Abstract
We have shown previously that fibrin(ogen) binds fibroblast growth factor 2 (FGF-2) and potentiates stimulation of endothelial-cell (EC) proliferation. We have now used 2 FGF-2 mutants differing only in the 5 residues constituting the binding site to characterize the importance of this interaction in angiogenesis. The nonbinding (2212) and binding (221*2) mutants stimulated EC proliferation by 2.2 ± 0.4-fold and 2.9 ± 0.3-fold over control, respectively, and both were similar to wild-type (wt) FGF-2 (2.5 ± 0.3-fold). Proliferation was augmented by fibrinogen to 5.3 ± 1.2-fold and 4.8 ± 0.8-fold with wtFGF-2 and 221*2, whereas no augmentation occurred with 2212 and fibrinogen. Using a placental explant model in a fibrin matrix, wtFGF-2 resulted in 2.6 ± 0.9-fold more growth over control, and 221*2 increased growth 3.3 plus or minus 0.9-fold. Vessel outgrowth with 2212 was minimal and comparable to control. Similarly, fibrinogen potentiated wtFGF-2 or 221*2-mediated angiogenesis in the chicken chorioallantoic membrane model. In a mouse Matrigel implant model, fibrinogen significantly increased angiogenesis with either wtFGF-2 or 221*2, whereas there was no augmentation with 2212. These results demonstrate that binding of FGF-2 to fibrin(ogen) mediated by the 5-residue FGF-2-fibrin(ogen) interactive site is required for augmented angiogenesis.
Introduction
Angiogenesis is a complex process resulting in the development of new capillaries from pre-existing vessels and plays a critical role in a variety of pathologic conditions characterized by neovascularization including diabetic retinopathy, tumor growth, metastasis, and inflammatory diseases.1 Angiogenesis is also one aspect of the response to injury that requires a coordinated interaction of the hemostatic and inflammatory systems and is regulated by cytokines and growth factors that act locally to regulate cellular proliferation and tissue repair. In this response, the activation of the hemostatic system results in platelet accumulation at the site of injury, and exposure of blood to tissue factor also leads to the formation of thrombin. Thrombin then cleaves fibrinopeptides from fibrinogen converting it to fibrin, which helps prevent blood loss and also serves as a temporary matrix to support tissue healing and remodeling. The role of fibrin in the cellular response is not passive as a structural matrix only, but rather it plays an active role through specific receptor-mediated interactions with cells of the blood and vessel wall. These result in fibrin-specific responses of endothelial cells (ECs) including adhesion and spreading,2 proliferation,3 protein synthesis,4 and secretion.5
Cytokines and growth factors are produced in response to injury and also act locally to modulate cell responses to vascular damage. Important among these are members of the fibroblast growth factor (FGF) family that exert a variety of effects on many cells and organ systems.6 One member of this family, FGF-2, plays an important role in vascular responses by increasing EC proliferation,7 stimulating migration,8,9 and promoting angiogenesis.1,10 FGF-2 also increases secretion of collagenase,11 urokinase plasminogen activator,12 and urokinase plasminogen activator receptor13 from ECs, thereby facilitating proteolytic degradation of the extracellular matrix required for cell migration and angiogenesis. The role of FGF-2 in vessel injury and repair is further supported by evidence that FGF-2 is released from vessel wall cells after injury14 and that FGF-2 mRNA is up-regulated in atherosclerotic arteries15 and following vessel injury.16
The need for fibrin to support EC spreading, migration, and angiogenesis and the potent stimulation of the same responses by FGF-2 suggest that these processes may be interrelated. This concept is supported by evidence that fibrin clots are a good matrix for FGF-2-stimulated angiogenesis in vitro.17,18 We have shown previously that FGF-2 binds specifically and with high affinity to fibrinogen and fibrin and that fibrinogen potentiates the ability of FGF-2 to stimulate EC proliferation.19,20 We have localized the binding site for fibrinogen to 5 amino acid residues on FGF-2 including Phe95, Ser100, Asn102, Arg107, and Arg109. FGF-1/2 cassette interchange mutants have been expressed including 2212, which contains the third cassette from FGF-1 and exhibits no affinity for fibrinogen, and 221*2 with exchange of the 5 residues constituting the binding site from FGF-2 into the corresponding site in FGF-1 and conferring high-affinity binding.21 We have now used these mutants to characterize the importance of the FGF-2-fibrinogen interaction on angiogenesis stimulated by FGF-2.
Materials and methods
Cell culture
Primary ECs were obtained as described previously,22 seeded on 0.2% wt/vol gelatin-coated 25-cm2 tissue culture flasks, and cultured in McCoy 5A medium (Flow Laboratories, McLean, VA) containing 20% fetal bovine serum (FBS) and 50 μg/mL endothelial-cell growth supplement (ECGS; Collaborative Research, Bedford, MA), until they reached confluence within 2 to 3 days. The cells were passaged up to 2 times before use and then placed in suspension by trypsinization of monolayers. Cells were suspended by rinsing in Hanks balanced salt solution followed by brief incubation with trypsin-EDTA (Gibco Life Technologies, Grand Island, NY). The cells were pelleted by centrifugation for 10 minutes at 500g and resuspended in McCoy 5A medium in the absence of serum. This wash procedure was repeated twice prior to use in experimental protocols.
Protein preparation
Human fibrinogen was obtained from Enzyme Research Laboratories (South Bend, IN) and copurifying fibronectin was removed by gelatin-Sepharose chromatography. Residual fibronectin remaining after gelatin-Sepharose chromatography was further depleted by immunoaffinity chromatography as described elsewhere.2 The fibronectin concentration was determined by enzyme-linked immunosorbent assay (ELISA; American Diagnostica, Greenwich, CT) and represented less than 0.02% of the total protein. Vitronectin (VN) and gelatin were purchased from Sigma (St Louis, MO). FGF-1 and FGF-2 proteins were prepared by expressing the FGF-1 constructs into Escherichia coli strain BL21(DE3)plysS, and FGF-2 and FGF-2 mutant constructs into E coli strain BL21-codonplus, and protein was eluted from the His-tagged column as described previously.21
3H-thymidine incorporation
Approximately 2 × 104 ECs suspended in McCoy 5A medium supplemented with 20% FBS and 50 μg/mL ECGS were plated in gelatin-coated 12-well, non-tissue culture-treated plates (Becton Dickinson, Franklin Lakes, NJ) and allowed to adhere for 6 hours. The medium was then removed, and the cells were washed twice with serum-free McCoy 5A medium. Serum-free medium was then added containing 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN) and 1 μCi/mL (0.037 MBq) 3H-thymidine (New England Nuclear, Boston, MA). To this medium was added 25 ng/mL FGF-2 or FGF-2 mutants in the presence or absence of 100 μg/mL fibrinogen. After incubation at 37°C for 24 hours, nonadherent cells were removed by washing twice with ice-cold phosphate-buffered saline (PBS). To each well, 500 μL 10% ice-cold trichloroacetic acid (TCA) was added, and precipitates were collected on a filter using a manifold. Filters were washed twice with ice-cold 5% TCA, followed by 95% ethanol, allowed to air dry, and then suspended in scintillation fluid. Acid precipitable counts were quantitated using a scintillation counter.
Placental explant angiogenesis
A placental explant model was prepared as previously described.17 Briefly, superficial vessels were excised from the apical surface of human placentas within 24 hours of elective cesarean sections. The vessels were cut into 1- to 2-mm fragments using fine dissecting forceps and placed into Hanks balanced salt solution containing 2.5 μg/mL amphotericin (Gibco Life Technologies). Then 0.5 U/mL thrombin (Calbiochem-Novabiochem, La Jolla, CA) was added to each well of a 48-well culture plate, followed by 0.5 mL/well of 3 mg/mL fibrinogen in Medium 199 (Gibco Life Technologies). One vessel fragment was placed quickly in the center of the well before clot formation. After gel formation, 0.5 mL/well Medium 199 supplemented with 20% FBS, l-glutamine, gentamicin (Gibco Life Technologies), and amphotericin was added. The plates were incubated at 37°C for 18 days, and medium was changed every 2 days. New vessel growth was observed microscopically and photographed every second day using a Nikon digital camera with a Sigma 28-105 nm lens (Nikon, Tokyo, Japan). Vessel growth was quantified using Image-Pro Plus 3.0 (Media Cybernetics, Silver Spring, MD).
Chicken CAM angiogenesis
Eggs of 8-day-old chick embryos were opened with a round window that allowed direct access to underlying chorioallantoic membrane (CAM). A filter paper disk saturated with wtFGF-2 or FGF-2 mutants (200 ng/disk) in the presence or absence of fibrinogen (20 μg/disk) or an equal aliquot of PBS was applied to the top of CAM. After 72 hours of incubation, the disk was removed and vessel formation photographed using a digital camera (Nikon, Tokyo, Japan). After 72 hours of incubation, vessel growth was quantitated by imaging the CAM with a Spot camera (Diagnostic Instruments, Sterling Heights, MI) mounted on an Olympus Vanox microscope (Olympus, Shinjuku-ku, Tokyo, Japan) linked to a Dell computer (Dimension XPS H266 model) running Image Pro Plus 3. The vessel length was measured using the length tool. Data were collected into a Microsoft Excel spreadsheet for calculation of mean and variance.
Matrigel plug angiogenesis
Matrigel plug assays were performed as described previously with modification.23 Briefly, an aliquot (0.5 mL) of Matrigel supplemented with wtFGF-2 or FGF-2 mutants (500 ng/mL) in the presence or absence of fibrinogen (500 μg/mL) was injected subcutaneously into the ventral region of 4-week-old C57BL/6 female mice. After 10 days, Matrigel plugs were removed, fixed in 3% formalin, embedded in paraffin, sectioned, and stained with trichrome. Sections were photographed using an Olympus Vanox-T microscope equipped with a digital camera and a 10 ×/0.17 objective lens (Olympus, Tokyo, Japan). In a parallel experiment, Matrigel plugs were removed and separated from the skin, and the hemoglobin content of the plugs was measured by using a hemoglobin assay kit (Sigma) according to the manufacturer's instructions.24 Vessel growth was quantitated using Image Pro Plus 3.0 image analysis software. The animal studies protocol was approved by the University of Rochester Committee on Animal Resources.
Statistics
Results are expressed as mean ± SE. Statistical significance of differences in means between groups was determined using a 2-tailed Student t test or analysis of variance (ANOVA). P values less than or equal to .05 were considered significant for our analysis. All experiments were repeated at least 3 times, each in triplicate.
Results
We have shown previously that FGF-2 binds specifically and with high affinity to fibrinogen and fibrin19 and that fibrinogen potentiates the proliferative capacity of FGF-2.20 We expressed several FGF-2 mutants including 2212 that contained residues from FGF-1 and 221*2 with exchange of 5 residues (Phe95, Ser100, Asn102, Arg107, and Arg109) from FGF-2 into the corresponding sites of FGF-1. 2212 exhibited no affinity for fibrinogen, whereas 221*2 bound fibrinogen with comparable affinity to wtFGF-2, indicating that these residues represent an interactive site. To characterize the biologic activity of FGF-2 mutants in the presence or absence of fibrinogen, ECs were cultured on gelatin-coated wells in the presence of 25 ng/mL wtFGF-2 or FGF-2 mutants with or without 10 μg/mL fibrinogen in the medium, and proliferation was measured as 3H-thymidine incorporation (Figure 1A). Cell proliferation increased 2.2 ± 0.4-fold with 2212 and 2.9 ± 0.3-fold with 221*2 over medium alone (P < .05 for each), and this was similar to wtFGF-2 (2.5 ± 0.3-fold). In the presence of fibrinogen, EC proliferation was increased further to 5.3 ± 1.2-fold over medium alone with 221*2, and this was similar to wt FGF-2 (4.8 ± 0.8-fold (P < .05 for each). No further increase in proliferation was observed with 2212 in the presence of fibrinogen.
To determine the specificity of fibrinogen in potentiating the activity of FGF-2 or its mutants, ECs were incubated in the presence of 10 μg/mL fibrinogen, VN, or gelatin (10 μg/mL) with or without 25 ng/mL 2212, 221*2, or wtFGF-2, and proliferation was measured after 24 hours (Figure 1B). VN, gelatin, or fibrinogen had no effect on EC proliferation in the absence of growth factor, and proliferation was similar to medium alone. Fibrinogen potentiated the activity of 221*2, but VN and gelatin had no effect on the activity of 221*2 or 2212, indicating the specificity of fibrinogen in potentiating EC proliferation induced by either FGF-2 or 221*2.
EC proliferation in the presence of FGF-2 mutants. (A) ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma, 1 μCi (0.037 MBq) 3H-thymidine with 25 ng/mL of wtFGF-2 or FGF-2 mutants (2212 or 221*2) in the presence or absence of 100 μg/mL fibrinogen (FBG) for 24 hours. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. (B) To characterize specificity, the same experiment was conducted comparing the response with 100 μg/mL fibrinogen, gelatin (GEL), or vitronectin (VN). Neither gelatin nor vitronectin significantly increased the response to FGF-2. Results are the mean ± SD of 3 different experiments.
EC proliferation in the presence of FGF-2 mutants. (A) ECs were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma, 1 μCi (0.037 MBq) 3H-thymidine with 25 ng/mL of wtFGF-2 or FGF-2 mutants (2212 or 221*2) in the presence or absence of 100 μg/mL fibrinogen (FBG) for 24 hours. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. (B) To characterize specificity, the same experiment was conducted comparing the response with 100 μg/mL fibrinogen, gelatin (GEL), or vitronectin (VN). Neither gelatin nor vitronectin significantly increased the response to FGF-2. Results are the mean ± SD of 3 different experiments.
We investigated the role of this interaction physiologically using 3 models of angiogenesis. In the first model, small pieces of fresh placental vessel were embedded into a fibrin gel formed in serum, which was observed for new vessel outgrowth (Figure 2). During culture in medium alone, without added growth factor, little vessel growth occurred (Figure 2A), but addition of 5 ng/mL FGF-2 resulted in 2.6 ± 0.9-fold more growth over control (Figure 2B; P < .02). However, addition of 2212 did not support growth, which was comparable to control (1.1 ± 0.3-fold; Figure 2C). Vessel outgrowth was 3.3 ± 0.9-fold with 221*2 and was similar to wtFGF-2 (Figure 2D). These findings indicate that the ability of FGF-2 to bind fibrin is essential for formation of vascular structures in a fibrin matrix.
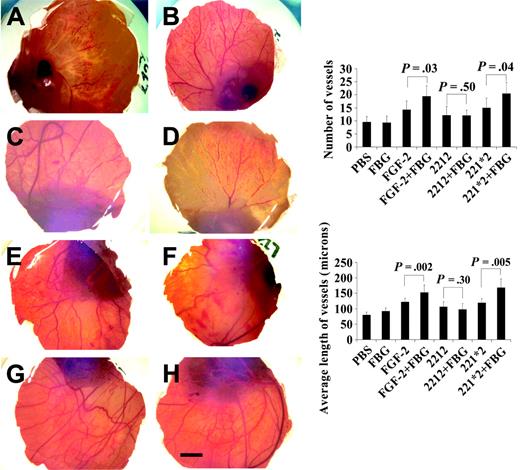
We extended these studies using the second model of angiogenesis on the chicken CAM with similar findings. Filter discs soaked with (200 ng/disc) wtFGF-2 or mutants, 2212 or 221*2, in the presence or absence (20 μg/disc) of fibrinogen were placed on 8-day-old CAM. After 72 hours, each CAM was fixed and photographed (Figure 3). Results showed greater new vessel growth with both wtFGF-2 and the mutant 221*2 in the presence of fibrinogen (Figure 3G-H) than in its absence (Figure 3C,E). No increase was observed with the nonbinding mutant 2212 and fibrinogen (Figure 3F) compared with 2212 alone (Figure 3D). Significantly greater vessel growth, measured as either the number of vessels or the average length, occurred with wtFGF-2 and with 221*2 in the presence of fibrinogen than in its absence (P < .05 for both). Vessel growth was similar with 2212 and fibrinogen as compared to 2212 alone.
Effect of FGF-2 mutants on new vessel formation in a placental explant model. The growth of vessels from human placental vessel fragments cultured in a fibrin gel with Medium 199 and 10% FBS for 18 days, in the presence or absence of FGF-2 or FGF-2 mutants is shown. (A) Control; (B) wtFGF-2; (C) fibrinogen nonbinding mutant (2212); (D) fibrinogen-binding mutant (221*2). Right panel: New vessel growth was quantified using image analysis software. Both wtFGF-2 and 221*2 supported significantly increased vessel growth over medium alone (P < .05 for both). The results are the mean ± SE of 3 separate experiments.
Effect of FGF-2 mutants on new vessel formation in a placental explant model. The growth of vessels from human placental vessel fragments cultured in a fibrin gel with Medium 199 and 10% FBS for 18 days, in the presence or absence of FGF-2 or FGF-2 mutants is shown. (A) Control; (B) wtFGF-2; (C) fibrinogen nonbinding mutant (2212); (D) fibrinogen-binding mutant (221*2). Right panel: New vessel growth was quantified using image analysis software. Both wtFGF-2 and 221*2 supported significantly increased vessel growth over medium alone (P < .05 for both). The results are the mean ± SE of 3 separate experiments.
Effect of fibrinogen, FGF-2, and FGF-2 mutants on new vessel formation in the chicken CAM model. Filter discs soaked in PBS (A), fibrinogen (FBG) (B), FGF-2 (C), non-fibrinogen-binding FGF-2 mutant 2212 (D), fibrinogen-binding FGF-2 mutant 221*2 (E), 2212 plus FBG (F), 221*2 plus FBG (G), and wtFGF-2 plus FBG (H). FGF-2 (200 ng/disc) and FBG (20 μg/disc) in a total volume of 20 μL were applied on 8-day-old CAMs. After 72 hours of incubation at 37°C, filters were removed, and each CAM was fixed and photographed. A CAM with a filter disc containing PBS without growth factors was used as control. Bar represents 100 μm. Right column: Quantitation of new vessel formation in the chicken CAM model. The number of new vessels and branches (top) and the average length of new vessels (bottom) were quantified using image analysis software. Seven embryos in each group were used. Data represent mean ± SE.
Effect of fibrinogen, FGF-2, and FGF-2 mutants on new vessel formation in the chicken CAM model. Filter discs soaked in PBS (A), fibrinogen (FBG) (B), FGF-2 (C), non-fibrinogen-binding FGF-2 mutant 2212 (D), fibrinogen-binding FGF-2 mutant 221*2 (E), 2212 plus FBG (F), 221*2 plus FBG (G), and wtFGF-2 plus FBG (H). FGF-2 (200 ng/disc) and FBG (20 μg/disc) in a total volume of 20 μL were applied on 8-day-old CAMs. After 72 hours of incubation at 37°C, filters were removed, and each CAM was fixed and photographed. A CAM with a filter disc containing PBS without growth factors was used as control. Bar represents 100 μm. Right column: Quantitation of new vessel formation in the chicken CAM model. The number of new vessels and branches (top) and the average length of new vessels (bottom) were quantified using image analysis software. Seven embryos in each group were used. Data represent mean ± SE.
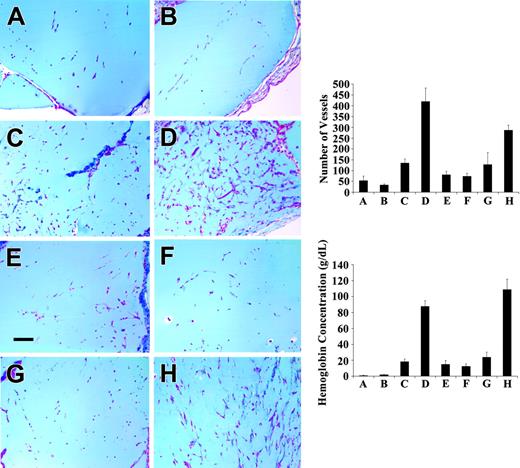
The third model examined the effect of fibrinogen binding on FGF-2-stimulated angiogenesis in vivo, using the Matrigel plug assay. New vessel growth was induced by wtFGF-2 or FGF-2 mutants, with and without fibrinogen mixed with Matrigel, which was injected subcutaneously in mice (Figure 4). After 10 days, FGF-2 (Figure 4C) induced a significantly greater angiogenic response than observed with controls (Figure 4A), or with fibrinogen alone (Figure 4B; P < .005 for both). However, fibrinogen and FGF-2 together had significantly more vessel growth (Figure 4D) than FGF-2 alone (Figure 4C; P < .001). Significantly greater vessel growth also occurred with the fibrinogen-binding mutant 221*2 in the presence of fibrinogen (Figure 4H) than in its absence (Figure 4G; P < .01). No increase in vessel growth was observed with 2212 and fibrinogen (Figure 4E) as compared to 2212 alone (Figure 4F). Growth of new vessels was also quantitated by measuring the hemoglobin content in the Matrigel implants (Figure 4 lower right panel). Hemoglobin reflects the amount of blood in the plug and provides an estimate of the extent of vascularization. There was significantly increased hemoglobin when fibrinogen was added to either wtFGF-2 or 221*2 (P < .001 for both). In contrast, no increase in hemoglobin content was observed when fibrinogen was added to 2212.
Effect of fibrinogen on FGF-2-induced angiogenesis in Matrigel plugs in C57-BL6 mice. Matrigel supplemented with PBS (A), fibrinogen (500 μg/mL) (B), wtFGF-2 (500 ng/mL) (C), FGF-2 and fibrinogen (D), 2212 (E), 2212 plus fibrinogen (F), 221*2 (G), or 221*2 plus fibrinogen (H) was injected subcutaneously into C57BL/6 female mice. After 10 days, the Matrigel plugs were removed, fixed in 3% formalin, embedded in paraffin, sectioned, and stained with trichrome. Bar represents 100 μm. Top right panel: vessel growth was quantitated by counting the number of new vessels and branches and using image analysis software. Ten mice were used in each group. Data represent mean ± SEM. Bottom right panel: The hemoglobin content in Matrigel plugs removed at 10 days was measured using Drabkin reagent. Hemoglobin reflects the amount of blood in the plugs and provides an estimate of the amount of vascularization. Results are shown as mean ± SE.
Effect of fibrinogen on FGF-2-induced angiogenesis in Matrigel plugs in C57-BL6 mice. Matrigel supplemented with PBS (A), fibrinogen (500 μg/mL) (B), wtFGF-2 (500 ng/mL) (C), FGF-2 and fibrinogen (D), 2212 (E), 2212 plus fibrinogen (F), 221*2 (G), or 221*2 plus fibrinogen (H) was injected subcutaneously into C57BL/6 female mice. After 10 days, the Matrigel plugs were removed, fixed in 3% formalin, embedded in paraffin, sectioned, and stained with trichrome. Bar represents 100 μm. Top right panel: vessel growth was quantitated by counting the number of new vessels and branches and using image analysis software. Ten mice were used in each group. Data represent mean ± SEM. Bottom right panel: The hemoglobin content in Matrigel plugs removed at 10 days was measured using Drabkin reagent. Hemoglobin reflects the amount of blood in the plugs and provides an estimate of the amount of vascularization. Results are shown as mean ± SE.
Discussion
The findings presented demonstrate the importance of the fibrin(ogen)-FGF-2 interaction for formation of vascular structures in a fibrin(ogen)-rich environment. The ability of wtFGF-2 and 2 mutants, 2212 and 221*2, to support EC proliferation was similar in the absence of fibrinogen. However, fibrinogen augmented the activity of wtFGF-2 and 221*2, but had no effect on 2212, which lacked the fibrin(ogen) interactive site.21 Similar results were found in 3 models of angiogenesis including an ex vivo placental explant model in fibrin, the chicken CAM, and the mouse Matrigel implant model. Notably, 2212 failed to support angiogenesis above control levels in the placental explant model, indicating that the high-affinity fibrin interaction is essential for vessel development in a fibrin matrix. In both the CAM and the Matrigel implant models, addition of fibrinogen to either wtFGF-2 or 221*2 significantly augmented the angiogenic response. There was no augmentation of activity with 2212 in the presence of fibrinogen. These findings link the hemostatic and matrix functions of fibrinogen with the EC regulatory activities of these growth factors. They are consistent with our previous reports that FGF-2 binds fibrin(ogen) with high affinity19 and that this interaction has functional significance by increasing EC proliferation,20 providing protection from proteolysis,25 increasing urokinase plasminogen activator (u-PA) activity26 and allowing prolonged EC growth on a fibrin matrix.27
We have used 3 different models of angiogenesis to evaluate the role of fibrinogen and FGF-2. The placental explant model is ex vivo, but it has the advantage of supporting vessel growth in a human fibrin matrix. Also, vessels derive from a human tissue explant. The chicken CAM model represents growth at an early stage of development, is technically simple, and allows accurate quantitation. The Matrigel plug model demonstrates vessel ingrowth in an intact mammal. It uses Matrigel, an artificial substrate, but allows accurate quantitative measurements of new vessel growth in Matrigel plugs and permits introduction of human fibrinogen into the gel.
EC responses to injury and angiogenesis are dependent on both growth factor stimulation and interactions with matrix components. Fibrinogen may serve as a matrix-binding site for FGF-2 because it is found in both normal and atherosclerotic arterial walls.28,29 Fibrin formation occurs following hemostatic activation at sites of vessel injury or inflammation, and it both contributes to the hemostatic plug and also provides a provisional matrix to support local cell responses. Binding of FGF-2 to fibrinogen may serve to localize activity, prevent proteolytic inactivation, and augment EC activation. Other interactions between matrix components and growth factors are also important in coordinating EC responses. For example, FGF-2 binds to extracellular matrix heparan sulfate proteoglycans30 through lower affinity interactions than those for fibrinogen binding,19 and the association with heparan sulfate proteoglycans is physiologically important in protecting FGF from proteolytic degradation31 and providing a local reservoir that can be released by enzymes that degrade proteoglycans. Additionally, heparan sulfate increases the binding affinity of FGFs for specific tyrosine kinase receptors and facilitates receptor activation, possibly through promoting dimerization.32
The mechanism of enhancement of FGF-2 activity by fibrin(ogen) may be related to alterations in receptor interactions. We have shown previously that antibodies to the fibrinogen receptor, αvβ3, completely block the activity of FGF-2 to stimulate EC proliferation in the presence of fibrinogen, but had no effect on the activity of free FGF-2.33 This may be due to colocalization of αvβ3 and FGFR1 when ECs are exposed to fibrinogen and FGF-2, suggesting a basis for coordinated activation and leading to an augmented response. Other examples demonstrate the importance of interactions between receptors for growth factors and integrins in modulating cellular activity. For example, VEGF up-regulates integrin expression on ECs, and surface-immobilized growth factor is recruited to cell-substratum contacts34 and modulates the cell-adhesive capacity of αvβ3.35 Fibronectin binds to and enhances the activity of VEGF.36 Also, αvβ3 associates with insulin and platelet-derived growth factor (PDGF) receptors and thereby potentiates their biologic activity.37 Ligand-mediated integrin clustering induces aggregation of FGFR38 and stimulates phosphorylation of PDGF-β receptors.39 FGFR and αvβ3 have been demonstrated to cooperate in EC signaling,40 and combined growth factor and integrin activation may be required for sustained cell activation.40 The effects on receptor activation and cell signaling of colocalized FGFR and αvβ3 in the presence of fibrin(ogen)-bound FGF-2 will require further investigation.
Defects in FGF-2-/- and in fibrinogen-/- mice may be due in part to abnormal angiogenesis. Vascular development appears to be normal in both, and tumor vessel structure is normal in fibrinogen-/- mice. However, wound healing is abnormal in both,41,42 and fibrinogen deficiency significantly diminishes the metastatic capacity of tumors.43 Both of these abnormalities are unexplained mechanistically and could be related to poor vessel growth. Previous reports also support a role for αvβ3 in angiogenesis. Thus, β3-/- mice appear to have enhanced angiogenesis,44 but inhibition of αvβ3 blocks angiogenesis in multiple models45-47 consistent with our findings.
The binding of FGF-2 to fibrin(ogen) has implications regarding the distribution and actions of FGF-2 within the vasculature. FGF-2 is present normally in plasma at a concentration up to 0.6 pM, and elevated levels up to 6 pM can be found in patients after cardiopulmonary bypass,48 chronic liver disease,49 sarcoma,50 and various carcinomas.51 At normal plasma concentrations of fibrinogen (7 μM) and FGF-2 (up to 6 pM) nearly all FGF-2 should be bound to fibrinogen considering the dissociation constants (Kds) in the nanomolar range.19 However, other FGF-2 binding proteins, α2-macroglobulin52 and soluble forms of FGFR,53 have also been identified in blood. The binding of FGF-2 to α2-macroglobulin involves formation of covalent bonds and is slow, requiring up to 4 hours to reach completion.52 Three soluble truncated forms of the high-affinity cell receptor FGFR1 have also been identified in plasma as binding proteins for FGF-2,53 but neither the plasma concentration nor binding affinities have been described. Fibrin formation at sites of vascular injury may contribute to localizing FGF-2 at these locations.
The potential to manipulate angiogenesis therapeutically is now being realized clinically, and new strategies to either inhibit or stimulate new vessel growth appear promising.54,55 The association of FGF-2 with fibrin(ogen) is relevant to the development of these therapeutic approaches. Because of its high plasma concentration, binding to fibrinogen will affect distribution of FGF-2 if administered systemically. Fibrin binding may also be important therapeutically. For example, in the successful initial trial in coronary artery disease,56 FGF-1 was injected locally near the site of vascular anastomosis where fibrin formation would be expected. Although FGF-1 does not bind fibrin, a similar approach using FGF-2 or another fibrin(ogen)-binding growth factor such as VEGF57 or IL-1β58 would serve to localize and possibly increase the local effect, contributing to neovascularization. Notably, VEGF also binds to fibrin(ogen) with high affinity and retains activity in the bound form.57 Inhibition of VEGF-stimulated angiogenesis is emerging as a clinically important approach to inhibiting angiogenesis in malignant disease.59 Fibrin deposition is a prominent feature in some malignancies60 and specific binding of VEGF to fibrin within the tumor may modify response to the inhibitor. The binding of FGF-2 to fibrin(ogen), and the demonstrated effects on EC proliferation and angiogenesis, suggest a new level of coordination between the hemostatic system and cell regulatory growth factors in the vascular response to injury. Binding of the growth factor to fibrin would serve to localize and possibly increase its local effect, contributing to neovascularization.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-06-2460.
Supported in part by grant HL-30616 from the National Heart Lung and Blood Institute, National Institutes of Health, and by a Grant-In-Aid from the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Jennifer Jodeksnis for expert technical assistance and Dr Lurong Zhang for helping with chicken CAM experiments. The assistance of Loel Turpin for Matrigel injections in mice and David Pasternack for quantitation of angiogenesis is acknowledged gratefully.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal