Abstract
A quantitative in vivo angiogenesis model employing collagen onplants placed on the chick embryo chorioallantoic membrane (CAM) has been used in this study to assess the spatial and temporal associations between neutrophil-like inflammatory cells, namely chicken heterophils, and the development of new blood vessels. Previously we have demonstrated that monocytes/macrophages infiltrating the onplants were associated with extracellular matrix remodeling and angiogenesis, in particular by delivering MMP-13 collagenase. By introducing chicken gelatinase B (chMMP-9) as a specific marker for heterophils, we now show that the onset and extent of angiogenesis induced by purified growth factors or by human HT-1080 fibrosarcoma cells correlated with the initial influx of chMMP-9–positive heterophils. This early heterophil arrival was followed by the infiltration of monocytes/macrophages and appeared to sustain further blood vessel formation. The disruption of inflammatory cell influx by 2 mechanistically distinct anti-inflammatory drugs, cortisone and ibuprofen, significantly inhibited angiogenesis, indicating a functional involvement of these inflammatory cells in new blood vessel development. A direct addition of isolated heterophils or purified chMMP-9 into the HT-1080 onplants engrafted into cortisone- or ibuprofen-treated embryos reversed the antiangiogenic effects of the drugs. The exogenously added heterophils induced in vivo a further infiltration of endogenous heterophils and monocytes and dramatically rescued the impaired angiogenesis, highlighting the importance of early inflammatory leukocytes in tumor-induced angiogenesis. Moreover, purified heterophils incorporated into onplants lacking growth factors or tumor cells induced angiogenesis in nontreated embryos, further indicating a direct proangiogenic role for neutrophil-like leukocytes.
Introduction
Tumor-induced angiogenesis occurs in a complex microenvironment in which a balance between antiangiogenic and proangiogenic factors has been shifted in favor of mediators promoting the expansion of preexisting blood vessels.1,2 One of the possible mechanisms underlying the angiogenic switch is a production by tumor cells of inflammatory mediators responsible for leukocyte recruitment. In turn, infiltrated leukocytes release an array of highly active mediators such as cytokines, chemokines, and growth factors, which further contribute to tumor progression by inducing tumor cells to produce additional angiogenic factors. Correspondingly, chronic inflammation and infiltration by leukocytes has been linked to neovascularization at the sites of human tumor development. Additional data suggest that inflammation can even be a prerequisite for the development of certain malignancies.3-13 Inhibition of tumor development and tumor angiogenesis by anti-inflammatory drugs, including those targeting cyclooxygenase 2 (COX-2), further suggests an active role of infiltrating leukocytes in neovascularization.2,14-21
Monocytes/macrophages, mast cells, neutrophils, and T lymphocytes represent the major types of infiltrating leukocytes found around and within solid tumors.3,5,7,8,10,11 During inflammatory responses, neutrophils are usually the first recruited effectors that produce potent chemotactic factors, likely guiding further influx of other inflammatory leukocytes such as monocytes/macrophages and T lymphocytes.8,11,22-25 Altogether, inflammatory conditions strongly contribute to a microenvironment favoring tumor growth and neovascularization.
In addition to cytokines and growth factors, inflammatory leukocytes also release a number of proteases, including matrix metalloproteinases (MMPs).4,7,11,26-28 MMPs are critical for remodeling of the tissue extracellular matrix (ECM) and are involved in many steps of tumor progression, including tumor angiogenesis.29,30 Recent evidence points to the critical and specific functions of MMPs derived from activated stromal cells and inflammatory leukocytes at sites of tumor development.7,8,11,28,31-36 Although there is no strict specificity, distinct subsets of tumor-associated leukocytes are often linked to production of characteristic MMPs.28 In this regard, infiltrating neutrophils constitute a particular source of MMP-9 (gelatinase B) because it is presynthesized and stored in specialized granules and thus is ready to be released rapidly at the site of inflammation.26,37-42 Other types of inflammatory leukocytes such as monocytes/ macrophages and mast cells produce a number of MMPs, including MMP-9, which functionally contribute to angiogenesis triggered by inflammation25,43,44 and tumor growth.4,7,34,35,45
To investigate the role of specific inflammatory MMP-carrying leukocytes in growth factor– and tumor-induced angiogenesis, we employed the chick embryo system involving collagen onplants placed on the top of the chorioallantoic membrane (CAM).46,47 In this model, preexisting blood vessels of the underlying CAM are induced by exogenously added growth factors or tumor cells to sprout and grow upward into a 3-dimensional collagen graft. These new blood vessels anastomose, generating a complete blood-circulating angiogenic network. Using the CAM/collagen onplant model, we have previously shown that the onset of angiogenesis is critically dependent on a stromal collagenase, chicken MMP-13 (chMMP-13), imported by hematopoietic cells of monocyte/macrophage lineage.48
In this study we analyzed the role of host heterophils (the avian analog of mammalian neutrophils49 ) in angiogenesis induced by purified angiogenic growth factors (bFGF and VEGF) or, alternatively, by human tumor cells (HT-1080 fibrosarcoma) incorporated into the collagen onplant. Detailed morphologic and immunohistochemical examination indicated that heterophils rapidly enter the collagen/CAM tissue and that levels of angiogenesis in the onplants correlated positively with the heterophil influx. These inflammatory granulocytes were the main, if not the only, cell type positive for chicken MMP-9 in the embryo, indicating that chMMP-9 can be used as a marker for heterophils and suggesting that heterophils import chMMP-9 protein to the sites of angiogenesis. Influx of heterophils into collagen onplants was followed by appearance of chMMP-13–positive monocyte/macrophages. The disruption of inflammatory cell influx by cortisone and the nonsteroidal inhibitor ibuprofen, 2 mechanistically distinct anti-inflammatory drugs,15,20,50 resulted in significant inhibition of tumor-induced angiogenesis, indicating that the inflammatory leukocytes are required to sustain new blood vessel formation. A direct addition of isolated heterophils or purified chMMP-9 into onplants placed atop the CAM of ibuprofen-treated embryos completely reversed the anti-inflammatory effects of the drug and rescued angiogenesis, highlighting the importance of the influx of cytokine- and MMP-bearing inflammatory cells during growth factor– and tumor-induced angiogenesis.
Materials and methods
CAM angiogenesis assay
CAM angiogenesis assays were performed using shell-free 10-day-old chick embryos as described.46,48 Fertilized avian-leukosis–negative White Leghorn chicken eggs (Charles River Labs, North Franklin, CT) were incubated in a rotating incubator at 38°C and 85% humidity. At day 3, the contents of the eggs were transferred to sterile plastic weigh boats, covered with square Petri dishes, and kept in a stationary incubator at 38°C with 85% humidity.
Collagen onplants were prepared as described.46,48 Briefly, 8 volumes of 3.0 mg/mL type I rat tail collagen (BD Biosciences, Franklin Lakes, NJ) were neutralized with 1 volume of 10 × phosphate-buffered saline (PBS) and 1 volume of 0.15 M NaOH/0.25 M HEPES buffer (pH 7.4). Two volumes of neutralized collagen were combined with one volume of PBS containing 1 mg/mL bovine serum albumin (BSA) (control onplants). To induce angiogenesis, angiogenic growth factors bFGF and VEGF were added at a final concentration of 16.7 μg/mL and 5 μg/mL, respectively (PeproTech, Rocky Hill, NJ). Alternatively, HT-1080 fibrosarcoma cells (ATCC, Rockville, MD) were used to induce angiogenesis (5 × 104 cells per onplant). In the tumor-cell–induced angiogenesis assays, neutralized collagen was prepared with 10 × Dulbecco modified Eagle medium (DMEM), and 2 volumes of neutralized collagen were combined with 1 volume of HT-1080 cell suspension in DMEM with 1 mg/mL BSA.
To assemble onplants, 30 μL of the final collagen mixture was placed atop 2 layers of nylon mesh (Tetko, Kansas City, MO) and allowed to polymerize at 37.5°C for 90 minutes. Solidified onplants were grafted on top of the CAM of day-10 chick embryos incubated ex ovo. Where indicated, the onplant-bearing animals were treated systemically with cortisone (0.5 mg/embryo; Sigma, St Louis, MO) or ibuprofen (0.75 mg/embryo; Cayman Chemical, Ann Arbor, MI). Both anti-inflammatory drugs were prepared in DMEM supplemented with 1% methylcellulose and injected at 0.1 mL per embryo under the CAM twice: immediately prior to placing the onplants on day 10 and a second time on day 12. Hydroxamate MMP inhibitor GM6001 (Calbiochem, La Jolla, CA) was either added directly over the onplants (5 μLof 25 μM solution per onplant) or injected under the CAM for systemic treatment (10 μL of 1.25 mM solution) at the time of grafting and 48 hours later. Recombinant chicken tissue inhibitor of metalloproteinase-2 (TIMP-2)51 was incorporated into the collagen onplant mixture to a final concentration of 2.85 μM. chMMP-9 was purified from serum-free conditioned medium from HD11 mononuclear cells as described52 and added at 100 ng per onplant. Angiogenesis was scored at 66 to 72 hours using a stereomicroscope (Olympus, Melville, NY), as described.46,48 Blood vessels, visualized within the grids of the top mesh (ie, above the lower mesh) were regarded as angiogenic. Data are presented as percentage of angiogenic grids (number of grids containing blood vessels over the total number of grids scored).
Isolation of heterophils from peripheral blood
Heterophils were isolated from the peripheral blood of 17-day-old chick embryos essentially as described.49 Blood was collected from the allantoic vein into 50 mM EDTA in PBS, layered over a discontinuous 1.077/1.119 Ficoll-Hypaque gradient, and centrifuged at 250g for 45 minutes at 22°C. Erythrocytes were collected from the cell pellet and resuspended in PBS supplemented with 1.0% chicken serum (PBS/CS). Heterophils were collected from the 1.077/1.119 interface and the 1.119 band, placed into a siliconized tube, and washed with PBS/CS. Heterophils comprised 85% to 95% of the total leukocytes in the isolated preparations. Following centrifugation at 250g for 7 minutes, erythrocytes and heterophils were resuspended at 2 × 107 cells/mL in PBS/20 mM HEPES and kept on ice until use.
Immunofluorescent staining and immunohistochemistry
Collagen onplants with the underlying CAM and samples of normal CAM from the embryos containing no onplants were snap-frozen on dry ice in the optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) or fixed with Zn-formalin and embedded in paraffin.
For immunofluorescent analyses, 20-μm cryosections were placed on poly-L-lysine–coated slides, fixed in 4% paraformaldehyde, and blocked in PBS with 10% normal goat serum (NGS) (NGS/PBS). Staining was performed using 0.3 μg/mL rabbit anti–chMMP-9 antibody52 or mouse monoclonal antibody (mAb) CVI-ChNL-68.1 (ID-DLO; Lelystad, The Netherlands) against chicken macrophages (1:10 000). After washing, sections were incubated with the secondary Alexa546-conjugated goat anti–mouse IgG or goat anti–rabbit IgG (1:1000 in NGS/PBS; Molecular Probes, Eugene, OR). Detection of the mouse mAb was performed using the Renaissance Tyramide Signal Amplification Kit (NEN, Boston, MA). The slides were incubated with 2 mg/mL RNAse (Labscientific, Livingstone, NJ) for 2 hours and then mounted in ProLong antifade reagent containing the green nucleic acid dye YO-PRO-1 iodide (1:400). Images were captured using a Zeiss Axioskop (Carl Zeiss, Thornwood, NY) microscope equipped with a 20×/0.75 objective lens, a 64×/1.4 oil-immersion objective lens, and a cooled charge-coupled device (CCD) Camera (Diagnostic Instruments, Santa Barbara, CA). Images were processed using Adobe Photoshop 6.0 software (San Jose, CA).
For immunohistochemical analysis of chMMP-9 expression, cryosections were fixed with cold methanol. Detection of chMMP-13 was performed on paraffin-embedded sections treated with 10 mM citrate buffer for antigen retrieval. Endogenous peroxidase was blocked with 0.3% hydrogen peroxidase. Nonspecific binding was blocked with PBS/2% BSA/5% NGS. Slides were incubated overnight at 4°C with 2 μg/mL rabbit anti–chMMP-9 or anti–chMMP-13 antibodies48 or murine mAb 29-7 reacting with a yet unidentified surface antigen of human cells. After washing, the slides were incubated for 1 hour with secondary biotinylated goat anti–rabbit or anti–mouse IgG (1:1000), followed by incubation with HRP-NeutrAvidin conjugate for 30 minutes (Pierce, Rockford, IL) and then with a DAB chromogenic substrate. Sections were counterstained with Mayer hematoxylin. Digital images were captured under low (4×/0.1 and 10×/0.3) and higher (20×/0.5 and 40×/0.75) magnifications using the Olympus BX60 microscope equipped with a digital video camera. The quantitation of chMMP-9–positive heterophils or chMMP-13–positive monocytes in individual onplants or normal CAM was performed on × 20 images overlaid with a 9 × 7 square grid. Positively stained cells were scored in the grids overlapped with tissue. Data are presented as the number of chMMP-9–positive heterophils or chMMP-13–positive monocytes per grid (mean ± SEM).
Protein extraction from the onplant tissue
Samples of normal CAM and collagen onplants were harvested at the indicated time points. A total of 4 to 6 onplants from 3 to 5 embryos were combined, frozen on dry ice, and kept at -80°C. The samples were thawed on ice, cut into pieces, and homogenized at 4°C in 0.6 mL extract buffer (0.1 M Tris; 1.0% SDS; 10 mM EDTA; 10 μg/mL each of aprotinin, pepstatin, and leupeptin; 1 mM PMSFT; pH 8.0). Extracts were cleared by centrifugation at 16 000g for 20 minutes at 4°C. Protein content was determined using the protein assay kit (BCA; Pierce).
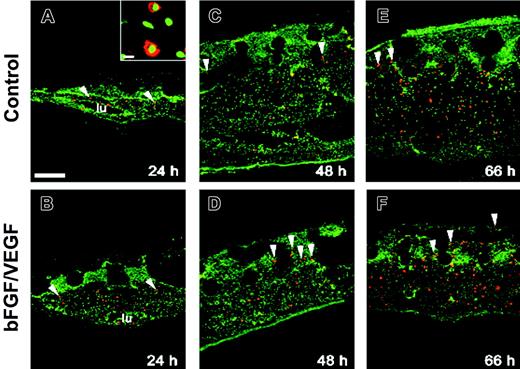
Angiogenesis in the collagen CAM onplants is accompanied by infiltration of chMMP-9–positive heterophils and accumulation of chMMP-9 protein. Collagen onplants were supplemented with buffer alone (control onplants: panels A, C, E) or angiogenic growth factors (bFGF/VEGF: panels B, D, F, H). Collagen onplants with the underlying CAM were harvested at 24 hours (A-B), 48 hours (C-D), or 66 hours (E-F, H), embedded in OCT compound, and frozen. Cryosections were immunostained for chMMP-9 with an affinity-purified rabbit polyclonal antibody (red). Tissue sections were counterstained with YO-PRO-1 iodide resulting in green-stained cell nuclei. The blank circular areas represent areas previously occupied by the 2-tiered nylon meshes, which are frequently displaced during tissue processing. At low magnification, scattered chMMP-9 staining appears to be associated with individual cells or small clusters of cells (arrowheads). The insets in panels A and B illustrate at higher magnification heterophils with distinctively shaped nuclei (green) and cytoplasmic chMMP-9–positive granules (red). * indicates specific staining of the extracellular fibrils for chMMP-9 in panel F. lu denotes the lumens of large blood vessels in panels B, C, and E; #, the bright ribbonlike staining at the upper border of the collagen-air interface in panel A. In panel H, a 66-hour growth factor–containing onplant is chMMP-9 negative after the chMMP-9 antibody was immunodepleted by preincubation with purified chMMP-9, confirming the specificity of the staining in the other panels. Bar represents 200 μm. The scatter graph (G) illustrates the levels of angiogenesis in control and growth factor–containing (bFGF/VEGF) onplants scored at 66 hours and presented as a fraction of grids with newly developed blood vessels (mean ± SEM). *P < .001. (I) Expression of chMMP-9 protein in the collagen CAM onplants. At 3 hours (lanes 2-3), 17 hours (lanes 4-5), 26 hours (lanes 6-7), 50 hours (lanes 8-9), and 66 hours (lanes 10-11) the collagen onplants with (+) or without (-) angiogenic growth factors (bFGF/VEGF) were excised from the CAM, pooled, extracted, and analyzed by SDS-PAGE and Western blotting with chMMP-9 antibody. As a positive control, the chMMP-9 proenzyme produced by PMA-treated chicken monocytic cells (HD11) was run in lane 1 (2 μL of cell lysate). The position of 75-kDa chMMP-9 is indicated on the right.
Angiogenesis in the collagen CAM onplants is accompanied by infiltration of chMMP-9–positive heterophils and accumulation of chMMP-9 protein. Collagen onplants were supplemented with buffer alone (control onplants: panels A, C, E) or angiogenic growth factors (bFGF/VEGF: panels B, D, F, H). Collagen onplants with the underlying CAM were harvested at 24 hours (A-B), 48 hours (C-D), or 66 hours (E-F, H), embedded in OCT compound, and frozen. Cryosections were immunostained for chMMP-9 with an affinity-purified rabbit polyclonal antibody (red). Tissue sections were counterstained with YO-PRO-1 iodide resulting in green-stained cell nuclei. The blank circular areas represent areas previously occupied by the 2-tiered nylon meshes, which are frequently displaced during tissue processing. At low magnification, scattered chMMP-9 staining appears to be associated with individual cells or small clusters of cells (arrowheads). The insets in panels A and B illustrate at higher magnification heterophils with distinctively shaped nuclei (green) and cytoplasmic chMMP-9–positive granules (red). * indicates specific staining of the extracellular fibrils for chMMP-9 in panel F. lu denotes the lumens of large blood vessels in panels B, C, and E; #, the bright ribbonlike staining at the upper border of the collagen-air interface in panel A. In panel H, a 66-hour growth factor–containing onplant is chMMP-9 negative after the chMMP-9 antibody was immunodepleted by preincubation with purified chMMP-9, confirming the specificity of the staining in the other panels. Bar represents 200 μm. The scatter graph (G) illustrates the levels of angiogenesis in control and growth factor–containing (bFGF/VEGF) onplants scored at 66 hours and presented as a fraction of grids with newly developed blood vessels (mean ± SEM). *P < .001. (I) Expression of chMMP-9 protein in the collagen CAM onplants. At 3 hours (lanes 2-3), 17 hours (lanes 4-5), 26 hours (lanes 6-7), 50 hours (lanes 8-9), and 66 hours (lanes 10-11) the collagen onplants with (+) or without (-) angiogenic growth factors (bFGF/VEGF) were excised from the CAM, pooled, extracted, and analyzed by SDS-PAGE and Western blotting with chMMP-9 antibody. As a positive control, the chMMP-9 proenzyme produced by PMA-treated chicken monocytic cells (HD11) was run in lane 1 (2 μL of cell lysate). The position of 75-kDa chMMP-9 is indicated on the right.
Western blotting
Twenty-five micrograms of extracted protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4% to 20% or 8% gels. Resolved proteins were transferred to the nitrocellulose or PVDF membranes. After blocking with 5% nonfat dry milk, the membranes were incubated overnight at 4°C with 1 μg/mL anti–chMMP-9 antibody, washed and incubated for 1 hour at room temperature with HRP-conjugated goat or donkey anti–rabbit IgG, and developed with Super Signal West Pico Chemiluminescent Substrate (Pierce).
Zymography
A total of 5 μg of extracted protein was mixed with 10 × SDS sample buffer and ran on 8% SDS-polyacrylamide substrate gels containing 0.8% gelatin. Following PAGE, gels were washed twice in 2.5% Triton X-100 for 30 minutes at room temperature and incubated overnight at 37°C in 50 mM Tris, pH 7.4; 150 mM NaCl; 5 mM CaCl2. Gels were stained with Coomassie Blue R-250. Bands of gelatinolytic activity correspond to the areas devoid of blue staining.
Statistical analysis
The statistical differences between data sets were analyzed by Student t test using the Prizm program (GraphPad Software, San Diego, CA). The data are presented as scattered plots or bar graphs with the mean plus or minus SEM. Data were considered statistically significant when P was less than .05.
Results
Influx of chMMP-9–positive heterophils into the CAM during angiogenesis
In preliminary immunohistologic analysis of leukocyte-enriched fractions from peripheral blood of chick embryos, heterophils were identified as the only cells positively stained with a chMMP-9–specific antibody. In normal quiescent CAM tissue, heterophils were rarely found extravascularly and no chMMP-9 protein was associated with noncellular components (data not shown). However, a rapid influx of chMMP-9–positive heterophils was induced in the collagen CAM onplants (Figure 1). Twenty-four hours after grafting both control and growth factor–containing onplants, chMMP-9–positive cells were readily identified in the underlying CAM and in the tissue overlaying the collagen onplant (ie, 24-48 hours before earliest appearance of new blood vessels; Figure 1A-B). Heterophil morphology of chMMP-9–positive cells was confirmed at higher magnification by their lobulated nuclei and granular chMMP-9–specific staining in the cytoplasm (Figure 1A-B insets). The levels of heterophil infiltration were higher in the onplants supplemented with angiogenic growth factors, where heterophils were frequently found around blood vessels (Figure 1B arrows). By 48 hours, more chMMP-9–positive heterophils were identified in the upper regions of the CAM, suggesting further infiltration of the collagen onplant tissue (Figure 1C-D). Levels of chMMP-9 staining did not appear to change substantially between 48 and 66 hours (Figure 1E-F).
In addition to distinct cellular staining, chMMP-9 was also associated with ECM fibrils, especially in the growth factor–containing onplants (Figure 1F asterisks). Noncellular chMMP-9 staining was particularly strong at the air/collagen interface visualized as ribbonlike structures (Figure 1A #). Both the cellular and ECM immunostaining were completely abrogated by preabsorbing the chMMP-9 antibody with purified chMMP-9 protein (Figure 1H), indicating that both types of staining were specifically associated with chMMP-9 protein. Angiogenesis, scored at 66 hours, confirmed that the levels of angiogenesis were 4- to 5-fold higher in growth factor–supplemented collagen onplants (Figure 1G). Thus, heterophil infiltration preceded and paralleled the development of a new vascular network, suggesting a functional role of infiltrating chMMP-9–positive heterophils in CAM angiogenesis.
Cells of monocyte/macrophage lineage infiltrate the collagen CAM onplants. Collagen on-plants supplemented with buffer alone (A,C,E) or bFGF/VEGF (B,D,F) were harvested with the underlying CAM at 24 hours (A-B), 48 hours (C-D), and 66 hours (E-F), embedded in OCT compound, and frozen. Cryosections were immunostained with a chicken monocyte/macrophage-specific monoclonal antibody (red). The sections were counterstained with YO-PRO-1 iodide, resulting in green-stained cell nuclei. (A-B) At 24 hours, positively stained cells (arrowheads) were present only in the underlying CAM. (C-F) By 48 to 66 hours, positively stained monocytes/macrophages appeared also in the upper areas of onplants as isolated cells or clusters of cells. Bar represents 200 μm. At a higher magnification (A inset; bar represents 20 μm), the stained cells display macrophage-like morphologic characteristics (rounded or bean-shaped nuclei and a relatively high cytoplasm-to-nucleus ratio). lu denotes the lumens of large vessels. Large, unstained, circular areas in the upper portions of the sections represent the sites where the grids of nylon meshes were displaced during the processing of sections.
Cells of monocyte/macrophage lineage infiltrate the collagen CAM onplants. Collagen on-plants supplemented with buffer alone (A,C,E) or bFGF/VEGF (B,D,F) were harvested with the underlying CAM at 24 hours (A-B), 48 hours (C-D), and 66 hours (E-F), embedded in OCT compound, and frozen. Cryosections were immunostained with a chicken monocyte/macrophage-specific monoclonal antibody (red). The sections were counterstained with YO-PRO-1 iodide, resulting in green-stained cell nuclei. (A-B) At 24 hours, positively stained cells (arrowheads) were present only in the underlying CAM. (C-F) By 48 to 66 hours, positively stained monocytes/macrophages appeared also in the upper areas of onplants as isolated cells or clusters of cells. Bar represents 200 μm. At a higher magnification (A inset; bar represents 20 μm), the stained cells display macrophage-like morphologic characteristics (rounded or bean-shaped nuclei and a relatively high cytoplasm-to-nucleus ratio). lu denotes the lumens of large vessels. Large, unstained, circular areas in the upper portions of the sections represent the sites where the grids of nylon meshes were displaced during the processing of sections.
The kinetics of chMMP-9 protein expression in collagen CAM onplants during angiogenesis was examined by Western blotting (Figure 1I). chMMP-9 protein was essentially undetectable during the first day after placing the onplants onto the CAM. By 26 hours, chMMP-9 proenzyme was readily identified in both types of onplants but was 2- to 3-fold higher in growth factor–containing onplants compared with controls as judged by densitometry analysis of blots. This differential was also observed at 50 hours. Incubation up to 66 hours, when angiogenesis was scored, did not cause any substantial changes in chMMP-9 levels and the zymogen form continued to be the major species detected. These changes in chMMP-9 protein levels were consistent with and corresponded to the kinetics of heterophil influx, suggesting that heterophils constitute a major cellular source of chMMP-9 in the collagen CAM onplants.
Kinetics of monocyte/macrophage infiltration
Cells of monocyte/macrophage lineage represent another major inflammatory cell type associated with the angiogenic onplant tissue.46,48 Therefore, we analyzed whether monocyte/macrophage influx overlapped or coincided with the heterophil population during infiltration of collagen CAM onplants. Immunostaining with an antibody specific for chicken monocytes/macrophages showed that at 24 hours, fewer monocytes/macrophages were present within the onplant tissue compared with heterophils and were identified only in the underlying CAM (Figure 2A-B). At 48 hours, some monocytes/macrophages could be detected around the grids of the lower mesh (ie, at the collagen/CAM interface; Figure 2C-D arrowheads), but in general their numbers remained quite sparse. In both control and growth factor–supplemented onplants, the number of monocytes/macrophages significantly increased by 66 hours (Figure 2E-F). This was in contrast to the influx kinetics of heterophils, which after rapid influx leveled off between 48 and 66 hours. Thus, monocytes/macrophages and heterophils represent 2 distinct inflammatory leukocyte populations infiltrating the collagen CAM tissue with different kinetics during angiogenesis.
Quantitative analysis of heterophil influx
Heterophil infiltration was quantitatively assessed on cryosections of CAM collagen onplants immunohistochemically stained with anti–chMMP-9 as our newly established heterophil marker. Multiple digital images were captured at × 4 to × 20 magnification and then analyzed quantitatively by scoring for the frequency and density of chMMP-9–positive heterophils (Figure 3A). The examination of these cells at × 40 magnification confirmed that all the scored cells were indeed heterophils, characterized by multilobed nuclei (counterstained to appear blue) and granular cytoplasm containing the brownish-stained antigen (Figure 3B).
Kinetics of heterophil influx into collagen CAM onplants. Samples of normal CAM from embryos without onplants (NCAM) and collagen onplants supplemented with angiogenic growth factors (bFGF/VEGF) or buffer alone (control) were excised with the underlying CAM, embedded in OCT compound, and frozen. Cryosections were immunohistochemically stained with chMMP-9 antibody and counterstained with Mayer hematoxylin. Digital images were collected at × 10, × 20, and × 40 magnifications and analyzed. (A) Representative sections of control (left panel) and growth factor–supplemented (right panel) collagen onplants harvested at 72 hours (original magnification × 10). Arrows point to dark brown–stained cells and cell clusters confirmed to be chMMP-9–positive heterophils. (B) At an original magnification of × 40, morphologic features of chMMP-9–positive cells are consistent with the characteristics of heterophils (ie, multilobed nuclei [bluish] and granular cytoplasm [brownish]). (C) Kinetics of heterophil influx into collagen CAM onplants was determined over the 72-hour time course. The original × 20 magnification images of normal CAM (NCAM), control, and growth factor–containing (bFGF/VFGF) onplants were overlaid with a 9 × 7 square grid and analyzed (data from 9 to 39 images per time point from 2 independent experiments). chMMP-9–positive heterophils were counted in the squares occupied with tissue. Data are presented as the mean ± SEM from the numbers of chMMP-9–positive cells per square. *P < .001, determined in the 2-tailed Student t test.
Kinetics of heterophil influx into collagen CAM onplants. Samples of normal CAM from embryos without onplants (NCAM) and collagen onplants supplemented with angiogenic growth factors (bFGF/VEGF) or buffer alone (control) were excised with the underlying CAM, embedded in OCT compound, and frozen. Cryosections were immunohistochemically stained with chMMP-9 antibody and counterstained with Mayer hematoxylin. Digital images were collected at × 10, × 20, and × 40 magnifications and analyzed. (A) Representative sections of control (left panel) and growth factor–supplemented (right panel) collagen onplants harvested at 72 hours (original magnification × 10). Arrows point to dark brown–stained cells and cell clusters confirmed to be chMMP-9–positive heterophils. (B) At an original magnification of × 40, morphologic features of chMMP-9–positive cells are consistent with the characteristics of heterophils (ie, multilobed nuclei [bluish] and granular cytoplasm [brownish]). (C) Kinetics of heterophil influx into collagen CAM onplants was determined over the 72-hour time course. The original × 20 magnification images of normal CAM (NCAM), control, and growth factor–containing (bFGF/VFGF) onplants were overlaid with a 9 × 7 square grid and analyzed (data from 9 to 39 images per time point from 2 independent experiments). chMMP-9–positive heterophils were counted in the squares occupied with tissue. Data are presented as the mean ± SEM from the numbers of chMMP-9–positive cells per square. *P < .001, determined in the 2-tailed Student t test.
During the entire 72-hour incubation, chMMP-9–positive heterophils were absent or rare in normal CAM from embryos containing no onplants (Figure 3C). In contrast, heterophils were identified in the underlying CAM of control onplants at 14 hours and steadily increased until 48 hours and then slightly declined at 72 hours. Remarkably, in the presence of angiogenic growth factors, chMMP-9–positive heterophils were first identified as early as 2 hours after placing onplants on the CAM (ie, when no heterophils were observed in the control onplants). Within the next 12 hours, the number of heterophils in growth factor–containing onplants increased so rapidly that their frequency exceeded 3-fold that observed in control onplants.
Tumor-induced angiogenesis in collagen CAM onplants
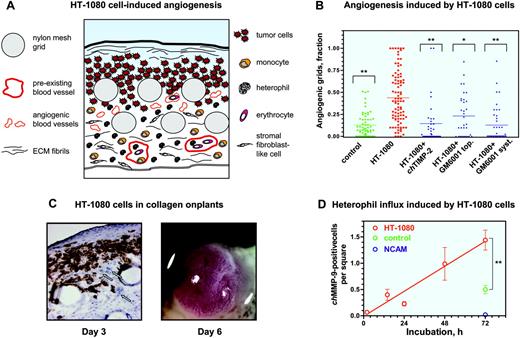
Our studies indicate that the varying levels of neovascularization induced by angiogenic growth factors correlated well with the levels of leukocyte influx, including heterophils and monocytes. We next verified whether such influx of inflammatory cells would be reproduced during angiogenesis induced by malignant tumor cells. Therefore, the purified growth factors were replaced in the collagen onplants with 5 × 104 human fibrosarcoma HT-1080 cells. The diagram in Figure 4A schematically represents the tumor angiogenic system introduced in this study and depicts the tissue and cellular components of a collagen CAM onplant after 3 days of angiogenic stimulation.
Levels of angiogenesis induced by HT-1080 cells were on average 4- to 5-fold higher than those induced in control collagen onplants (Figure 4B) and were comparable with the maximum levels of growth factor–induced angiogenesis (Figure 1G). Importantly, similar to growth factor–induced angiogenesis,46,48 angiogenesis induced by HT-1080 cells was sensitive to inhibitors of MMPs, using both natural (chicken TIMP-2) and synthetic (GM6001) inhibitor compounds delivered either topically or systemically (Figure 4B). The wide variation and range in angiogenic scoring of individual onplants depicted in the scatter graph (Figure 4B) is typical for measurements in highly complex in vivo systems. Nevertheless, the P values (< .005) clearly indicate significant difference between the experimental variables.
To visualize tumor cells within the complex vascular/stromal tissue of the collagen onplants, we stained cryosections with mAb 29-7, which reacts with a surface antigen of human cells (Figure 4C left panel). Based on the 29-7 antigen distribution, 3 days after placing collagen onplants on the CAM, HT-1080 cells spread beneath the air/collagen interface among the infiltrating stromal cells and between the grids of the nylon meshes (Figure 4C left panel, circles), whereas the underlying CAM was generally free of human tumor cells. Newly formed blood vessels induced by the tumor cells were present throughout the underlying CAM (Figure 4C left panel, arrows).
If the HT-1080 onplants were incubated on the CAM of chick embryos for 3 additional days after angiogenic scoring (ie, 6 days after onplant grafting), large primary tumors formed on the top of the onplant (Figure 4C right panel). These tumors were highly vascularized and yielded secondary micrometastatic foci in the liver and lungs (data not shown). Thus, incorporation of 5 × 104 HT-1080 cells into a collagen onplant resulted in a pronounced MMP-dependent angiogenic response in 3 days (Figure 4B) and the development of invasive, vascularized malignant tumors in 3 to 6 days (Figure 4C), demonstrating that the modified CAM/onplant assay indeed functions as a quantitative tumor angiogenesis model.
Angiogenesis and heterophil influx induced by human tumor cells in collagen CAM onplants. (A) Schematic presentation of tissue and cellular components of a collagen onplant containing HT-1080 cells 3 days after grafting on the CAM. Tumor cells could be visualized in the upper portions of the onplant, around and between 2 layers of grids from the nylon meshes. The grids are often displaced during tissue processing, leaving empty circles in the tissue sections. Large, preexisting blood vessels containing nucleated erythrocytes and leukocytes are located in the underlying CAM, which is bordered by the endoderm layer. Newly formed blood vessels, which are the ones scored in the angiogenic assay, are identified mostly between or directly below the grids and usually are close to tumor cells. Angiogenic vessels are often filled with blood cells, indicating the existence of an established, complete circulation. Stromal fibroblast-like cells of the CAM mesoderm are numerous and infiltrate the entire collagen onplant, including the very top portions of the onplant. Lower and middle portions of the onplant are infiltrated with inflammatory cells such as monocytes/macrophages and heterophils. These cells could be identified morphologically by immunohistochemical staining with chMMP-13– and chMMP-9–specific antibodies, respectively. The matrix components could be identified as a fine network of fibrils present throughout the entire onplant and a thick ribbonlike structure at the collagen/air interface on the top of onplant. (B) Collagen onplants supplemented with buffer alone (control) or 5 × 104 HT-1080 cells (HT-1080) were placed on the CAM and scored 72 hours later for levels of angiogenesis. Recombinant chicken TIMP-2 was incorporated into onplants at 2.85 μM. Hydroxamate MMP inhibitor GM6001 was added either topically (top; 5 μLof 25 μM solution) or systemically (syst; 10 μL of 1.25 mM solution) at the time of onplant grafting and 48 hours later. At 72 hours, the angiogenic response was determined as a fraction of grids containing newly formed blood vessels. Statistical significance between the groups of onplants was determined by comparison with nontreated HT-1080 cell–containing onplants. **P < .001; *P = .004. (C) At 72 hours (day 3), HT-1080 cell–containing onplants were harvested, embedded in OCT compound, and frozen. HT-1080 cells were identified in cryosections (left panel) after immunohistochemical staining with mAb 29-7 recognizing a human cell surface antigen (original magnification × 20). Human tumor cells (brown) are located at the top and in between the grids of onplants, whereas underlying CAM appears mainly devoid of tumor cells. Displaced nylon mesh grids appear as empty circular structures. Some newly formed blood vessels are indicated by arrows. By day 6 of incubation, highly vascularized HT-1080 tumors are generated on the top of the onplant grids (right panel). (D) Kinetics of heterophil influx into collagen CAM onplants supplemented with HT-1080 cells. The numbers of chMMP-9–positive heterophils were determined over a 72-hour time course in the × 20 images of tissue cryosections stained with the chMMP-9 antibody as described in Figure 3. For comparison, samples of 72-hour control onplants (control) and normal CAM (NCAM) were included in quantitation. Data are presented as the mean ± SEM of heterophil numbers per square scored in 7 to 14 images per time point. **P < .001.
Angiogenesis and heterophil influx induced by human tumor cells in collagen CAM onplants. (A) Schematic presentation of tissue and cellular components of a collagen onplant containing HT-1080 cells 3 days after grafting on the CAM. Tumor cells could be visualized in the upper portions of the onplant, around and between 2 layers of grids from the nylon meshes. The grids are often displaced during tissue processing, leaving empty circles in the tissue sections. Large, preexisting blood vessels containing nucleated erythrocytes and leukocytes are located in the underlying CAM, which is bordered by the endoderm layer. Newly formed blood vessels, which are the ones scored in the angiogenic assay, are identified mostly between or directly below the grids and usually are close to tumor cells. Angiogenic vessels are often filled with blood cells, indicating the existence of an established, complete circulation. Stromal fibroblast-like cells of the CAM mesoderm are numerous and infiltrate the entire collagen onplant, including the very top portions of the onplant. Lower and middle portions of the onplant are infiltrated with inflammatory cells such as monocytes/macrophages and heterophils. These cells could be identified morphologically by immunohistochemical staining with chMMP-13– and chMMP-9–specific antibodies, respectively. The matrix components could be identified as a fine network of fibrils present throughout the entire onplant and a thick ribbonlike structure at the collagen/air interface on the top of onplant. (B) Collagen onplants supplemented with buffer alone (control) or 5 × 104 HT-1080 cells (HT-1080) were placed on the CAM and scored 72 hours later for levels of angiogenesis. Recombinant chicken TIMP-2 was incorporated into onplants at 2.85 μM. Hydroxamate MMP inhibitor GM6001 was added either topically (top; 5 μLof 25 μM solution) or systemically (syst; 10 μL of 1.25 mM solution) at the time of onplant grafting and 48 hours later. At 72 hours, the angiogenic response was determined as a fraction of grids containing newly formed blood vessels. Statistical significance between the groups of onplants was determined by comparison with nontreated HT-1080 cell–containing onplants. **P < .001; *P = .004. (C) At 72 hours (day 3), HT-1080 cell–containing onplants were harvested, embedded in OCT compound, and frozen. HT-1080 cells were identified in cryosections (left panel) after immunohistochemical staining with mAb 29-7 recognizing a human cell surface antigen (original magnification × 20). Human tumor cells (brown) are located at the top and in between the grids of onplants, whereas underlying CAM appears mainly devoid of tumor cells. Displaced nylon mesh grids appear as empty circular structures. Some newly formed blood vessels are indicated by arrows. By day 6 of incubation, highly vascularized HT-1080 tumors are generated on the top of the onplant grids (right panel). (D) Kinetics of heterophil influx into collagen CAM onplants supplemented with HT-1080 cells. The numbers of chMMP-9–positive heterophils were determined over a 72-hour time course in the × 20 images of tissue cryosections stained with the chMMP-9 antibody as described in Figure 3. For comparison, samples of 72-hour control onplants (control) and normal CAM (NCAM) were included in quantitation. Data are presented as the mean ± SEM of heterophil numbers per square scored in 7 to 14 images per time point. **P < .001.
Influx of chMMP-9–positive heterophils during tumor-induced angiogenesis
We next verified whether tumor-induced angiogenesis in CAM collagen onplants would be accompanied by infiltration of specific leukocytes. Since heterophils were the first to influx CAM collagen onplants, kinetics of chMMP-9–positive cells in HT-1080 onplants was analyzed and quantified between 2 and 72 hours (Figure 4D). In contrast to the growth factor–supplemented onplants, chMMP-9–positive heterophils were very rare in HT-1080 cell–containing onplants 2 hours following grafting. However, heterophils were readily identified at 12 hours, indicating that a few hours were required to accumulate effective levels of angiogenic factors and heterophil attractants within the HT-1080 onplants. Nevertheless, a significant increase in heterophils occurred in the onplants containing HT-1080 cells prior to and during the neovascular growth period (Figure 4D). In control onplants, the frequency of chMMP-9–positive heterophils at 72 hours was significantly lower than that of HT-1080 onplants (Figure 4D), which closely correlated with the different levels of angiogenesis achieved at this time point (Figure 4B).
Depletion of heterophil influx during tumor cell–induced angiogenesis by cortisone
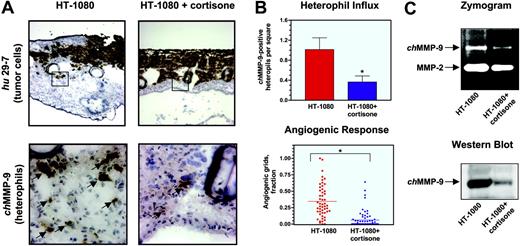
To analyze whether angiogenesis induced by tumor cells depended on the influx of inflammatory leukocytes, infiltration of leukocytes into HT-1080 onplants was prevented by treatment with the anti-inflammatory drug cortisone. HT-1080 cells in cortisone-treated and nontreated onplants were visualized with the human-specific mAb 29-7. Cortisone treatment did not appear to affect the growth and spread of tumor cells in the onplants although it caused a generalized thinning of the CAM tissue (Figure 5A top panels). However, immunohistochemical staining of cortisone-treated and nontreated onplants with the chMMP-9–specific antibody (Figure 5A bottom panels) and quantitation of the resulting images (Figure 5B top panel) confirmed that the frequency of chMMP-9–positive heterophils in cortisone-treated onplants was decreased approximately 3-fold compared with nontreated controls.
Western blot and zymography analyses confirmed that chMMP-9 content was correspondingly lower in collagen onplants treated with cortisone as determined by the diminished lysis zone typical for the chMMP-9 region in a zymograph and, more specifically, by the near absence of a chMMP-9–specific band in the Western blot (Figure 5C top and bottom panels, respectively). Densitometry of the zymograph and Western blot images demonstrated a corresponding 60.2% ± 7.9% and 81.6% ± 3.9% decrease in chMMP-9 protein in cortisone-treated onplants. In agreement with the suggestion that the presence of tumor-associated leukocytes is critical for proper development of the angiogenic network, cortisone caused a severe suppression of angiogenesis in collagen onplants containing HT-1080 cells (Figure 5B bottom panel). Levels of angiogenesis were diminished by 80% in cortisone-treated animals, establishing a strong correlation between inflammatory cell influx and neovascularization.
Modulation of inflammatory cell influx by a COX inhibitor, ibuprofen
To extend and corroborate our findings with cortisone-treated onplants, we used a nonsteroidal anti-inflammatory agent, ibuprofen, a potent pan-COX inhibitor. HT-1080 onplants from control embryos and ibuprofen-treated embryos were analyzed for levels of angiogenesis and degree of infiltration by chMMP-9–positive heterophils and chMMP-13–positive monocytes (Figure 6). At a dose of 0.75 mg/embryo, which calculates to approximately 50 μM, ibuprofen caused a 50% to 70% reduction of angiogenesis. The gross morphology of onplants and the distribution of HT-1080 cells within the onplants from ibuprofen-treated embryos appeared similar to that of untreated animals (Figure 6A top panels). This finding indicated that the antiangiogenic effect of ibuprofen was not associated with a direct inhibition of HT-1080 cell proliferation. This indication was confirmed by the similar size of tumors developed on top of the onplants 6 to 7 days after grafting on the CAM (data not shown). In further agreement, ibuprofen did not cause significant effects on HT-1080 cell proliferation in vitro at a dose range of 10 to 100 μM (data not shown). Moreover, when growth factor–supplemented onplants were placed on the CAM of ibuprofen-treated embryos, levels of angiogenesis were diminished almost to control levels (data not shown), further demonstrating the effects of ibuprofen on host tissues.
Inhibition of heterophil influx and angiogenesis in HT-1080 onplants by cortisone. Onplants containing HT-1080 cells (5 × 104 cells per onplant) were placed on the top of the CAM of 10-day-old chick embryos, nontreated or injected with cortisone (0.5 mg/embryo). (A) At 72 hours, nontreated (left panels) and cortisone-treated (right panels) HT-1080 onplants with the underlying CAM were harvested, embedded in OCT compound, and frozen. Cryosections were immunohistochemically stained with mAb 29-7 (top panels) or anti–chMMP-9 antibody (bottom panels). As judged by the 29-7 staining (original magnification × 4), expanding HT-1080 cells (brown) are localized at the top of the onplants and in between grids. Boxed areas are presented at higher magnification (original magnification × 40) of the adjacent sections stained with the chMMP-9–specific antibody. The chMMP-9–positive staining is associated with single heterophils and heterophil clusters (arrows). (B) Cortisone reduces the heterophil influx (top graph) and angiogenic response (bottom graph) in the collagen onplants containing HT-1080 cells. Cryosections of nontreated and cortisone-treated collagen HT-1080 onplants harvested at 72 hours were stained with the chMMP-9 antibody. The chMMP-9–positive heterophils were scored in × 20 images. Data are presented as the mean ± SEM of chMMP-9–positive heterophil numbers per square. Levels of angiogenesis were scored at 66 hours and presented as percentage of grids with newly formed blood vessels. *P < .001. (C) Cortisone diminishes chMMP-9 protein levels in collagen CAM onplants containing HT-1080 cells. Nontreated (HT-1080) and cortisone-treated (HT-1080+cortisone) onplants were harvested at 72 hours and snap-frozen on dry ice. Extracted proteins were separated by SDS-PAGE on gelatin-containing 8% polyacrylamide gels for zymography analysis (5 μg/lane; top panel) or separated by 8% SDS-PAGE, transferred to a membrane support, and immunoblotted with anti–chMMP-9 antibody (25 μg/lane; bottom panel).
Inhibition of heterophil influx and angiogenesis in HT-1080 onplants by cortisone. Onplants containing HT-1080 cells (5 × 104 cells per onplant) were placed on the top of the CAM of 10-day-old chick embryos, nontreated or injected with cortisone (0.5 mg/embryo). (A) At 72 hours, nontreated (left panels) and cortisone-treated (right panels) HT-1080 onplants with the underlying CAM were harvested, embedded in OCT compound, and frozen. Cryosections were immunohistochemically stained with mAb 29-7 (top panels) or anti–chMMP-9 antibody (bottom panels). As judged by the 29-7 staining (original magnification × 4), expanding HT-1080 cells (brown) are localized at the top of the onplants and in between grids. Boxed areas are presented at higher magnification (original magnification × 40) of the adjacent sections stained with the chMMP-9–specific antibody. The chMMP-9–positive staining is associated with single heterophils and heterophil clusters (arrows). (B) Cortisone reduces the heterophil influx (top graph) and angiogenic response (bottom graph) in the collagen onplants containing HT-1080 cells. Cryosections of nontreated and cortisone-treated collagen HT-1080 onplants harvested at 72 hours were stained with the chMMP-9 antibody. The chMMP-9–positive heterophils were scored in × 20 images. Data are presented as the mean ± SEM of chMMP-9–positive heterophil numbers per square. Levels of angiogenesis were scored at 66 hours and presented as percentage of grids with newly formed blood vessels. *P < .001. (C) Cortisone diminishes chMMP-9 protein levels in collagen CAM onplants containing HT-1080 cells. Nontreated (HT-1080) and cortisone-treated (HT-1080+cortisone) onplants were harvested at 72 hours and snap-frozen on dry ice. Extracted proteins were separated by SDS-PAGE on gelatin-containing 8% polyacrylamide gels for zymography analysis (5 μg/lane; top panel) or separated by 8% SDS-PAGE, transferred to a membrane support, and immunoblotted with anti–chMMP-9 antibody (25 μg/lane; bottom panel).
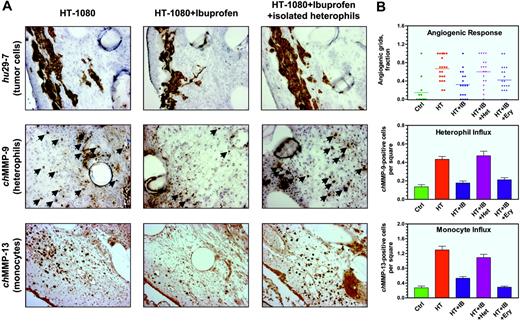
Immunohistochemical analysis of the HT-1080 onplant tissue from embryos treated with ibuprofen demonstrated a substantial reduction of infiltration by chMMP-9–positive heterophils and chMMP-13–positive monocytes/macrophages (Figure 6A middle and bottom panels, respectively). Quantification of digital images demonstrated 53.9% ± 9.4% inhibition of heterophil influx by ibuprofen in 4 independent experiments. Concomitantly, influx of chMMP-13–positive monocytes/macrophages was reduced by 55.2% ± 9.7% (n = 3). The diminished infiltration of both types of inflammatory leukocytes correlated well with overall inhibition (64.9% ± 6.8%, n = 9) of the angiogenic response in ibuprofen-treated embryos (Figure 6B).
Rescue of antiangiogenic effects of ibuprofen and cortisone
Since heterophils were the first leukocytes infiltrating the onplant tissue and their infiltration was prevented by anti-inflammatory agents, it is suggested that the inhibitory effects of cortisone and ibuprofen might be overcome by the incorporation of purified heterophils into the onplants. Incorporation of as few as 5 × 104 purified heterophils indeed completely reversed the anti-inflammatory effects of ibuprofen on HT-1080 cell–containing onplants, rescuing both the angiogenic response and the influx of endogenous inflammatory leukocytes (Figure 6B). In 5 independent experiments, levels of angiogenesis decreased by ibuprofen to 44.9% ± 8.0% of nontreated control were brought back to 96.4% ± 14.8% by exogenous heterophils. Angiogenic rescue was accompanied by a complete restoration of infiltration by chMMP-9–positive heterophils and chMMP-13–positive monocytes (100.2% ± 15.9%, n = 4; and 113.2% ± 26.4%, n = 3 of untreated levels, respectively; Figure 6A right panels and corresponding bar graphs in Figure 6B). Importantly, the density of heterophils, identified in the rescued onplant tissue 3 days after incubation on the CAM, exceeded approximately 10- to 20-fold the initial density of the exogenously added purified heterophils. This suggests an induction of endogenous heterophil influx, likely in an autocrine manner. In contrast, addition of 5 × 104 purified erythrocytes per onplant did not overcome the inhibitory effects of ibuprofen on angiogenesis and infiltration by inflammatory cells (Figure 6B), indicating that the addition of nucleated, but noninflammatory cells, cannot bring about the rescue. Similarly to ibuprofen-treated animals, the addition of purified heterophils to HT-1080 cell–containing onplants grafted in embryos treated with cortisone also restored angiogenesis and infiltration by chMMP-9–positive heterophils (data not shown).
Modulation of angiogenesis in HT-1080 onplants: inhibition of inflammatory cell influx by ibuprofen and its rescue by exogenous heterophils. (A) Collagen onplants containing 5 × 104 HT-1080 cells were incubated on the CAM of nontreated embryos (left panels) or embryos treated systemically with ibuprofen (middle and right panels). In addition, a subset of ibuprofen-treated embryos was engrafted with HT-1080 onplants containing 5 × 104 purified heterophils isolated from peripheral blood (right panels). At 72 hours, onplants were harvested and frozen in OCT compound or fixed in formalin. Cryosections were immunohistochemically stained with mAb 29-7 to visualize human cells (top panels) or chMMP-9–specific antibody to visualize heterophils (arrows, middle panels). Paraffin-embedded sections were immunohistochemically stained with chMMP-13–specific antibody to visualize monocytes/macrophages as brown rounded cells (bottom panels). (B) Angiogenic response and the heterophil and monocyte influxes were scored at 72 hours in control onplants (ctrl) and onplants containing 5 × 104 HT-1080 cells (HT), which were placed on nontreated embryos or embryos systemically treated with ibuprofen (IB). Isolated heterophils (Het) or erythrocytes (Ery) were added at a concentration of 5 × 104 cells per onplant to the 2 subsets of HT-1080 onplants grafted on the CAM of ibuprofen-treated embryos. Angiogenic response was determined as a fraction of onplant grids containing the newly formed blood vessels (top graph; bar indicates mean). Influx of heterophils was determined as a tissue density of chMMP-9–positive cells scored in × 20 tissue images (middle graph). Influx of monocytes was determined as a tissue density of chMMP-13–positive cells scored in × 20 tissue images (bottom graph). Data are presented as the mean ± SEM. Shown is a representative of 3 independent experiments. Statistical significance was confirmed (P < .05) for each variable in comparison with the previous experimental group as depicted in the scatter and bar graphs.
Modulation of angiogenesis in HT-1080 onplants: inhibition of inflammatory cell influx by ibuprofen and its rescue by exogenous heterophils. (A) Collagen onplants containing 5 × 104 HT-1080 cells were incubated on the CAM of nontreated embryos (left panels) or embryos treated systemically with ibuprofen (middle and right panels). In addition, a subset of ibuprofen-treated embryos was engrafted with HT-1080 onplants containing 5 × 104 purified heterophils isolated from peripheral blood (right panels). At 72 hours, onplants were harvested and frozen in OCT compound or fixed in formalin. Cryosections were immunohistochemically stained with mAb 29-7 to visualize human cells (top panels) or chMMP-9–specific antibody to visualize heterophils (arrows, middle panels). Paraffin-embedded sections were immunohistochemically stained with chMMP-13–specific antibody to visualize monocytes/macrophages as brown rounded cells (bottom panels). (B) Angiogenic response and the heterophil and monocyte influxes were scored at 72 hours in control onplants (ctrl) and onplants containing 5 × 104 HT-1080 cells (HT), which were placed on nontreated embryos or embryos systemically treated with ibuprofen (IB). Isolated heterophils (Het) or erythrocytes (Ery) were added at a concentration of 5 × 104 cells per onplant to the 2 subsets of HT-1080 onplants grafted on the CAM of ibuprofen-treated embryos. Angiogenic response was determined as a fraction of onplant grids containing the newly formed blood vessels (top graph; bar indicates mean). Influx of heterophils was determined as a tissue density of chMMP-9–positive cells scored in × 20 tissue images (middle graph). Influx of monocytes was determined as a tissue density of chMMP-13–positive cells scored in × 20 tissue images (bottom graph). Data are presented as the mean ± SEM. Shown is a representative of 3 independent experiments. Statistical significance was confirmed (P < .05) for each variable in comparison with the previous experimental group as depicted in the scatter and bar graphs.
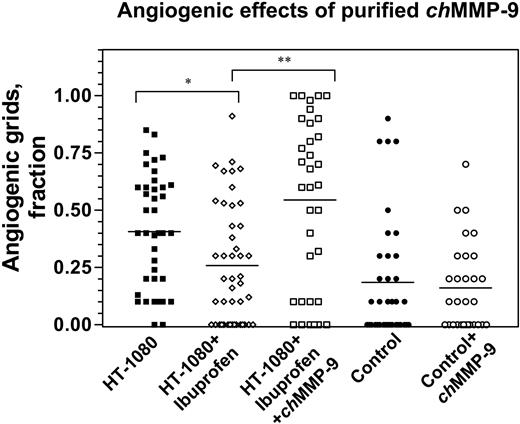
Given that heterophils have been the major cell type associated with and delivering chMMP-9 in the angiogenic onplant tissue (Figures 1 and 3), we next verified whether a direct addition of purified chMMP-9 to the onplants would overcome the antiangiogenic effects of ibuprofen on HT-1080 cell–induced angiogenesis. In 2 independent experiments, angiogenesis in ibuprofen-treated animals was inhibited by 36.5% (Figure 7). Although not as acute as the inhibition in the experiments presented in Figure 6, this inhibition of angiogenesis was statistically significant (P = .009) and was fully overcome by the addition of 100 ng of purified chMMP-9 (P < .001). This finding indicates that purified chMMP-9, possibly in conjunction with other angiogenic factors, provides a means to restore angiogenesis diminished by the anti-inflammatory drugs such as ibuprofen. The results of the rescue by chMMP-9 suggested that the enzyme itself might be able to directly elicit angiogenic responses. However, no proangiogenic effects of purified chMMP-9 were demonstrated when 100 ng of the enzyme was incorporated directly into control collagen onplants that were not supplemented with any growth factors or tumor cells (Figure 7). Therefore, chMMP-9 alone apparently is not sufficient to induce angiogenesis in this experimental system but rather requires cooperation with other factors provided possibly by tumor cells in a setting where inflammatory cell influx has been compromised.
Angiogenic effects of purified chMMP-9. Control and HT-1080 cell–containing onplants were supplemented with either buffer or 100 ng purified chMMP-9 and placed on the CAM of nontreated embryos or embryos treated with ibuprofen. At 72 hours, angiogenesis was scored in the onplants to determine fraction of grids containing newly formed blood vessels. *P = .009; **P = .001.
Angiogenic effects of purified chMMP-9. Control and HT-1080 cell–containing onplants were supplemented with either buffer or 100 ng purified chMMP-9 and placed on the CAM of nontreated embryos or embryos treated with ibuprofen. At 72 hours, angiogenesis was scored in the onplants to determine fraction of grids containing newly formed blood vessels. *P = .009; **P = .001.
To directly assess the proangiogenic capacity of inflammatory heterophils, purified heterophil preparations were incorporated into control onplants containing no growth factors or HT-1080 cells. The addition of only 5 × 104 heterophils per onplant substantially induced angiogenesis, resulting in an approximately 3-fold increase in levels of angiogenesis in 3 independent experiments (Figure 8A-B top panels). Correspondingly, this induced angiogenic response was accompanied by increased levels of infiltration by endogenous chMMP-9–positive heterophils and chMMP-13–positive monocytes (2.9-fold and 2.5-fold, respectively; Figure 8A-B middle and bottom panels, respectively). The addition of isolated erythrocytes to control onplants failed to significantly induce angiogenesis and leukocyte infiltration (data not shown). These experiments demonstrate that relatively low numbers of exogenously added heterophils not only rescue drug-inhibited tumor angiogenesis but also exhibit an inherent capacity to induce a substantial proangiogenic response in vivo in the absence of added growth factors and/or tumor cells.
Discussion
A wealth of compelling evidence spatially and temporally links cancer progression and tumor angiogenesis with the infiltration of tumor sites by inflammatory leukocytes.3 The critical role of persistent inflammation in tumor development has been convincingly demonstrated by the anticancer effects of long-term use of anti-inflammatory drugs in humans.18-20,53,54 In agreement, specific functions of tumor-associated leukocytes were experimentally confirmed in several animal models,13,34-36,45,55-57 clearly suggesting a proangiogenic role of inflammatory leukocytes. However, the actual scenario and kinetics of early leukocyte influx as well as interrelationships between specific inflammatory cell types during the initial stages of tumor-induced angiogenesis is still not fully understood.
Induction of angiogenesis and inflammatory cell influx by purified heterophils. (A) Collagen onplants supplemented with buffer alone (control) or 5 × 104 heterophils isolated from peripheral blood were engrafted on the CAM of 10-day-old embryos incubated ex ovo. Onplants were harvested and fixed in formalin or frozen in OCT compound at 72 hours after the angiogenic response was scored. Paraffin sections stained with hematoxylin and eosin (H&E) indicate similar tissue composition of the onplants (top panels). Arrows point to some of newly formed angiogenic vessels visualized more frequently, especially between the grids, in the onplants supplemented with heterophils. Cryosections were immunohistochemically stained with chMMP-9–specific antibody to visualize heterophils (arrows, middle panels). In addition, paraffin sections were immunohistochemically stained with anti–chMMP-13 antibody identifying brown rounded cells of monocyte/macrophage lineage (bottom panels). (B) Control onplants with and without exogenously added heterophils were scored for angiogenesis (top graph; bar indicates mean), heterophil influx (middle graph), and infiltration by monocytes (bottom graph) as described in Figure 7. Data are presented as means ± SEM. One of 3 independently performed experiments is shown. A statistically significant difference was confirmed (P < .01) for the 2 experimental groups depicted in the scatter and bar graphs.
Induction of angiogenesis and inflammatory cell influx by purified heterophils. (A) Collagen onplants supplemented with buffer alone (control) or 5 × 104 heterophils isolated from peripheral blood were engrafted on the CAM of 10-day-old embryos incubated ex ovo. Onplants were harvested and fixed in formalin or frozen in OCT compound at 72 hours after the angiogenic response was scored. Paraffin sections stained with hematoxylin and eosin (H&E) indicate similar tissue composition of the onplants (top panels). Arrows point to some of newly formed angiogenic vessels visualized more frequently, especially between the grids, in the onplants supplemented with heterophils. Cryosections were immunohistochemically stained with chMMP-9–specific antibody to visualize heterophils (arrows, middle panels). In addition, paraffin sections were immunohistochemically stained with anti–chMMP-13 antibody identifying brown rounded cells of monocyte/macrophage lineage (bottom panels). (B) Control onplants with and without exogenously added heterophils were scored for angiogenesis (top graph; bar indicates mean), heterophil influx (middle graph), and infiltration by monocytes (bottom graph) as described in Figure 7. Data are presented as means ± SEM. One of 3 independently performed experiments is shown. A statistically significant difference was confirmed (P < .01) for the 2 experimental groups depicted in the scatter and bar graphs.
To experimentally address some of the essential questions regarding the putative proangiogenic role of specific inflammatory leukocytes, we have modified a previously described growth factor–induced in vivo angiogenesis model employing collagen CAM onplants.46,48 In the modified assay, sprouting of angiogenic vessels from the preexisting CAM vasculature is induced by human tumor cells. This model provides an accurate and reproducible measure of new vasculature formation and also allows for analysis of host infiltrating leukocytes in the tumor-induced angiogenic process. Not only is the observed angiogenesis dependent on MMP-mediated collagen remodeling but it also coincides with a rapid influx of MMP-bearing leukocytes: first, chMMP-9–positive heterophils, followed by chMMP-13–positive monocytes/macrophages.
Early heterophil arrival into collagen onplants is a part of a broader infiltration scenario comprising influx of a variety of host cells, including myofibroblasts, angiogenic capillaries, and blood vessels, into the original onplant space. The early infiltrating chicken heterophils were shown to be a major source of leukocyte-derived chMMP-9 protein. In the absence of exogenous growth factors, low levels of chMMP-9–positive heterophil infiltration were associated with the low angiogenic response in the control onplants. More importantly, increased levels of heterophil infiltration correlated closely with high levels of angiogenesis induced in the collagen onplants by growth factors or tumor cells, whereas inhibition of heterophil influx by anti-inflammatory agents directly associated with diminished levels of angiogenesis.
During angiogenesis in collagen CAM onplants, cleavage, remodeling, and degradation of collagen occur coordinately.46,48 Although heterophils are the earliest leukocytes to appear in the onplant tissue, substantial influx of monocytes/macrophages in the underlying CAM tissue was detected approximately 24 hours later after the initial wave of infiltrating heterophils. That these events were temporally linked was indicated by the observation that the addition of purified heterophils to control onplants was followed shortly by an induced influx of monocytes. Functional distinction between the 2 leukocyte populations is also indicated by different MMPs mediating ECM remodeling at the angiogenic site: chMMP-13 collagenase, critical for the cleavage of fibrillar collagen, is delivered by monocytes/macrophages,48 whereas chMMP-9 gelatinase, likely degrading the denatured collagen and possibly cleaving other polypeptides, is imported specifically by the heterophils.
In mammals, inhibition of neutrophil influx into various inflamed tissues correspondingly decreased both MMP-9 levels and neovascularization.25,41,43 Similarly, diminished macrophage infiltration and lack of MMP-9 in MMP-9-/- mice were associated with a decrease in angiogenesis, specifically in microvessel density and capillary branching.44 In the present study, evidence for a critical role of chMMP-9–positive heterophils in tumor-induced angiogenesis was provided by experiments where leukocyte influx into HT-1080 cell–containing onplants was abrogated by 2 potent, mechanistically distinct anti-inflammatory agents, cortisone or ibuprofen.15,50,58 In cortisone-treated animals, an inhibited development of angiogenic capillaries and blood vessels was associated with significant decrease in the levels of heterophil influx as well as chMMP-9 protein, providing an experimental link between infiltrating heterophils, chMMP-9 delivery, and tumor-induced angiogenesis.
The effects of the nonsteroidal anti-inflammatory drug ibuprofen on HT-1080 cell–induced angiogenesis corroborate the cortisone effects and expand our data on coordinated inhibition of angiogenesis and heterophil infiltration. Ibuprofen, known to target both tumor and inflammatory cells,58 was used at concentrations that did not affect HT-1080 cell growth and distribution but very effectively inhibited influx of the inflammatory leukocytes, namely chMMP-9–positive heterophils and chMMP-13–positive monocytes. Concomitantly, this inhibition of inflammatory cell influx was accompanied by a corresponding decrease in angiogenic response, indicating a proangiogenic role of inflammatory leukocytes in tumor-induced angiogenesis. Surprisingly, only limited quantitative data are available in the literature to support a causal relationship between coordinated inhibition of tumor angiogenesis and leukocyte infiltration by anti-inflammatory drugs, including COX inhibitors.59,60 Therefore, the present study provides a strong experimental link for a direct correlation between the tripartite effects of anti-inflammatory drugs, inhibition of leukocyte influx, and inhibition of tumor-induced angiogenesis.
The present study also provides direct evidence that specific inflammatory leukocytes (ie, neutrophil-like heterophils) could themselves be highly proangiogenic in vivo. The addition of only 5 × 104 heterophils directly into collagen onplants, not supplemented with any exogenous growth factors or tumor cells, induced a strong stimulation of new blood vessel formation. Further support for a direct causal effect of inflammatory cells on malignant progression is illustrated by the complete rescue of ibuprofen-inhibited angiogenesis in tumor cell–containing onplants by the addition of purified heterophils. Remarkably, exogenously added heterophils not only restored the levels of accompanying leukocyte infiltration of onplants but also the levels of tumor-induced neovascularization. To our knowledge, this is the first demonstration that these inflammatory leukocytes are directly proangiogenic. In a mammalian system, stimulatory effects of a different inflammatory leukocyte type on tumor growth and angiogenesis were recently demonstrated when purified mast cells were mixed with plasmacytoma cells before implantation into the mice.57
It is not clear what factors are responsible for the proangiogenic role of inflammatory heterophils. It is unlikely that the simple physical presence of exogenous live cells elicited the observed effects, since nucleated chicken erythrocytes clearly failed to stimulate or rescue angiogenesis. It is more likely that the released products of heterophils are involved in their proangiogenic functions. Like their mammalian counterparts, heterophils are terminally differentiated leukocytes that do not synthesize their products in response to specific stimuli but store presynthesized mediators, including MMP-9, in granules for rapid release at sites of inflammation or angiogenesis.
There is a great deal of evidence linking mammalian MMP-9 to neovascular tissue function as an angiogenic switch as a mediator of tissue remodeling and/or as a catalytic provider of bioactive cytokines, regulators, and inhibitory peptides.32-36,40,45,61 Thus, chMMP-9 found in the earliest arriving cell type that infiltrates the newly stimulated CAM vascular tissue could be considered as a prime catalytic effector molecule. Unlike the interstitial collagenase chMMP-13, which stimulates angiogenesis when added to the collagen onplants,48 purified chMMP-9 did not by itself stimulate angiogenesis in the CAM assay. However, purified chMMP-9 was capable of rescuing angiogenesis compromised by anti-inflammatory effects of ibuprofen. Therefore, other intrinsic factors released by heterophils, possibly acting in tandem with catalytic chMMP-9, are likely involved in the proangiogenic action of these inflammatory leukocytes. It would be premature at this point to speculate on the molecular identity of released products of the avian heterophils that might be functionally active in CAM vascular tissue or CAM tumor tissue. However, the facile yet quantitative model described herein for both growth factor– and tumor-induced angiogenesis might in the near future allow for screening isolated secreted products of heterophils for their ability to stimulate angiogenesis and/or to induce the further influx of specific inflammatory cells.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-04-1458.
Supported by grants from the National Institutes of Health (grants CA55852 and CA105412) and American Heart Association Fellowship (0225103Y).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 3. Kinetics of heterophil influx into collagen CAM onplants. Samples of normal CAM from embryos without onplants (NCAM) and collagen onplants supplemented with angiogenic growth factors (bFGF/VEGF) or buffer alone (control) were excised with the underlying CAM, embedded in OCT compound, and frozen. Cryosections were immunohistochemically stained with chMMP-9 antibody and counterstained with Mayer hematoxylin. Digital images were collected at × 10, × 20, and × 40 magnifications and analyzed. (A) Representative sections of control (left panel) and growth factor–supplemented (right panel) collagen onplants harvested at 72 hours (original magnification × 10). Arrows point to dark brown–stained cells and cell clusters confirmed to be chMMP-9–positive heterophils. (B) At an original magnification of × 40, morphologic features of chMMP-9–positive cells are consistent with the characteristics of heterophils (ie, multilobed nuclei [bluish] and granular cytoplasm [brownish]). (C) Kinetics of heterophil influx into collagen CAM onplants was determined over the 72-hour time course. The original × 20 magnification images of normal CAM (NCAM), control, and growth factor–containing (bFGF/VFGF) onplants were overlaid with a 9 × 7 square grid and analyzed (data from 9 to 39 images per time point from 2 independent experiments). chMMP-9–positive heterophils were counted in the squares occupied with tissue. Data are presented as the mean ± SEM from the numbers of chMMP-9–positive cells per square. *P < .001, determined in the 2-tailed Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-04-1458/4/m_zh80010688940003.jpeg?Expires=1767865219&Signature=E7Y~-vMOaCJAwB9EMSb~uqN8YjpW8hxXHdJuyx0Qc7N1qX7EIcxc2478Wii6fJD5QrcutarZMY9cdiuK7ujDEc5MzcVgZEioccXH5J~8dPNTrILxJ4mGYMcsFBYfXiS6qZxg6gFY-CqyoQLPCv0X3rpjo4ThLtl6VcYrY7PeBNsg7w772P2t8oyCRbIAmt4ld8e6EYQjNLkOX5la6gTy-POwLX5VUYsPW2eT37cun~DEGj1esyVs~l2XChn2qY4maMa-2F~avdhxjz0OqdTKSCYanfLHQawSPMMbfxeRtYH~orZ2xiu7J9QlbGVknOvqbCKVR1oMeGtBk-b6RZ--Uw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal