Abstract
Patients with thalassemia major often report that they do not maintain their usual pretransfusion hemoglobin concentration during the summer season. We collected 3977 pretransfusion hemoglobin values, amount of blood transfused, and intertransfusion intervals for 94 patients with thalassemia major from 4 centers worldwide. We also assessed the hematocrits of blood donors, the hemoglobin content of units transfused in one center, and the local mean monthly temperatures during the periods of data collection. Pretransfusion hemoglobin levels were significantly lower during the summer in all centers except the one center where monthly temperatures have the least variation throughout the year. A similar relationship to temperature was observed for the hematocrits of blood donors and the hemoglobin content of donor units. This study confirms that pretransfusion hemoglobin levels in patients with thalassemia major are lower in the summertime. Possible mechanisms include expansion of plasma volume with resultant hemodilution in the patient and lower hemoglobin content in donor blood.
Introduction
Thalassemia major is a severe defect of hemoglobin synthesis that requires regular transfusion of erythrocytes every 2 to 4 weeks. Patients with thalassemia in different parts of the world commonly complain that their hemoglobin levels are lower during the summertime than the rest of the year. Seasonal variation of hemoglobin levels and hematocrit in healthy individuals has been described1-3 but has not been studied in transfusion-dependent patients. We sought to verify if, indeed, there are seasonal differences in the hemoglobin concentrations of patients with transfusion-dependent thalassemia.
Study design
We collected data regarding hemoglobin concentrations, the amount of transfused blood (measured as pure red cell mass; hematocrit 100%), and the intertransfusion intervals for 94 patients with thalassemia major from centers in Ferrara, Northern Italy (16 patients); Cagliari, Sardinia, Italy (21 patients); and Sydney, Australia (40 patients) followed for 3 years from January 2001 to December 2003, and in Philadelphia (17 patients) from January 2000 to December 2003. Forty-nine patients were male (52.1%). The mean age was 27 years (Ferrara: mean, 29 years, range, 21-35 years; Cagliari: mean, 27 years, range, 20-35 years; Philadelphia: mean, 22 years, range, 8-43 years; Sydney: mean, 27 years, range, 19-43 years). A total of 3977 pretransfusion hemoglobin values were collected. In Ferrara, we also collected the hemoglobin concentrations of the units of packed cells transfused during the same time interval. To examine seasonal variation in healthy individuals, we collected venous hematocrits, measured by automated counter (Coulter LH750, Fullerton, CA), of volunteers who presented as potential blood donors in Ferrara. All hematocrits obtained during predonation screening were used in order to include values below the minimum criterion for blood donation. Mean monthly temperatures for the years of the study were obtained from the Meteorological Branch of the Italian Air Force for Ferrara and Cagliari, from the Northeast Regional Climate Center for Philadelphia, and from the Bureau of Meteorology NSW for Sydney. Approval for the study entitled Seasonal Variation of Pretransfusion Hemoglobin Levels in patients with Thalassemia major was obtained from the institutional review board of the Azienda Ospedaliera Universitaria of Ferrara. Informed consent was provided according to the Declaration of Helsinki.
Statistical analyses were performed separately for each of the 4 study centers. For descriptive purposes, mean hemoglobin levels were calculated by calendar month. To test the hypothesis that hemoglobin levels are lower during the summer, data were combined into 4 seasons defined by equinoxes and solstices. To account for the lack of independence of the multiple observations, data were analyzed using a cross-sectional time-series general linear model. To account for possible confounding effects of age, sex, and differing interval between consecutive transfusions, and to account for potential variability in the amount of blood transfused, multivariate analyses were adjusted for those variables. Finally, to investigate the effect of temperature variability on hemoglobin distribution, the models were also adjusted for average monthly temperature.
Results and discussion
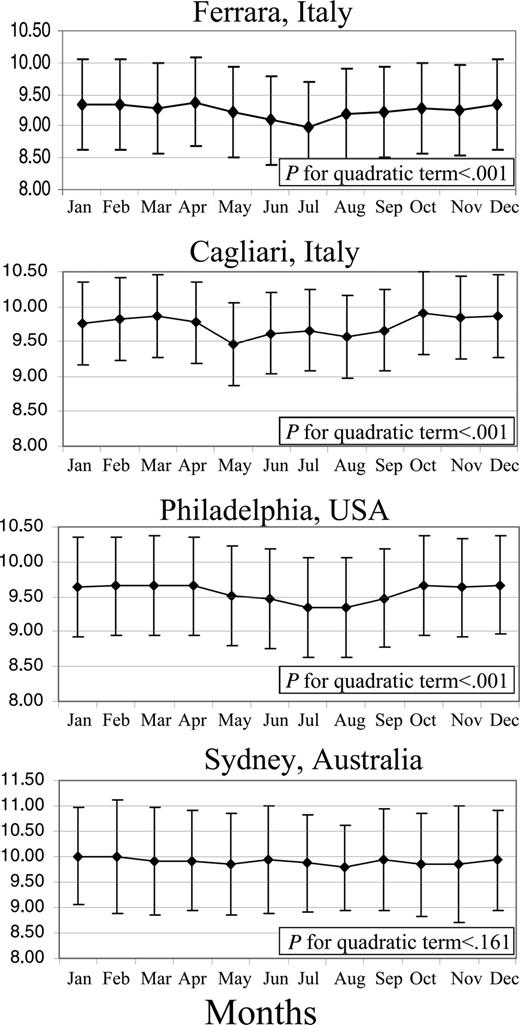
Figure 1 shows the mean pretransfusion hemoglobin levels of patients by calendar month and by center. In Ferrara, Cagliari, and Philadelphia, but not in Sydney, there was a nonlinear relationship between month and mean hemoglobin level, with lower levels during the summer months (P for quadratic term < .001 for the 3 centers). When months were grouped into seasons, the unadjusted mean pretransfusion hemoglobin levels were also significantly lower during the summer than other seasons in all centers except Sydney (Table 1 Model 1). In multivariate analysis, adjusted for age, sex, interval between transfusions, and amount of blood transfused during the previous visit, the seasonal difference in mean pretransfusion hemoglobin remained significant (Table 1 Model 2). However, when mean monthly temperature was included in the multivariate model (Table 1 Model 3), the differences in hemoglobin levels between seasons were strongly attenuated and no longer significant.
Crude and adjusted mean hemoglobin values by season and clinical center
. | . | Model 1 . | . | Model 2 . | . | Model 3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. patients . | Crude mean (SEM) . | P . | Adjusted mean*(SEM) . | P . | Adjusted mean†(SEM) . | P . | |||
| Ferrara, Italy | ||||||||||
| Summer | 254 | 9.13 (0.10) | – | 9.12 (0.9) | – | 9.25 (0.10) | – | |||
| Fall | 233 | 9.29 (0.10) | .001 | 9.29 (0.10) | .001 | 9.24 (0.10) | .995 | |||
| Winter | 220 | 9.36 (0.10) | <.001 | 9.38 (0.10) | .000 | 9.25 (0.10) | .994 | |||
| Spring | 241 | 9.28 (0.10) | .001 | 9.28 (0.10) | .001 | 9.32 (0.10) | .162 | |||
| Cagliari, Italy | ||||||||||
| Summer | 376 | 9.68 (0.08) | – | 9.70 (0.15) | – | 9.71 (0.15) | – | |||
| Fall | 380 | 9.88 (0.08) | .001 | 9.88 (0.15) | .001 | 9.85 (0.15) | .001 | |||
| Winter | 386 | 9.83 (0.08) | .001 | 9.84 (0.14) | .001 | 9.74 (0.15) | .541 | |||
| Spring | 387 | 9.64 (0.08) | .230 | 9.64 (0.15) | .128 | 9.75 (0.15) | .388 | |||
| Philadelphia, PA | ||||||||||
| Summer | 144 | 9.39 (0.11) | – | 9.38 (0.12) | – | 9.51 (0.13) | – | |||
| Fall | 140 | 9.70 (0.11) | .001 | 9.65 (0.18) | .001 | 9.61 (0.12) | .362 | |||
| Winter | 117 | 9.62 (0.12) | .002 | 9.64 (0.12) | .001 | 9.48 (0.14) | .837 | |||
| Spring | 143 | 9.56 (0.11) | .016 | 9.59 (0.12) | .002 | 9.61 (0.12) | .248 | |||
| Sydney, Australia‡ | ||||||||||
| Summer | 271 | 9.96 (0.11) | – | 9.98 (0.11) | – | 9.97 (0.11) | – | |||
| Fall | 224 | 9.88 (0.11) | .207 | 9.90 (0.11) | .285 | 9.90 (0.11) | .343 | |||
| Winter | 230 | 9.85 (0.11) | .112 | 9.86 (0.11) | .107 | 9.86 (0.11) | .388 | |||
| Spring | 231 | 9.87 (0.11) | .190 | 9.84 (0.11) | .06 | 9.84 (0.11) | .144 | |||
. | . | Model 1 . | . | Model 2 . | . | Model 3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. patients . | Crude mean (SEM) . | P . | Adjusted mean*(SEM) . | P . | Adjusted mean†(SEM) . | P . | |||
| Ferrara, Italy | ||||||||||
| Summer | 254 | 9.13 (0.10) | – | 9.12 (0.9) | – | 9.25 (0.10) | – | |||
| Fall | 233 | 9.29 (0.10) | .001 | 9.29 (0.10) | .001 | 9.24 (0.10) | .995 | |||
| Winter | 220 | 9.36 (0.10) | <.001 | 9.38 (0.10) | .000 | 9.25 (0.10) | .994 | |||
| Spring | 241 | 9.28 (0.10) | .001 | 9.28 (0.10) | .001 | 9.32 (0.10) | .162 | |||
| Cagliari, Italy | ||||||||||
| Summer | 376 | 9.68 (0.08) | – | 9.70 (0.15) | – | 9.71 (0.15) | – | |||
| Fall | 380 | 9.88 (0.08) | .001 | 9.88 (0.15) | .001 | 9.85 (0.15) | .001 | |||
| Winter | 386 | 9.83 (0.08) | .001 | 9.84 (0.14) | .001 | 9.74 (0.15) | .541 | |||
| Spring | 387 | 9.64 (0.08) | .230 | 9.64 (0.15) | .128 | 9.75 (0.15) | .388 | |||
| Philadelphia, PA | ||||||||||
| Summer | 144 | 9.39 (0.11) | – | 9.38 (0.12) | – | 9.51 (0.13) | – | |||
| Fall | 140 | 9.70 (0.11) | .001 | 9.65 (0.18) | .001 | 9.61 (0.12) | .362 | |||
| Winter | 117 | 9.62 (0.12) | .002 | 9.64 (0.12) | .001 | 9.48 (0.14) | .837 | |||
| Spring | 143 | 9.56 (0.11) | .016 | 9.59 (0.12) | .002 | 9.61 (0.12) | .248 | |||
| Sydney, Australia‡ | ||||||||||
| Summer | 271 | 9.96 (0.11) | – | 9.98 (0.11) | – | 9.97 (0.11) | – | |||
| Fall | 224 | 9.88 (0.11) | .207 | 9.90 (0.11) | .285 | 9.90 (0.11) | .343 | |||
| Winter | 230 | 9.85 (0.11) | .112 | 9.86 (0.11) | .107 | 9.86 (0.11) | .388 | |||
| Spring | 231 | 9.87 (0.11) | .190 | 9.84 (0.11) | .06 | 9.84 (0.11) | .144 | |||
For statistical comparison, summer was taken as the reference category.
– indicates no P value.
Cross-sectional time-series general linear model adjusted for age, sex, interval between transfusions, and amount of blood transfused during the previous visit
Cross-sectional time-series general linear model adjusted for the same variables as Model 2 and also monthly average temperature
For centers in Sydney, summer was defined as being December through February; fall, March through May; winter, June through August; and spring, September through November
Pearson correlation coefficients between mean monthly temperature and hemoglobin level were -0.15 (P < .001) for Ferrara, -0.14 (P < .001) for Philadelphia, -0.13 (P < .001) for Cagliari, and 0.04 (P = .271) for Sydney, showing a significant inverse correlation between temperature and hemoglobin level. A similar trend was observed for the hemoglobin content (ie, hemoglobin concentration × volume) of 1345 units of blood transfused in Ferrara and for 24 888 hematocrits of 5491 volunteer blood donors.
Mean monthly pretransfusional hemoglobin level (±SD) of thalassemia patients transfused in 4 different centers from 2001 to 2003 (2000-2003 in Philadelphia). A nonlinear relationship between month and mean hemoglobin level is present in all centers except Sydney, where the mean of the monthly temperatures has a smaller standard deviation during the year.
Mean monthly pretransfusional hemoglobin level (±SD) of thalassemia patients transfused in 4 different centers from 2001 to 2003 (2000-2003 in Philadelphia). A nonlinear relationship between month and mean hemoglobin level is present in all centers except Sydney, where the mean of the monthly temperatures has a smaller standard deviation during the year.
Finally, we found that the standard deviation of mean monthly temperatures in Philadelphia (mean and SD = 14.3°C ± 8.4°C), Ferrara (14.8°C ± 8.07°C), and Cagliari (17.9°C ± 6.09°C) were significantly different from that in Sydney (18.9°C ± 3.4°C) (Bartlett test for equal variances: χ2 [3 DF] = 23.9; P < .001).
Our study confirms the belief held by many patients with transfusion-dependent β-thalassemia that their pretransfusion hemoglobin levels show seasonal variation, with a nadir in the summer. This seasonal variation was noted in the 3 centers that experience significant temperature variation between seasons. The seasonal difference in pretransfusion hemoglobin levels was most marked in Ferrara and Philadelphia, less marked in Cagliari, and absent in Sydney, in accordance with the degree of variability of mean monthly temperature during the year. The nadir in hemoglobin level occurred in May in Cagliari where the temperature reaches summertime values earlier than in the other centers (data not shown). We have also demonstrated that the hemoglobin content of blood transfused to the patients in Ferrara was significantly lower in the summer than during the rest of the year, as was the hematocrit of volunteer blood donors.
Seasonal variation of hemoglobin level and hematocrit in healthy individuals has been described. A large cross-sectional study from Israel reported significantly lower values of hemoglobin and hematocrit in August.1 In another study, deferral of blood donors for low hematocrit was more frequent with increasing outdoor air temperature.2 A recent review, based on results from 18 studies including 24 793 subjects, reported that hematocrit values were on average 3% lower in summer than winter.3 This phenomenon may be attributed, at least in part, to hemodilution in warm weather, secondary to increased plasma volume expansion.2,4,5 The increase in plasma volume during the summer has been calculated to be 5.5% in healthy nonsmokers.6 The correlation that we found between hemoglobin level and seasonal temperature supports the theory that lower summertime hemoglobin level is more likely a consequence of heat acclimatization than an endogenous circannual rhythm.1
Although one study found that seasonal changes in blood volume and total hemoglobin level were attributable to changes in the length of the day rather than changes in temperature,7 most studies attribute the hemodilutional effect to heat.
The lower summertime hemoglobin level in patients with thalassemia may result from 2 mechanisms: an endogenous hemodilution effect of plasma volume expansion with higher temperatures, and the lower hemoglobin content of transfused donor blood in the summer months. In healthy individuals, the seasonal decrease in hemoglobin level is usually inconsequential, but for patients with thalassemia, the decrease may accentuate the symptoms of anemia, especially when compounded by the additional cardiovascular demands of coping with hotter weather. The results of our study may be helpful to clinicians when counseling patients and in planning transfusion volumes and schedules. The results also suggest that measurement of the hematocrit of individual units of packed red blood cells is important for the accurate assessment of transfusional iron loading.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-03-1231.
Supported in part by grants from the Ministry of the Instruction and University Research (C.B.-P.) and grant L.R. 30.4.1990, n.11 (R.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal