experiments are expressed as median percent input of net migration, maximum and minimum values. *P = .021 and .013 for CXCL12 at the B-cell/hMSC 1:1 and 1:10 ratios, respectively, and .032 for CXCL13 at a 1:1 ratio.
hMSCs down-regulate B-cell expression of CXCR4, CXCR5, and CCR7 and chemotaxis to CXCL12 and CXCL13. (A-C left panels) Purified B cells were cultured 24 hours without or with hMSCs at 1:1 or 1:10 ratios in the presence of CpG, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. After culture, B cells were stained with anti-CXCR4, anti-CXCR5, or anti-CCR7 mAbs in combination with CD19 mAb and analyzed by flow cytometry. Results are expressed as median percent positive cells, first and third quartiles, obtained from 7 different experiments. *P = .036 for CXCR4 and .035 for CXCR5 at a B-cell/hMSC 1:1 ratio; **P = 0.003 for CXCR4 at a 1:10 ratio and .002 for CCR7 at a 1:1 ratio. (A-C right panels) Chemotaxis of activated B cells cultured with or without hMSCs at a 1:1 or 1:10 ratio in response to CXCL12, CXCL13, and CCL19, that is, the ligands of CXCR4, CXCR5, and CCR7, respectively, or medium alone. Migrated B cells were identified by CD19 staining. Results from 5 different experiments are expressed as median percent input of net migration, maximum and minimum values. *P = .021 and .013 for CXCL12 at the B-cell/hMSC 1:1 and 1:10 ratios, respectively, and .032 for CXCL13 at a 1:1 ratio.
B-cell expression of costimulatory molecules is unaffected by incubation with hMSCs. Purified B cells were cultured for 24 hours with or without hMSCs at a 1:1 ratio, in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. After culture, B cells were stained with anti-HLA-DR, anti-CD40, anti-CD86, and anti-CD80 mAbs in combination with CD19 mAb and analyzed by flow cytometry. Results are expressed as median percent positive cells, maximum and minimum values obtained from 7 different experiments.
B-cell expression of costimulatory molecules is unaffected by incubation with hMSCs. Purified B cells were cultured for 24 hours with or without hMSCs at a 1:1 ratio, in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. After culture, B cells were stained with anti-HLA-DR, anti-CD40, anti-CD86, and anti-CD80 mAbs in combination with CD19 mAb and analyzed by flow cytometry. Results are expressed as median percent positive cells, maximum and minimum values obtained from 7 different experiments.
Discussion
MSCs have been shown to exert in vitro immunosuppressive activities on activated T cells.6,7,9,26,27 Based on these properties we recently used murine MSCs to successfully treat a T-cell-mediated experimental model of multiple sclerosis.10 Others have demonstrated that MSCs have immunomodulatory properties impairing maturation and function of dendritic cells.11-13
In this study, we show for the first time that hMSCs inhibit in vitro human B-cell proliferation, differentiation to antibody-secreting cells, and chemotaxis. B cells isolated from PB of healthy individuals were stimulated with anti-immunoglobulin antibodies, rCD40L, the CpG oligonucleotide 2006, and a cocktail of B-cell tropic cytokines. Such a cocktail provides an optimal trigger for most B-cell effector functions,16 thus allowing investigation of the direct effects of hMSCs on B-cell activities.
Following culture with hMSCs, B-cell proliferation and differentiation to IgM-, IgG-, and IgA-producing cells were significantly impaired. The effects of hMSCs on B cells appeared to be strongly influenced by the relative in vitro concentrations, as suggested by a gradual loss of inhibition observed at lower MSC/B-cell ratios. MSCs also appeared to affect B-cell proliferation and differentiation differently, likely due to diverse transcriptional pathways involved in such processes. Because T-cell activation is strongly inhibited by MSCs even at low concentrations of MSCs,10 we cannot exclude that their in vivo effect on B cells may be stronger when T-cell help is required.
Similarly to that observed for T cells,10 on MSC interaction, B cells did not proliferate and did not undergo apoptosis, but accumulated in the G0-G1 phases of the cell cycle.
MSC-mediated inhibition of murine B-cell proliferation has been reported in 2 studies. In the first, T cell-depleted splenocytes were stimulated with anti-CD40 antibodies and IL-4 and MSCs were found to exert a potent inhibitory effect at a MSC/B-cell ratio of 1:10.28 It is likely that the minimal effect of hMSCs we observed at low concentration depends on the maximal B-cell activation we routinely obtain in our experimental conditions,16 compared to the weaker trigger used by Glennie and coworkers.28
In the second murine study, unfractionated splenocytes were activated with pokeweed mitogen, which triggers both T- and B-cell proliferation. These experimental conditions do not allow us to dissect the latter events and to conclude unambiguously that B-cell proliferation was inhibited by MSCs.29
The similar inhibition of B-cell proliferation observed in this study when B cells and hMSCs were seeded together or physically separated by a filter in the same well indicates that the inhibitory effect was mediated by soluble factors. These factors appear to be released by hMSCs on direct cross-talk with B cells, suggesting that hMSCs require activation signals from B cells to exert their suppressive action. Conversely, supernatants from pure hMSCs at confluence did not inhibit B-cell proliferation. Among soluble factors that have been involved in the immunosuppressive activity of hMSCs, transforming growth factor β1 and hepatocyte growth factor,6 prostaglandin E2,11 and indoleamine 2,3-dioxygenase30 are consistent candidates.
Because it was previously shown that hMSCs down-regulated HLA-DR and the CD80 and CD86 costimulatory molecules in human mature myeloid dendritic cells,11-13 we investigated whether similar changes were induced in B cells, which also belong to professional antigen-presenting cells (APCs). No significant down-regulation of HLA-DR, CD80, CD86, or CD40 was observed in B cells cultured with hMSCs, suggesting that the APC function of B cells was spared. Furthermore, hMSCs did not significantly inhibit TNF, IFN-γ, IL-4, and IL-10 production by B cells.
Finally, we investigated whether hMSCs can modulate the expression of chemokine receptors and their subsequent migratory properties to the respective ligands. Migration of B lymphocytes to and positioning in secondary lymphoid organs is tightly regulated by constitutively expressed lymphoid chemokines, that is, CXCL12 that binds CXCR4, CXCL13 that interacts with CXCR5, and CCL19 and CCL21, both of which bind CCR7.22-24
CXCR4 is involved in early B-cell lymphopoiesis, B-cell adhesion to lymph node high endothelial venules, centroblast localization to the dark zone of germinal centers, and bone marrow migration of plasmablasts originating from the latter structures.31-37 CXCR5 attracts antigen-activated B cells to the germinal centers and promotes B-cell entry into Peyer patches.33,38 CCR7 mediates B-cell adhesion to lymph node high endothelial venules in combination with CXCR4 and trafficking of memory B cells to the T-cell areas of secondary lymphoid organs.33,39
Here we show that CXCR4, CXCR5, and CCR7 B-cell expression was down-regulated following incubation with hMSCs at a 1:1 ratio. CXCR4 and CXCR5 down-regulation was paralleled by decreased B-cell chemotaxis in response to CXCL12 and CXCL13, respectively. Notably, phenotypic and functional down-modulation of CXCR4, but not of CXCR5, was still detected when hMSCs and B cells were cocultured at a 1:10 ratio, suggesting that marrow-derived MSCs may preferentially target CXCR4, which plays a major role for homing and retention of stem cells in the bone marrow.32,40
In conclusion, hMSCs interfere with human B-cell function by acting at multiple levels, that is, proliferation, differentiation to antibody-producing cells, and chemotaxis. The recent report that murine MSCs injected in vivo home to lymph nodes and spleen where they cluster around T cells10 supports the hypothesis that B cells may also be targeted by MSCs in the T-cell area of secondary lymphoid organs.
Thus, the inhibition of most B-cell effector functions, combined with the profound suppression of T-cell activation, further supports the use of hMSCs for the treatment of immune-mediated disorders and sets the stage for the possible use of hMSCs as cell therapy for autoimmune diseases, including those in which B cells play a major role.
Prepublished online as Blood First Edition Paper, September 1, 2005; DOI 10.1182/blood-2005-07-2657.
Supported by grants from the Italian Foundation for Multiple Sclerosis (A.U.), Istituto Superiore di Sanità (A.U.) “National Program on Stem Cells,” the Fondazione Cassa di Risparmio di Genova e Imperia (CARIGE; A.U. and G.L.M.), and the Ministero della Salute, Ricerca Corrente (V.P.).
V.P. and A.U. share equal credit for senior authorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dr Angela Pistorio for the help in statistical analysis of the results. The excellent secretarial assistance of Mrs Chiara Bernardini is acknowledged.

![Figure 1. hMSCs inhibit B-cell proliferation. (A) B cells purified from PB of healthy donors were cocultured for 3 days with allogeneic hMSC suspensions at 1:1 and 1:2 ratios in the presence of CpG 2006, rCD40L, anti-immunoglobulin antibodies, IL-2, and IL-4. Cell proliferation was assessed by [3H]-thymidine incorporation. Median counts per minute, maximum and minimum values, from 9 independent experiments are shown. **P = .008; *P = .036. (B) B-cell-enriched populations cultured for 3 days with or without hMSCs at a 1:1 ratio in the presence of stimuli were double-stained with CD19 mAb and annexin V and analyzed by flow cytometry. Results are median percent CD19, annexin V+ cells, and maximum and minimum values from 3 independent experiments. (C) B-cell-enriched populations cocultured with (right panel) or without (left panel) hMSCs at a 1:1 ratio in the presence of stimuli were fixed, permeabilized, and stained with CD19 and anti-Ki67 mAb. A representative experiment is shown. Gated CD19+ B cells were stained with anti-Ki67 mAb (black histograms) or its isotypic control (white histograms). Results are shown as percentage of positive cells. The differences between the histograms of left and right panels are likely related to changes in B-cell shape caused by their coculture with hMSCs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-07-2657/4/m_zh80010688560001.jpeg?Expires=1765049926&Signature=xNvVoWMcGA5MGYsAvcSiofyngV00gF1O0xWUluIUKaegPWfAsFmlKp48dCalXAujY54ZeOqsRbDYMeLvpRq4Ak7UjTbo9A0wvcrNQN6b3IM5lKL2hTm9skn-C7xaVZ19RpqokzK2l9Er-LDldJTN2ZZZMtR2AsDaC5wUeF3wCEvJ6MzcWgBv1i2y-nOEc9F4qPMlRnON91KZtjBgecBN1WEemSOzABmDHKmLtlmzhpfzPQEhbpSdV0CQZCEiVic~bLw7JrRyPL-RA-kUdrOSU2WcuQbtygzmOz1WGiuZ1ATNAClOAnTH~n~HI0RwnAS2OpClBp5Nfa9~oNtZQXH5qQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. hMSCs inhibit B-cell proliferation through the release of soluble factors. hMSCs and B cells at a 1: 1 ratio were seeded in 24-well plates on the opposite sides of a 5-μm pore-size polycarbonate membrane (transwell) and cultured for 3 days. Aliquots of the same hMSC and B-cell suspensions were cocultured at the same ratio for the same time. Control B cells were cultured alone. Stimuli were CpG 2006, rCD40L, anti-immunoglobulin, IL-2, and IL-4. B-cell proliferation was assessed by [3H]-thymidine incorporation. Results are expressed as median counts per minute, maximum and minimum values from 5 different experiments. **P = .009.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-07-2657/4/m_zh80010688560002.jpeg?Expires=1765049926&Signature=MuUxPC-g~Bm9qGgyTOLPVIhitZCsJb1kvq56cILgCGBPbdopr71jqFi6L7tTVDgrw8wqShMMav0BzYrj5triZTNXwJ2q7keW~tPbxECtqhT9T74K0oyYGkFU51Dw905Ao-OJKSbUDAbyh2CSQpUozICSWOx-FoQzKbX2r33OSsBLTSDqBWQu6pDViHwKw3KgQRpRuPnbloM5VDP~FoyU-erjGnfsoWi7oGAi2BOv3ZxEEJ0dTXJeO7E84YzsSFLu-gSOz98yyv3aWdN2~EZ5cdtcY2yYnAdngpGc7hYCZCNJ2Sy4kU-b1wUR-WUNVqdnPYIX-j1svRhj5Q4zJyVPDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal