Abstract
Pentraxins are soluble pattern recognition receptors with a dual role: protection against extracellular microbes and autoimmunity. The mechanisms by which they accomplish these tasks are not yet fully understood. Here we show that the prototypic long pentraxin PTX3 is specifically recruited at both sides of the phagocytic synapse between dendritic cells (DCs) and dying cells and remains stably bound to the apoptotic membranes (estimated half-time > 36 hours). Apoptotic cells per se influence the production of PTX3 by maturing DCs. When both microbial stimuli and dying cells are present, PTX3 behaves as a flexible adaptor of DC function, regulating the maturation program and the secretion of soluble factors. Moreover a key event associated with autoimmunity (ie, the cross-presentation of epitopes expressed by apoptotic cells to T cells) abates in the presence of PTX3, as evaluated using self, viral, and tumor-associated model antigens (vinculin, NS3, and MelanA/MART1). In contrast, PTX3 did not influence the presentation of exogenous soluble antigens, an event required for immunity against extracellular pathogens. These data suggest that PTX3 acts as a third-party agent between microbial stimuli and dying cells, contributing to limit tissue damage under inflammatory conditions and the activation of autoreactive T cells.
Introduction
The humoral arm of innate immunity includes the soluble pattern recognition receptors of the pentraxin family, which are characterized by a cyclic pentameric structure. The classic short pentraxins, C-reactive protein (CRP) and serum amyloid protein (SAP), are produced by the liver in response to inflammatory mediators like interleukin-6.1 They play an important role in antimicrobial responses and in the clearance of cellular debris.2 The prototypic long pentraxin is pentraxin 3 (PTX3), which shares structural homology with the short pentraxins in the carboxy terminal domain. A 174-amino acid-long amino terminal domain differentiates PTX3 from CRP and SAP (for details see Garlanda et al3 ).
PTX3 was first identified as an IL-1β-inducible gene in endothelial cells4 and a TNF-α-stimulated gene in fibroblasts.5 Elevated levels of PTX3 have been reported in infectious and inflammatory conditions.6-11 Mouse models indicate a nonredundant role of PTX3 in immunity to fungal infections, especially pulmonary aspergillosis, and in female fertility.12-14 Smooth muscle cells, adipocytes, mononuclear phagocytes, and dendritic cells (DCs) produce PTX3 as well.15-19
Myeloid DCs are major producers of PTX3 in response to toll-like receptor (TLR) ligands.17 DCs play a key role in the activation of major histocompatibility complex (MHC)-restricted adaptive immune responses: most nucleated cells present peptides derived from the processing of endogenous proteins in association with MHC class I molecules. Cytotoxic T lymphocytes thus identify the cells that present microbial epitopes as infected and lyse them, limiting or preventing the replication of intracellular pathogens. Only endogenous proteins have access to this presentation pathway, thus avoiding that cytotoxic T cells kill bystander cells endocytosing exogenous microbial proteins. The ability to process and cross-present antigens expressed by dying cells, therefore exogenous, into the MHC class I pathway20 endows DCs with the ability to initiate immune responses against microbes that do not directly infect them.21,22 Cross-presentation has initially been characterized in transplantation; it can occur in other circumstances, including organ and systemic autoimmune diseases20,23-30 and cancer.31-34
Innate factors regulate the immunogenicity of dying cells.35-37 Pentraxins efficiently bind to dying cells.38-40 They are required for the clearance of chromatin and cell debris27-41 and for effective protection against autoimmunity.3,42-48 This function is in apparent contrast with the involvement of pentraxins in protection against extracellular bacteria or fungi.3 The mechanisms by which pentraxins on the one hand restrict the activation of autoreactive lymphocytes and on the other promote antimicrobial immunity are not yet characterized. In this study we demonstrate that PTX3 behaves as a flexible regulator of the function of DCs: it limits the production of TNF-α and IL-10 and the upregulation of costimulatory molecules in response to microbial ligands. In sharp contrast, in the presence of dying cells, PTX3 enhances the production of cytokines but actively restricts the cross-presentation of antigens derived from the processing of the dying cells. PTX3 therefore favors immediate protection when acute inflammation and extensive cell death coexist while limiting the onset, and possibly the maintenance,30 of autoimmune phenomena.
Patient, materials, and methods
Reagents
Human PTX3 was purified from Chinese hamster ovary (CHO) cells stably and constitutively expressing the protein, as described.49 Biotinylation was performed as described.49 Biotinylated PTX3 was analyzed in the native state in 5% to 10% gradient polyacrylamide gel electrophoresis (PAGE). Gels were stained with silver nitrate. The concentrations of PTX3 in culture supernatants were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) method based on the MNB4 PTX3-specific monoclonal antibody and on biotinylated rabbit PTX3-specific polyclonal IgG (Alexis, Vinci, Italy). This assay is highly sensitive and specific; no cross-reactions were observed with other pentraxins and in particular with the CRP or SAP.50 The recombinant NS31187-1465 protein of HCV (HCV-NS3), expressed in Escherichia coli and affinity purified as described in the manufacturer's instructions, was purchased by Biodesign International (Saco, ME). Human CRP was purchased from Calbiochem (EMD Biosciences, La Jolla, CA).
Cell lines
HeLa and NIH-3T3 fibroblasts were purchased from ATCC (Manassas, VA). H9T cells were kindly donated by Dr M. Ferrarini (University of Genoa, Italy). NIH-3T3 cells were transduced as previously described. Lymphoblastoid cell lines (EBV) were propagated from healthy HLA-A2 donors and induced to express the NS3 viral antigen upon NS3-expressing vaccinia virus (VV-NS3) infection (5 plaque-forming units [PFUs]/cell). Lines infected with wild-type VV (WT-VV) were used as controls. A retroviral vector coding for the MelanA/MART1 tumor differentiation antigen was constructed as described for the M3-CSM vector.51 The MelanA/MART1 cell-surface marker vector encodes the full-length MelanA/MART1 cDNA52 under the control of the long terminal repeat (LTR) and the truncated form of the human low-affinity nerve growth factor receptor (ΔLNGFR) driven by the SV40 promoter. The ecotropic murine fibroblast cell line E86 was transiently transfected with 10 μg LAaSΔN plasmid by standard calcium-phosphate method. Infection of the amphotropic murine packaging cell line Am12 by supernatant of 48 cultures of transfected E86 cells was performed for 16 hours in the presence of 8 μg/mL polybrene. Infected packaging cells were selected for ΔLNGFR expression by immunoselection with magnetic beads coated with the NGFR-specific mAb 20.4. Transduction of NIH-3T3 cells was performed by cultivating them in the presence of retroviral-containing supernatant and 8 μg/mL of polybrene. The percentage of infection was evaluated by fluorescence-activated cell sorter (FACS) analysis for LNGFr expression.
Antigen-presenting cells
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats of healthy HLA-A2+ donors by sedimentation over Ficoll Hypaque as described.24,25 Monocytes were differentiated into DCs by culture in RPMI-1640 supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 1.5 mM L-glutamine, and 10% heat-inactivated fetal calf serum (FCS; tissue culture medium [TCM]) in the presence of GM-CSF (800 U/mL) and IL-4 (800 U/mL). The phenotype of the DCs was monitored by flow cytometry, using fluorochrome-labeled monoclonal antibodies against CD1a, CD14, CD40, CD83, CD86, HLA-DR, and HLA-ABC surface markers (BD Biosciences Pharmingen, San Diego, CA). Maturation of DCs was induced by stimulation with lipopolysaccharide (LPS; 10-1000 ng/mL; Sigma, St Louis, MO). On day 6 the TCM was replaced with fresh medium or with the synthetic serum-free X Vivo-15 medium (BioWhittaker, Cambrex, NJ). When indicated, PTX3 or CRP were added (final concentration 50 μM). Apoptotic cells (1 × 106/mL) were added with a final DC-apoptotic cell ratio of 1:4. After 24 to 48 hours the cells were harvested and the supernatants collected and frozen. Experiments were repeated using DCs from at least 6 different donors.
Antigen-specific T-cell propagation and characterization
The MT27-35 (MelanA/MART1) peptide-specific CD8+ T cells were obtained by using a protocol previously described.52 Briefly, PBMCs from HLA-A2+ healthy donors were challenged with autologous and activated B lymphoblasts pulsed with 100 μg/mL of MT27-35 in medium containing 10% human serum, 1000 U/mL IL-6 (Genzyme, Cambridge, MA), and 10 ng/mL IL-12 (Hoffmann-La Roche, Nutley, NJ). Autologous, CD8+-enriched (> 90%) responder cells were purified from autologous PBMCs by magnetic sorting (Miltenyi GmBH, Bergisch-Gladbach, Germany) according to the manufacturer's recommendations and restimulated after 8 days and propagated in the presence of 10 U/mL of IL-2 (Chiron, Siena, Italy). Antigen specificity was verified in a standard 51Cr-release assay53 or tested in an enzyme-linked immunospot (ELIspot) assay for the IFN-γ spot formation. HLA-A2-restricted vinculin-specific CD8+ T-cell lines were generated by stimulation of peripheral blood lymphocytes from HLA-A2+ healthy donors with autologous DCs pulsed with the HLA-A2-binding vinculin823-831 peptide and repeated stimulation as described.25 Briefly, immature HLA-A2+ DCs (2.5 × 105 cells/well) were exposed to vinculin823-831 peptide (100 μg/mL) for 18 hours. Positively isolated autologous CD8+ T cells (1 × 106 cells/well) were added to the cultures in the presence of CD40L-transfected J558L cells (at a DC/J558L ratio of 1:1), kindly provided by Dr Peter Lane (University of Birmingham Medical School, Birmingham, United Kingdom), and primed CD8+ T cells expanded with IL-2 and tested in an ELIspot assay for the IFN-γ spot formation. Cells of CD8+ T-cell clone NS3-1 specific for HLA-A2-binding peptide NS3 (1406-1415) of HCVNS3 protein were obtained from an HCV-positive patient, maintained by repeated stimulation with phytohemagglutinin and allogeneic antigen-presenting cells, and propagated in the presence of IL-2, as described.54 Antigen specificity was verified in a standard 51Cr-release assay.
Induction and detection of apoptosis
Programmed cell death was triggered by UVC irradiation (254-nm wavelength) at the dose of 3.5 mW/cm2/s for 60 seconds (HeLa cells) or at the dose of 1.8m W/cm2/s for 45 seconds (fibroblasts). Actual induction of apoptosis was confirmed by flow cytometry, microscopy, and agarose gel electrophoresis, as described.55 When indicated, irradiated cells (1 × 106 cells/mL) were chased for different times (up to 24 hours) in TCM. The phase of apoptosis was verified evaluating the exposure of anionic phospholipids by flow cytometry using annexin V-fluorescein isothiocyanate (FITC) (Bender MedSystem, GmbH, Vienna, Austria), per manufacturer's instructions; membrane function was assessed by addition of the vital dye propidium iodide (1 μg/mL) immediately before FACS analysis. In preliminary experiments, the optimal cell concentration for apoptosis induction was verified per each cell line: HeLa cells were seeded at 1 × 106 cells/mL while 3T3 and 3T3-MelanA/MART1 fibroblasts were routinely plated at a concentration of 5 × 104 cells/mL. HLA-A2- lymphoblastoid cells (EBV) expressing or not the NS3 viral antigen and H9T cells were committed to apoptosis by treatment with 500 ng/mL anti-Fas monoclonal IgM antibodies for 6 hours (CH11 clone; Immunotech, Marseille, France; or Upstate Biotechnology, Lake Placid, NY).
Assessment of PTX3 binding to living and apoptotic cells
Apoptotic and viable HeLa cells (1 × 105 cells in 50 μL phosphate-buffered saline [PBS]) were incubated with biotinylated human PTX3 (50 μM) for 30 minutes at 4°C. DCs were incubated with biotinylated PTX3 for 30 minutes at 37°C. Bound PTX3 on cells was detected by subsequent incubation with streptavidin-FITC (Pharmingen) in PBS (final concentration 5 μg/mL) for 30 minutes at 4°C followed by flow cytometric analysis. Cells for confocal microscopy were prepared by incubating the cells with or without biotinylated PTX3 and stained as described for flow cytometric analysis. Cells were placed on polylysinated glass coverslips at room temperature for 45 minutes, washed with PBS containing calcium and magnesium, and fixed with 4% paraformaldehyde at room temperature for 15 minutes. Counterstaining for the nuclei was done by incubation with Hoechst 33342 (Molecular Probes, Eugene, OR) for 15 minutes. The coverslips were mounted on glass slides with Mowiol and preserved at 4°C until analysis. Images were visualized under a Leica TCS SP2 confocal microscope equipped with a 63 ×/1.4 oil-immersion objective lens (Leica, Heidelberg, Germany). Leica Confocal Software (LCS) was used to acquire images, and Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA) was used to process them.
Antigen presentation and cross-presentation assays
HLA-A2+ immature DCs (2 × 104) were incubated with 1 × 104 antigen-specific CD8+ T cells and 5 × 104 apoptotic (3T3 and 3T3-MelanA/MART1) cells per well in Iscoves modified Dulbecco medium (IMDM; Invitrogen) with 10% inactivated human serum and IL-2 for 24 hours. The synthetic peptide corresponding to residues 27-35 of the MelanA/MART1 antigen peptide (MT27-35; 3 μM) and PTX3 (100 μg/mL) was added. The supernatants were collected after 24 hours and assessed for IFN-γ by ELISA. For the vinculin-specific cross-priming, immature HLA-A2+ DCs (2.5 × 105 cells/well) were exposed to either apoptotic H9T cells (1.5 × 106 cells/well) or to synthetic vinculin823-831 peptide (100 μg/mL) in 24-well plates for 18 hours, in the presence or absence of PTX3 or CRP in different concentrations. Vinculin-specific CD8+ T-cell lines were tested in an ELIspot assay for the IFN-γ spot formation in response to a 24-hour stimulation with DCs (5 × 103 cells/well) that had been pulsed or not with apoptotic cells or vinculin peptide with or without PTX3/CRP. Denatured PTX3 was generated by heating at 56°C for 30 minutes and was used in the same concentrations as unmodified PTX3. When indicated, the effects of CRP (range tested 0-200 μg/mL) on the activation of vinculin-specific T cells were also evaluated. In the case of the HCV-NS3 protein cross-presentation, HLA-A2+ immature DCs were pulsed overnight with the indicated recombinant NS3Ag concentrations, in the presence or absence of PTX3. For analyzing cross-presentation of the antigen from apoptotic cells, HLA-A2+ DCs were pulsed overnight with the relevant peptide or apoptotic cells derived from either HLA-A2- VV-NS3-infected EBV-B cells (ratio 1:10), or their counterparts infected with WT-VV, in the presence or absence of PTX3. Then DCs (2 × 104 cells/well) were cocultured with HLA-A2-restricted NS31406-1415-specific CD8+ T-cell clone (2 × 104 cells/well). Cells were treated with Brefeldin-A (10 μg/mL; Sigma) after 2 hours and stained with tricolor (TC)-labeled anti-CD8 (Caltag Laboratories, Burlingame, CA). After a further 10 hours, cells were processed for intracellular detection of IFN-γ with FITC-labeled anti-IFN-γ (Becton Dickinson, BD Pharmingen), as previously described,56 and then analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA) using CellQuest software (Becton Dickinson).
Cytokines
The IL-10, IL-12, TNF-α, TGF-β, and IFN-γ ELISA kits were purchased from Duotech (R&D Systems, Minneapolis, MN) and the ELISAs were performed according to the manufacturer's instructions. The samples and standards were added in duplicates. For the TGF-β ELISA the samples were first treated as instructed in order to measure the active form of TGF-β.
Statistical analysis
Statistical analysis was performed using the 2-tailed Student t test for unpaired samples with unequal variance. P less than .05 was considered statistically significant.
Results
Apoptotic cells modulate the generation of PTX3 by maturing DCs and bind to PTX3
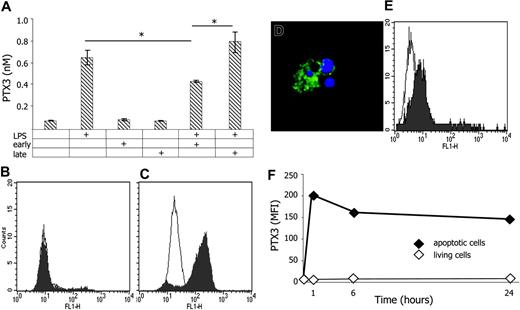
Monocyte-derived DCs challenged with endotoxin release PTX3 (Figure 1A). DCs also recognize specific moieties on dying cells, which influence their function.20-28 Neither cells in early apoptosis or in the later phases of the process (ie, after disruption of the plasma membrane) had any detectable effect on PTX3 generation by DCs (Figure 1A). In contrast, maturing DCs consistently produced significantly less PTX3 in the presence of early apoptotic cells. This effect was lost when late apoptotic cells were used (P < .01).
Once in the microenvironment, PTX3 anchors on both sides of the phagocytic synapse between DCs and apoptotic substrate. Viable immature DCs constitutively bind PTX3, as evaluated by flow cytometry (Figure 1E). This is a selective feature of DCs, since viable or early apoptotic monocytes, lymphocytes, neutrophils, or neoplastic cell lines (HeLa or Jurkat leukemia cells) did not associate to PTX3 (Figure 1B; not shown). However, they all acquired the ability to bind to PTX3 when they proceed through later phases of apoptosis (Figure 1C-D). PTX3 remained associated to late apoptotic cell domains over a 24-hour time span, even when the unbound soluble pentraxin was removed by repeated washings: estimated half residence time on the apoptotic plasma membrane longer than 36 hours (Figure 1F). Therefore when DCs are simultaneously exposed in vivo to microbes and apoptotic cells, PTX3 is generated as a third party that possibly modulates the interaction between the phagocyte and the apoptotic substrate.
PTX3 modulates the function of maturing DCs
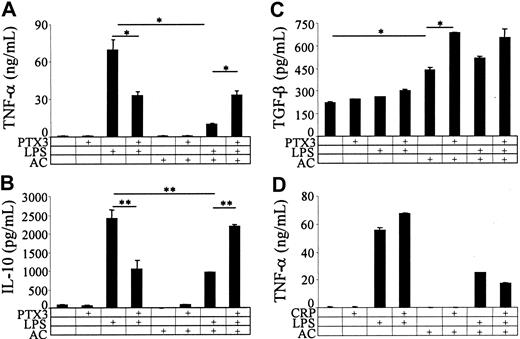
To verify whether PTX3 influences DCs maturation following challenge with bacterial moieties, we treated human DCs with LPS (10 ng/mL) and verified by ELISA the secretion of soluble factors (TNF-α, IL-10, and TGF-β) in the microenvironment and evaluated by flow cytometry the expression of molecules involved in T-cell activation/costimulation (CD86, MHC class I, and class II molecules). In parallel, we verified the effect of exogenous PTX3 and of apoptotic HeLa cells on these parameters. TLR4 activation by LPS resulted in the production of TNF-α and IL-10; TGF-β levels were not influenced (Figure 2A-C). In contrast, apoptotic cells per se induced the release of TGF-β by DCs (Figure 2C) and actively inhibited the secretion of TNF-α and IL-10 (Figure 2A-B) induced by LPS.
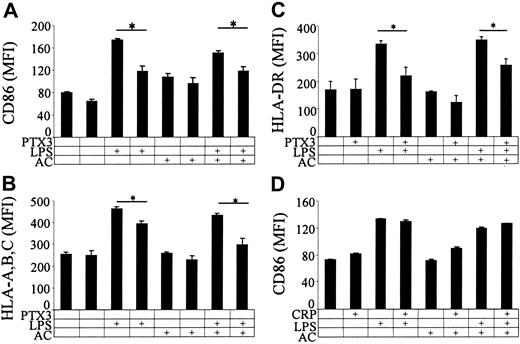
PTX3 per se did not exert any effect on immature DCs (Figure 2A-C). However, PTX3 inhibited the release of TNF-α and IL-10 by LPS-challenged DCs (Figure 2A-B). In contrast, PTX3 reversed the inhibition of TNF-α and IL-10 in the presence of apoptotic cells (Figure 2A-B). PTX3 also enhanced the production of TGF-β by DCs challenged with apoptotic cells (Figure 2C). The effect was specific, since the short pentraxin CRP did not elicit any change in the production of TNF-α (Figure 2D) or IL-10 (not shown) by stimulated DCs. In keeping with the effects on the secretory function (Figure 2A-D), PTX3 consistently inhibited the upregulation of membrane molecules (CD86, HLA-ABC, HLA-DR) induced by LPS (Figure 3A-C) in the presence or in the absence of apoptotic cells (Figure 3A-C). Also this parameter was unaffected by CRP (Figure 3D).
Apoptotic cells stably bind to PTX3 and influence its production by immature DCs. DCs challenged with LPS (10 ng/mL), early or late apoptotic cells, or a combination of these stimuli release PTX3 (y-axis; nM) in the microenvironment (A). Results representative of three independent experiments are depicted as mean ± SD of duplicate samples. *Statistically significant value; P < .05. Exogenous biotinylated PTX3 binds to late (C) but not to early (B) apoptotic Hela cells as evaluated by flow cytometry after addition of FITC-labeled streptavidin (filled profiles). The empty profiles indicate the fluorescence detected in the presence of the second-step reagent only. PTX3 binds to membrane domains of late apoptotic cells (green) and fails to recognize nuclear domains (DAPI, blue), as assessed by confocal scanning laser microscopy (D). Exogenous biotinylated PTX3 binds to viable immature DCs, as evaluated by flow cytometry after addition of FITC-labeled streptavidin (filled profile). The empty profile refers to the fluorescence in the presence of the second-step reagent only (E). PTX3 remains associated to apoptotic cells at different time points (x-axis, hours). Results are expressed as mean fluorescence intensity (MFI; y-axis) assessed by flow cytometry as described above on apoptotic (♦) or living (⋄) HeLa cells (F).
Apoptotic cells stably bind to PTX3 and influence its production by immature DCs. DCs challenged with LPS (10 ng/mL), early or late apoptotic cells, or a combination of these stimuli release PTX3 (y-axis; nM) in the microenvironment (A). Results representative of three independent experiments are depicted as mean ± SD of duplicate samples. *Statistically significant value; P < .05. Exogenous biotinylated PTX3 binds to late (C) but not to early (B) apoptotic Hela cells as evaluated by flow cytometry after addition of FITC-labeled streptavidin (filled profiles). The empty profiles indicate the fluorescence detected in the presence of the second-step reagent only. PTX3 binds to membrane domains of late apoptotic cells (green) and fails to recognize nuclear domains (DAPI, blue), as assessed by confocal scanning laser microscopy (D). Exogenous biotinylated PTX3 binds to viable immature DCs, as evaluated by flow cytometry after addition of FITC-labeled streptavidin (filled profile). The empty profile refers to the fluorescence in the presence of the second-step reagent only (E). PTX3 remains associated to apoptotic cells at different time points (x-axis, hours). Results are expressed as mean fluorescence intensity (MFI; y-axis) assessed by flow cytometry as described above on apoptotic (♦) or living (⋄) HeLa cells (F).
PTX3 inhibits cross-presentation of apoptotic-cell-derived antigens to CD8+ T cells
We assessed whether PTX3 influences the ability to cross-present antigens expressed by dying cells. We first focused on a characterized endogenous model antigen, the actin-based cytoskeleton component vinculin.25 Figure 4A shows that HLA-A2+ but not HLA-A2- DCs activated vinculin-specific CD8+ T cells with similar efficiency when loaded with the 823-831 synthetic vinculin peptide or with vinculin-expressing apoptotic cells. T-cell activation, as assessed evaluating the frequency of IFN-γ spot-forming CD8+ T cells upon cross-presentation, abated in the presence of PTX3 with a clear dose dependency (complete inhibition at around 60 μg/mL; Figure 4B). PTX3-elicited inhibition required conformational integrity, since denatured PTX3 failed to inhibit CD8+ T-cell activation (Figure 4B). In contrast, CRP did not influence the cross-presentation of vinculin after phagocytosis and processing of apoptotic cells (Figure 4D) or the direct presentation of the relevant synthetic sequence vinculin823-831 (Figure 4E).
PTX3 regulates the production of cytokines by maturing DCs. The ability of DCs to secrete TNF-α (y-axis; A), IL-10 (y-axis; B), and TGF-β (y-axis; C) was assessed in the presence of PTX3 (50 μg/mL), LPS (10 ng/mL), apoptotic cells (AC), or a combination of these stimuli. The effect of CRP (50 μg/mL) on TNF-α secretion (y-axis) by stimulated DCs in the presence or in the absence of apoptotic cells is also reported in panel D. *P < .05, **P < .01; significantly different from control. Results representative of three independent experiments are depicted as mean ± SD of duplicate samples.
PTX3 regulates the production of cytokines by maturing DCs. The ability of DCs to secrete TNF-α (y-axis; A), IL-10 (y-axis; B), and TGF-β (y-axis; C) was assessed in the presence of PTX3 (50 μg/mL), LPS (10 ng/mL), apoptotic cells (AC), or a combination of these stimuli. The effect of CRP (50 μg/mL) on TNF-α secretion (y-axis) by stimulated DCs in the presence or in the absence of apoptotic cells is also reported in panel D. *P < .05, **P < .01; significantly different from control. Results representative of three independent experiments are depicted as mean ± SD of duplicate samples.
PTX3 influences the membrane expression of CD86, MHC class I, and class II molecules by maturing DCs. The expression of CD86 (y-axis; A), MHC class I molecules (HLA-A, -B, -C; y-axis; B), and MHC class II molecules (HLA-DR; C) was assessed in the presence of PTX3 (50 μM), LPS (10 ng/mL), apoptotic cells, or a combination of these stimuli. The effect of CRP (50 μM) on CD86 expression (y-axis) in the presence or in the absence of apoptotic cells and LPS is also reported (D). *P < .01, significantly different from control. Results represent the mean ± SD of three independent experiments.
PTX3 influences the membrane expression of CD86, MHC class I, and class II molecules by maturing DCs. The expression of CD86 (y-axis; A), MHC class I molecules (HLA-A, -B, -C; y-axis; B), and MHC class II molecules (HLA-DR; C) was assessed in the presence of PTX3 (50 μM), LPS (10 ng/mL), apoptotic cells, or a combination of these stimuli. The effect of CRP (50 μM) on CD86 expression (y-axis) in the presence or in the absence of apoptotic cells and LPS is also reported (D). *P < .01, significantly different from control. Results represent the mean ± SD of three independent experiments.
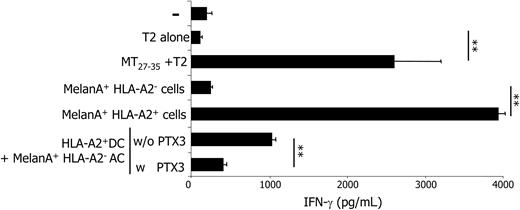
Vinculin is a ubiquitous antigen expressed by most mammalian cells. We decided to verify whether PTX3 influenced the cross-presentation of antigens with restricted tissue expression, like tumor or viral antigens. To this aim, we propagated CD8+ T cells specific for the tumor-associated antigen MelanA/MART1 from an HLA-A2+ donor. These cells secreted substantial amounts of IFN-γ when challenged with T2 cells pulsed with the relevant epitope (MelanA/MART127-35). MHC class I restriction was further demonstrated by the fact that HLA-A2+ but not HLA-A2- melanoma cell lines expressing the antigen were able to elicit IFN-γ secretion (Figure 5).
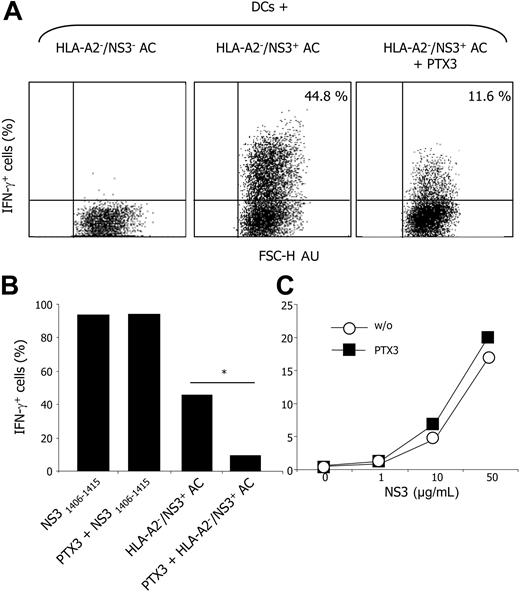
DCs efficiently activated MelanA/MART1-specific CD8+ T cells upon phagocytosis and processing of apoptotic murine fibroblasts expressing MelanA/MART1. This effect was specific since the processing of wild-type murine fibroblasts did not result in CD8+ T-cell activation (not shown). Moreover, MelanA/MART1+ mouse cells did not express the relevant restricting human MHC element, ruling out direct presentation. T-cell activation abated to background levels in the presence of PTX3 (Figure 5). We observed a similar inhibition in the cross-presentation of HCV-NS3-expressing apoptotic cells, but not in the presentation of the relevant NS31406-1415 peptide, in the presence of PTX3 (Figure 6). Cloned CD8+ T-cell activation was detectable when DCs had been pulsed with either VV-NS3-infected apoptotic cells or NS31406-1415 peptide but not when pulsed with control cells infected with WT-VV and committed to apoptosis. Again, cross-presentation was evident using HLA-A2- apoptotic cells, implicating processing and association of the produced epitopes with the MHC class I molecules of the phagocyte.55 Of importance PTX3 did not inhibit the presentation of the soluble viral antigen, internalized by macropinocytosis and presented by HLA-A2+ DCs, indicating that PTX3 does not influence the molecular machinery involved in the activation of T cells specific for soluble antigens.
PTX3 interferes with the cross-presentation of vinculin epitopes to autoreactive CD8+ T cells upon processing of apoptotic cells. Vinculin-specific, HLA-A2-restricted CD8+ T cells form IFN-γ spots (number/25 000; x-axis) when challenged with the specific synthetic epitope (vinculin823-831) or upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin (apoptotic cells [AC]; A). The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; y-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin were evaluated in the absence or the presence of native (▪) or denatured (□) PTX3 (μM; x-axis; B). Results are represented as mean ± SD of three independent experiments. The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; x-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin by HLA-A2- or HLA-A2+ DCs were evaluated. HLA-A2- DCs also failed to activate T cells when pulsed with the specific synthetic epitope (vinculin823-831; C). CRP (x-axis) did not influence the activation of vinculin-specific CD8+ T cells (IFN-γ spots number/25 000; y-axis) upon cross-presentation of apoptotic cells (D) or direct presentation of vinculin823-831 (E). **P < .001, significantly different from control.
PTX3 interferes with the cross-presentation of vinculin epitopes to autoreactive CD8+ T cells upon processing of apoptotic cells. Vinculin-specific, HLA-A2-restricted CD8+ T cells form IFN-γ spots (number/25 000; x-axis) when challenged with the specific synthetic epitope (vinculin823-831) or upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin (apoptotic cells [AC]; A). The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; y-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin were evaluated in the absence or the presence of native (▪) or denatured (□) PTX3 (μM; x-axis; B). Results are represented as mean ± SD of three independent experiments. The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; x-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin by HLA-A2- or HLA-A2+ DCs were evaluated. HLA-A2- DCs also failed to activate T cells when pulsed with the specific synthetic epitope (vinculin823-831; C). CRP (x-axis) did not influence the activation of vinculin-specific CD8+ T cells (IFN-γ spots number/25 000; y-axis) upon cross-presentation of apoptotic cells (D) or direct presentation of vinculin823-831 (E). **P < .001, significantly different from control.
PTX3 interferes with the cross-presentation of MelanA/MART1 to CD8+ T cells upon processing of apoptotic cells. MelanA/MART1-specific, HLA-A2-restricted CD8+ T cells secrete significant amounts of IFN-γ (x-axis; pg/mL) when challenged with T2 cells in the presence of the specific synthetic epitope (residues 27-35) or when challenged with HLA-A2+ (but not with HLA-A2- melanoma cell lines expressing the native antigen). Moreover, they secreted amounts of IFN-γ significantly higher than the background when challenged with DCs that phagocytosed and processed HLA-A2- apoptotic cells expressing MelanA/MART1; T-cell activation abated in the presence of PTX3 (50 μM). **P < .01, significantly different from control. Results are depicted as mean ± SD of samples analyzed in triplicate.
PTX3 interferes with the cross-presentation of MelanA/MART1 to CD8+ T cells upon processing of apoptotic cells. MelanA/MART1-specific, HLA-A2-restricted CD8+ T cells secrete significant amounts of IFN-γ (x-axis; pg/mL) when challenged with T2 cells in the presence of the specific synthetic epitope (residues 27-35) or when challenged with HLA-A2+ (but not with HLA-A2- melanoma cell lines expressing the native antigen). Moreover, they secreted amounts of IFN-γ significantly higher than the background when challenged with DCs that phagocytosed and processed HLA-A2- apoptotic cells expressing MelanA/MART1; T-cell activation abated in the presence of PTX3 (50 μM). **P < .01, significantly different from control. Results are depicted as mean ± SD of samples analyzed in triplicate.
Discussion
Most forms of tissue damage, infection, and neoplasia elicit a stereotypic response that comprises the synthesis of inflammatory factors both at hepatic and extrahepatic sites.3-57 Pentraxins in particular contribute to the response against microbes, sustaining the survival or enhancing the control of experimental infection with Streptococcus pneumoniae, Haemophilus influenzae, Salmonella enterica, Salmonella typhimurium, E coli, Plasmodium chabaudii, Pseudomonas aeruginosa, or Aspergillus fumigatus. However, in apparent contrast with this function, pentraxins behave as scavengers of cell debris and as immunosuppressants, limiting autoimmunity and leading in some cases to reversal of autoimmune-mediated tissue damage in vivo.3,47,48 The molecular mechanisms by which they accomplish these opposite functions (promoting protective immunity against pathogens while restricting noxious autoimmune responses) are not yet fully understood.
Pentraxins bind to cells in advanced apoptosis and to their by-products, which represent key antigens in systemic autoimmune diseases58 and an important substrate for immune responses against tumors or viruses that do not directly infect antigen-presenting cells.29 In this study we investigated whether the prototypic long pentraxin PTX3, which is generated in peripheral tissues under the control of primary proinflammatory stimuli,3 represents a third party regulating the interaction between dying cells and the most potent antigen-presenting cells, the DCs.
We found that this is indeed the case: PTX3 binds on one side specifically to the plasma membrane of maturing DCs (Figure 1E; Garlanda et al12 ). On the other it remains stably associated to the apoptotic membranes (Figure 1F) for the duration of the apoptotic program; postapoptotic debris only, which represent uneaten remnants of dying cells and are devoid of most cellular antigens,35 fail to bind to PTX3.38 This layer of PTX3 between phagocyte and prey influences the outcome of the interaction regulating the maturation program and the secretion of inflammatory factors, like TNF-α and IL-10, induced by environmental stimuli, like LPS (Figure 2). Finally, we observed that PTX3 edits the cross-presentation of epitopes expressed by apoptotic cells to autoreactive CD8+ T lymphocytes. This effect does not depend on the features of the antigen, since we obtained similar results with CD8+ T cells specific for different self, viral, and tumor-associated model antigens (vinculin, MelanA/MART1, and HCV-NS3; Figures 4, 5, 6). The effect is not only restricted to cross-presented antigens but also apparently quite selective, since the closely related short pentraxin CRP1-3 did not influence in the systems we used the cross-presentation of apoptotic cell-associated antigens or the events regulating DC maturation in the presence of dying cells (Figures 2, 3, 4). It is important to note that PTX3 did not influence the presentation of exogenous soluble antigens by DCs (Figure 6B), further highlighting the selective PTX3 capacity to constrain the apoptotic cell-DC interactions. Usually, particulate antigens, such as the cell-associated ones, are several-fold more efficiently cross-presented than soluble antigens.59 The efficient cross-presentation reported for bacteria, immune complexes, or apoptotic cells by DCs seems to be due to a more efficient phagocytic uptake of particulate antigens compared to fluid-phase uptake of soluble antigens or to phagosomes being more efficient than endosomes in exporting proteins into cytosol.60-64 T cells recognize antigen peptides bound to polymorphic MHC molecules. Both protective immune responses and autoimmunity stem from these interactions. Extensive cell death/tissue necrosis represents a challenge for the immune system, since autoantigens are released in a context in which antigen presentation is favored.65-67 The availability in situ of a censorship system induced by primary proinflammatory stimuli, which selectively hinders the activation of CD8+ T cells (most likely autoreactive) by cross-presentation of cell-associated antigens without affecting the inefficient possibly nondangerous cross-presentation of soluble antigens, could indeed prove an evolutionary advantage. Interestingly, patients with systemic autoimmunity display a remarkable defect in the generation of pentraxins during flares of the disease.39-68 The therapy of autoimmune diseases still largely relies on nonspecific immuno-suppression. A therapeutic role for pentraxins in human systemic autoimmune diseases has been proposed.47,48 Further studies are warranted to verify whether the effect we describe here can be exploited in vivo to limit pathogenic autoimmunity. Conversely, reversal of this effect may prove valuable for treatment of neoplastic diseases or chronic viral infections.
PTX3 interferes with the cross-presentation of HCV-NS3 epitopes to HCV-specific CD8+ T cells upon processing of apoptotic cells. DCs that phagocytosed and processed HLA-A2- apoptotic cells expressing the HCV-NS3 protein, but not DCs that phagocytosed control apoptotic cells, induced a brisk increase of IFN-γ production by NS31241-1260-specific, HLA-A2-restricted CD8+ T-cell clone, as evaluated by flow cytometry. This effect abated in the presence of exogenous PTX3 (50 μM). x-axis: forward scatter, FSC-H. Dot plots are gated on CD8+ cells and IFN-γ staining. Results are expressed as percentage of cells, as indicated in the top right quadrant. Results of 1 of 3 different experiments are shown (A). AU indicates arbitrary unit. Bars represent the fraction of antigen-specific CD8+ T cells expressing IFN-γ when challenged with DCs that had been pulsed with either HLA-A2- apoptotic cells expressing the NS3 antigen or the relevant NS31241-1260 peptide in the presence or in the absence of PTX3 (B). In contrast, no difference in the percentage of IFN-γ-positive cells (y-axis) was detectable when DCs presented the recombinant HCV-NS3 antigen (x-axis; μg/mL) in the absence (○) or in the presence (▪) of PTX3 (50 μM; C). *Statistically different from control.
PTX3 interferes with the cross-presentation of HCV-NS3 epitopes to HCV-specific CD8+ T cells upon processing of apoptotic cells. DCs that phagocytosed and processed HLA-A2- apoptotic cells expressing the HCV-NS3 protein, but not DCs that phagocytosed control apoptotic cells, induced a brisk increase of IFN-γ production by NS31241-1260-specific, HLA-A2-restricted CD8+ T-cell clone, as evaluated by flow cytometry. This effect abated in the presence of exogenous PTX3 (50 μM). x-axis: forward scatter, FSC-H. Dot plots are gated on CD8+ cells and IFN-γ staining. Results are expressed as percentage of cells, as indicated in the top right quadrant. Results of 1 of 3 different experiments are shown (A). AU indicates arbitrary unit. Bars represent the fraction of antigen-specific CD8+ T cells expressing IFN-γ when challenged with DCs that had been pulsed with either HLA-A2- apoptotic cells expressing the NS3 antigen or the relevant NS31241-1260 peptide in the presence or in the absence of PTX3 (B). In contrast, no difference in the percentage of IFN-γ-positive cells (y-axis) was detectable when DCs presented the recombinant HCV-NS3 antigen (x-axis; μg/mL) in the absence (○) or in the presence (▪) of PTX3 (50 μM; C). *Statistically different from control.
Prepublished online as Blood First Edition Paper, September 15, 2005; DOI 10.1182/blood-2005-03-1112.
Supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC; A.A.M, P.R.-Q., V.B., A.M., V.R.), the Fondazione Berlucchi (A.A.M.), the Ministero della Salute (A.A.M., V.B., A.M.), the European Community (EC) (Clearance of apoptotic cells: discovery of autoantigens and therapy for autoimmune diseases [APOCLEAR] project, P.R.-Q.; Dendritic Cells and Novel Immunotherapies [DC-THERA] and Integrated Functional Genomics in Mutant Mouse Models as Tools to Investigate the Complexity of Human Immunological Disease [MUGEN]; A.M.), and Telethon (A.M.).
P.B. designed and performed experiments and wrote the manuscript; A.P. designed and performed experiments; I.E.D. discussed the experimental strategy and performed experiments; P.R.-Q. supervised experiments and defined the experimental strategy; V.R. and G.P. provided vital reagents; R.F. and D.A. performed experiments; A.M. provided vital reagents and discussed the experimental strategy; V.B. defined the experimental strategy and supervised experiments; and A.A.M. defined the experimental strategy and wrote the manuscript.
P.B. and A.P. contributed equally to the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 4. PTX3 interferes with the cross-presentation of vinculin epitopes to autoreactive CD8+ T cells upon processing of apoptotic cells. Vinculin-specific, HLA-A2-restricted CD8+ T cells form IFN-γ spots (number/25 000; x-axis) when challenged with the specific synthetic epitope (vinculin823-831) or upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin (apoptotic cells [AC]; A). The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; y-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin were evaluated in the absence or the presence of native (▪) or denatured (□) PTX3 (μM; x-axis; B). Results are represented as mean ± SD of three independent experiments. The vinculin-specific CD8+ T cells forming IFN-γ spots (number/25 000; x-axis) upon phagocytosis and processing of HLA-A2- apoptotic cells expressing vinculin by HLA-A2- or HLA-A2+ DCs were evaluated. HLA-A2- DCs also failed to activate T cells when pulsed with the specific synthetic epitope (vinculin823-831; C). CRP (x-axis) did not influence the activation of vinculin-specific CD8+ T cells (IFN-γ spots number/25 000; y-axis) upon cross-presentation of apoptotic cells (D) or direct presentation of vinculin823-831 (E). **P < .001, significantly different from control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/1/10.1182_blood-2005-03-1112/4/m_zh80010688790004.jpeg?Expires=1769096395&Signature=vSPljcaRXdPFChq4kQFndMbYVBEH2MOA7B4NDj-iSLprGUqTGwJnVB5HBXS8IE12QzlkxKuIkTf5kkTt~KdrQ72Gl7lssQZDoHUqLQEnA2Rkv85XFneaQVkDBv7qDy0EP-tkq4NufD9eK1EG02fV3f6UKuWiT3ToFqeqtQmN6Ac4Zl5x98nsCR1IJeCI-ItHN7QJF5oN9lcD51~MC5AlicwFWomwJzbpqvyly~laqJSZnHmHvlPbxff8dhXRm9blP9Uy4P4x5JHzsGesYAlybKVzGoEI~zxKALDzla~wkgZ7Yi~B4Z2FTB7b8PqQU7jzagGYsaxA5s4-9TCoTaJd-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal