Abstract
Multiple myeloma (MM) is characterized by the production of monoclonal immunoglobulin and is associated with suppressed uninvolved immunoglobulins and dysfunctional T-cell responses. The biologic basis of this dysfunction remains ill defined. Because T regulatory (Treg) cells play an important role in suppressing normal immune responses, we evaluated the potential role of Treg cells in immune dysfunction in MM. We observed a significant increase in CD4+CD25+ T cells in patients with monoclonal gammopathy of undetermined significance (MGUS) and in patients with MM compared with healthy donors (25% and 26%, respectively, vs 14%); however, Treg cells as measured by FOXP3 expression are significantly decreased in patients with MGUS and MM compared with healthy donors. Moreover, even when they are added in higher proportions, Treg cells in patients with MM and MGUS are unable to suppress anti-CD3–mediated T-cell proliferation. This decreased number and function of Treg cells in MGUS and in MM may account, at least in part, for the nonspecific increase in CD4+CD25+ T cells, thereby contributing to dysfunctional T-cell responses.
Introduction
T regulatory (Treg) cells play an important role in the maintenance of self-tolerance, control of auto-immunity, and regulation of T-cell homeostasis, and they modulate overall immune responses against infectious agents and tumor cells.1 Natural Treg cells develop during normal T-cell maturation in the thymus and represent 5% to 10% of the CD4+ cell compartment in the peripheral blood.2 These cells express CD4 and CD25 surface antigens as well as CTLA-4, GITR, CD103, CD62L, CD69, CD134, CD71, CD54, and CD45RA.3 The suppressive activity of Treg cells is associated with the overexpression of FOXP3, a member of the forkhead/winged helix family, which acts as a transcriptional repressor.4 Treg cells suppress CD25-CD4+ T-cell proliferation on the basis of cell–cell contact and suppress immune responses by secreting immunosuppressive cytokines such as IL-10 and TGF-β.5
A significant impairment of T-cell function is observed in patients with multiple myeloma (MM) and patients with monoclonal gammopathy of undetermined significance (MGUS). Although phenotypic and functional aberrations in CD4 and CD8 cells have been described in MM and MGUS,6-9 the biologic basis for these abnormalities remains unclear. Because Treg cells play an important role in modulating normal immune responses, the abnormal Treg-cell activity in myeloma patients could contribute to immune dysfunction in MM and could provide a new target to enhance immune responses. Therefore, in this study we evaluated natural Treg-cell number and function in patients with MGUS and MM and compared them with those of healthy donors.
Study design
Phenotypic characterization
CD4+CD25+ Treg-cell numbers were analyzed by flow cytometric analyses in peripheral-blood mononuclear cells (PBMCs) collected from healthy donors, patients with MGUS, and patients with newly diagnosed MM. Approval for these studies was obtained from the institutional review board of the Dana-Farber Cancer Institute and the Veterans Administration (VA) Boston Healthcare System. Informed consent was provided according to the Declaration of Helsinki.
Measurement of FOXP3 expression
As FOXP3 is specifically expressed by Treg cells and is required for their suppressive activity, we analyzed the proportion of PBMCs expressing intracellular FOXP3 using anti-FOXP3 antibody (eBiosciences, San Diego, CA) using dual-color flow cytometry and multiphoton microscopy. Level of protein expression was quantitated by Western blotting and by real-time reverse transcription–polymerase chain reaction (RT-PCR) using previously described methods.10
Suppressive activity of T regulatory cells
To evaluate the function of Treg cells, PBMCs were first depleted of CD25+ T cells (which contain Treg cells) by positive selection using anti-CD25–coated microbeads (Miltenyi Biotech, Auburn, CA), according to the manufacturer's instructions.11 PBMCs depleted of CD25+ cells and control PBMCs containing CD25+ cells were stimulated with anti-CD3 antibody for 3 days, and proliferation was measured by 3H-thymidine uptake during the last 8 hours of culture. In a separate study, purified CD25+ cells were added in various proportions to PBMCs depleted of CD25+ cells to assess their effects on anti-CD3–induced T-cell proliferation.
Results and discussion
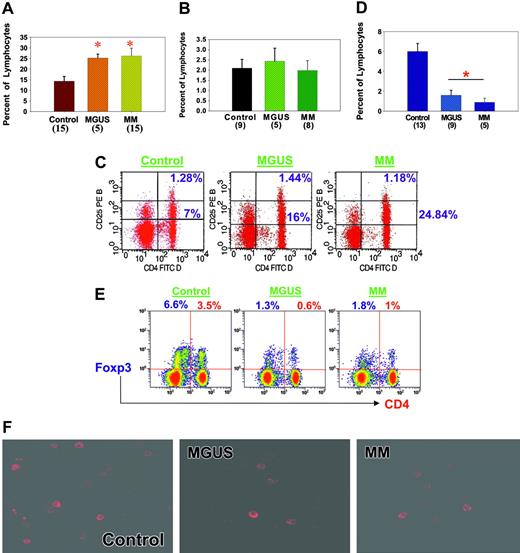
We evaluated the proportions of CD4+CD25+ cells in the peripheral blood of healthy donors and of patients with MGUS or MM. As seen in Figure 1A, the proportion of these cells in PBMCs was significantly elevated in MGUS (mean, 25% ± 1.8%; range, 20%-29%) and MM (mean, 26% ± 3.6%; range, 6%-51%) compared with healthy donors (mean, 14% ± 2.3%; range, 4%-28%) (P < .01). Because Treg cells and activated CD4 cells express CD4 and CD25,12 we next evaluated the proportions of cells expressing high levels of CD25, characteristic of cells with regulatory function. As seen in Figure 1B-C, we did not observe significant differences in the proportions of CD4+CD25high cells in PBMCs in patients with MGUS or MM compared with healthy donors.
Characterization of Treg cells in MGUS and MM compared with healthy donors. (A) PBMCs were isolated, incubated with anti-CD4 and -CD25 antibodies, and analyzed by flow cytometry. Results are expressed as percentages of lymphocytes. Number of samples analyzed in each category is given in parentheses. *P ≤ .01 by Student t test analysis. MGUS and MM patients had significantly greater numbers of CD4+CD25+ T cells than healthy donors. (B) CD4- and CD25-expressing T cells were isolated as described in panel A, and cells expressing high levels of CD25 were then analyzed. Results are expressed as percentages of lymphocytes expressing CD4 and CD25high. Number of samples analyzed in each category is given in parentheses. No significant increases in CD4+CD25high T-cell numbers were observed in MGUS or MM patients compared with healthy donors. (C) Representative example of flow cytometry data using healthy donor cells and cells from patients with MGUS and MM. Frequency of CD25+ cells in the lymphocyte gates was analyzed using anti-CD25 PE antibody along with anti-CD4 FITC antibody. Quadrants were established using isotype controls, and stained cells were analyzed using Cytomics FC 500 (Beckman-Coulter, Fullerton, CA) and CXP software. (D) PBMCs were isolated and incubated with anti-FOXP3 antibodies (eBiosciences) for intracellular staining and then were analyzed by flow cytometry. Cells in the lymphocyte gates were used for the analysis. Results are expressed as percentages of lymphocytes expressing FOXP3, and the number of samples analyzed in each category is given in parentheses. A significantly decreased number of FOXP3+ (P < .01) Treg cells were observed in MGUS and MM patients compared with healthy donors. (E) Frequency of FOXP3+ cells in the lymphocyte gates was analyzed using anti-FOXP3 PE antibody. Dual-color analysis (PE-FOXP3 and Pc5-CD4) was optimized and used in these studies. Quadrants were established using isotype controls, and stained cells were analyzed using Cytomics FC 500 (Beckman-Coulter) and CXP software. Data are representative of 5 separate experiments. (F) PBMCs were stained with anti-FOXP3 antibodies and then analyzed using multiphoton microscopy. (BioRad MRC 1024ES multiphoton system; Bio-Rad, Hercules, CA). A Zeiss Axiovert S 100 inverted microscope equipped with a high-quality water immersion 40×/1.2 numeric aperture C-Apochromat objective was used to obtain images (total magnification is 640×). Images were reconstructed using the Bio-Rad LaserSharp and/or MetaMorph software (MetaMorph Imaging Series, Universal Imaging, West Chester, PA). Higher frequencies of stained cells were observed in PBMCs from healthy donors than in PBMCs from MGUS and MM patients. Error bars in panels A, B, and D indicate SEM.
Characterization of Treg cells in MGUS and MM compared with healthy donors. (A) PBMCs were isolated, incubated with anti-CD4 and -CD25 antibodies, and analyzed by flow cytometry. Results are expressed as percentages of lymphocytes. Number of samples analyzed in each category is given in parentheses. *P ≤ .01 by Student t test analysis. MGUS and MM patients had significantly greater numbers of CD4+CD25+ T cells than healthy donors. (B) CD4- and CD25-expressing T cells were isolated as described in panel A, and cells expressing high levels of CD25 were then analyzed. Results are expressed as percentages of lymphocytes expressing CD4 and CD25high. Number of samples analyzed in each category is given in parentheses. No significant increases in CD4+CD25high T-cell numbers were observed in MGUS or MM patients compared with healthy donors. (C) Representative example of flow cytometry data using healthy donor cells and cells from patients with MGUS and MM. Frequency of CD25+ cells in the lymphocyte gates was analyzed using anti-CD25 PE antibody along with anti-CD4 FITC antibody. Quadrants were established using isotype controls, and stained cells were analyzed using Cytomics FC 500 (Beckman-Coulter, Fullerton, CA) and CXP software. (D) PBMCs were isolated and incubated with anti-FOXP3 antibodies (eBiosciences) for intracellular staining and then were analyzed by flow cytometry. Cells in the lymphocyte gates were used for the analysis. Results are expressed as percentages of lymphocytes expressing FOXP3, and the number of samples analyzed in each category is given in parentheses. A significantly decreased number of FOXP3+ (P < .01) Treg cells were observed in MGUS and MM patients compared with healthy donors. (E) Frequency of FOXP3+ cells in the lymphocyte gates was analyzed using anti-FOXP3 PE antibody. Dual-color analysis (PE-FOXP3 and Pc5-CD4) was optimized and used in these studies. Quadrants were established using isotype controls, and stained cells were analyzed using Cytomics FC 500 (Beckman-Coulter) and CXP software. Data are representative of 5 separate experiments. (F) PBMCs were stained with anti-FOXP3 antibodies and then analyzed using multiphoton microscopy. (BioRad MRC 1024ES multiphoton system; Bio-Rad, Hercules, CA). A Zeiss Axiovert S 100 inverted microscope equipped with a high-quality water immersion 40×/1.2 numeric aperture C-Apochromat objective was used to obtain images (total magnification is 640×). Images were reconstructed using the Bio-Rad LaserSharp and/or MetaMorph software (MetaMorph Imaging Series, Universal Imaging, West Chester, PA). Higher frequencies of stained cells were observed in PBMCs from healthy donors than in PBMCs from MGUS and MM patients. Error bars in panels A, B, and D indicate SEM.
Treg cells express FOXP3, a transcriptional factor required for regulatory and suppressive function.13 Therefore, we next used flow cytometry to define the proportions of cells expressing CD4 and FOXP3 in healthy donors and in patients with MGUS and MM. As seen in Figure 1D-E, although 6.0% ± 0.8% PBMCs from healthy donors expressed FOXP3, significantly reduced numbers of FOXP3+ PBMCs were detected in MGUS (1.6% ± 0.5%; P < .01) and MM (0.9% ± 0.4%; P < .01). This reduction in FOXP3-expressing cells observed by flow cytometry was further confirmed by immunohistochemistry using multiphoton microscopy (Figure 1F) and Western blot analysis (data not shown). Proportions of PBMCs expressing CTLA-4, a cell-surface molecule expressed by Treg cells, were also significantly reduced in patients with MGUS (0.9% ± 0.5%; P < .01) and MM (1% ± 0.6%; P < .01) compared with healthy donors (6.8% ± 0.6%; data not shown). These data, therefore, show significantly reduced numbers of Treg in patients with MGUS and MM compared with healthy donors.
Lack of suppression of T-cell proliferation by Treg cells in MGUS and myeloma. (A) Anti-CD3–mediated T-cell proliferation of PBMCs incubated with or without CD25+ T cells for 72 hours was measured by 3H-thymidine uptake during the last 8 hours of culture. Results are mean ± SEM CPM, and the number of samples analyzed is shown in parentheses. *P < .05 by Student t test analysis. Compared with healthy donor cells, Treg cells from MGUS and MM patients were unable to significantly suppress T-cell proliferation. (B) CD25+ T-cell–depleted PBMCs were cocultured with increasing numbers of CD25+ T cells at different ratios, and anti-CD3–mediated proliferation was then measured at 72 hours by 3H-thymidine uptake during the last 8 hours of culture. Adding excess CD25+ cells failed to suppress T-cell proliferation in patients with MGUS or MM compared with healthy donors. Results are from 1 of 7 representative experiments.
Lack of suppression of T-cell proliferation by Treg cells in MGUS and myeloma. (A) Anti-CD3–mediated T-cell proliferation of PBMCs incubated with or without CD25+ T cells for 72 hours was measured by 3H-thymidine uptake during the last 8 hours of culture. Results are mean ± SEM CPM, and the number of samples analyzed is shown in parentheses. *P < .05 by Student t test analysis. Compared with healthy donor cells, Treg cells from MGUS and MM patients were unable to significantly suppress T-cell proliferation. (B) CD25+ T-cell–depleted PBMCs were cocultured with increasing numbers of CD25+ T cells at different ratios, and anti-CD3–mediated proliferation was then measured at 72 hours by 3H-thymidine uptake during the last 8 hours of culture. Adding excess CD25+ cells failed to suppress T-cell proliferation in patients with MGUS or MM compared with healthy donors. Results are from 1 of 7 representative experiments.
Next, we evaluated the regulatory function of Treg cells in patients with MGUS and MM compared with healthy donors. To assess function, we measured the ability of Treg cells to suppress T-cell proliferation induced by soluble anti-CD3 antibody. PBMCs were activated by anti-CD3 antibody in the presence or absence of Treg cells (depleted using anti-CD25–coated microbeads), and proliferation was measured by 3H-thymidine uptake. In healthy donor PBMCs, proliferation was significantly suppressed in the presence of CD25+ cells (71 770 ± 8010) compared with proliferation in their absence (115 753 ± 10 113; P < .05) (Figure 2A). In contrast, CD25+ cells failed to significantly suppress PBMC proliferation in patients with MGUS and MM (29 813 ± 8396 vs 39 437 ± 7463 [P = NS] and 62 223 ± 10 175 vs 51 893 ± 12 361 [P = NS], respectively) (Figure 2A). To account for the reduced frequency of FOXP3+ Treg cells in patients with MGUS and MM, we evaluated cell activity after adding a proportionately higher number of Treg cells to CD25-depleted PBMCs. As can be seen in Figure 2B, adding even 10-fold more Treg cells did not suppress soluble anti-CD3–mediated T-cell proliferation in patients with MGUS or MM.
These results highlight important Treg-cell abnormalities in patients with MM and MGUS because natural Treg cells are significantly reduced in number and are dysfunctional. The biologic basis for the reduction and dysfunction of Treg cells in patients with MM and MGUS remains undefined. However, in a murine model of asthma, it has been demonstrated that IL-6 and soluble IL-6 receptor (sIL-6R) together decrease Treg-cell number and function in the lungs. Interestingly, interactions between MM cells and bone marrow stromal cells trigger the production of IL-6 and a number of cytokines and chemokines, including TNF-α, VEGF, IGF-1, SDF-1α, IL-1β, TGF-β, and MIP-1α/β with immunomodulatory activity.14,15 In patients with MM and MGUS, serum levels of IL-6 and sIL-6R are highly elevated and may, therefore, play important roles in Treg-cell development and function.16 Moreover, mice defective in TGF-β receptor II expression and signaling have low numbers of Treg cells.17 TGF-β has also been shown to inhibit IL-2–dependent T-cell proliferation.18 In patients with myeloma, TGF-β is induced by interactions between MM cells and the bone marrow stromal cells, which may modulate peripheral expansion and maintenance of Treg cells.
A second unresolved issue is how dysfunctional Treg cells affect immune function in MGUS and MM. The significantly elevated numbers of CD4+CD25+ cells, which are predominantly FOXP3-, suggest an increase in activated T cells in patients with MGUS and MM. Low Treg-cell numbers or function generate nonspecific immune responses or autoimmunity.5,19 If Treg cells are so low that immune responses are not suppressed, particularly at the terminal stages of an immune response, an increase in hyperreactive T cells may be seen, as in myeloma patients.7 This hyperactivity of T cells has been associated with defective TCR signaling20 and increased sensitivity to costimulatory signals.7 In contrast, recent studies in patients with breast and gastroesophageal cancers, metastatic melanoma, and Hodgkin lymphoma/chronic lymphocytic leukemia (CLL) have reported increased numbers of Treg cells that may suppress immune responses, leading to ineffective antitumor immune responses.21-26
These results identify potentially important mediators of immune dysfunction in myeloma. The presence of Treg-cell dysfunction in MGUS suggests a role for Treg cells even at an earlier stage in the development of disease. Evaluation of Treg cells at various stages in MGUS may provide further insight into the development and progression of MGUS to MM. Additionally, MM is reported to be associated with a number of autoimmune disorders, such as thyroid abnormalities,27 rheumatoid arthritis,28 and renal complications.29 It will be intriguing to study whether these conditions are the cause or the effect of dysfunctional Treg cells in MM. If hyperactive T cells are the source of immune dysfunction in patients with myeloma, improving Treg-cell number and function may provide a solid foundation for enhancing immune function and vaccination strategies in the future.
Prepublished online as Blood First Edition Paper, September 8, 2005; DOI 10.1182/blood-2005-08-3101.
Supported by Department of Veterans' Affairs Merit Review Awards and by a Leukemia and Lymphoma Society Scholar in Translational Research Award (N.C.M.); Multiple Myeloma Research Foundation Awards (N.C.M., K.C.A.); National Institutes of Health grants P50-100707, PO1-78378, (K.C.A., N.C.M.), and RO1-50947; and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Virginia M. Cumming and Suzan B. Lazo-Kallanian for their excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal