Abstract
Shp2 tyrosine phosphatase plays a critical role in hematopoiesis, and dominant active mutations have been detected in the human gene PTPN11, encoding Shp2, in child leukemia patients. We report here that although no such mutations were detected in 44 adult leukemia patients screened, Shp2 expression levels were significantly elevated in primary leukemia cells and leukemia cell lines, as compared with normal hematopoietic progenitor cells. The Shp2 protein amounts correlated well with the hyperproliferative capacity but were inversely associated with the differentiation degree of leukemia cells. Suppression of Shp2 expression induced apoptosis and inhibition of leukemic cell clonogenic growth. Notably, the majority of Shp2 was preferentially localized to the plasma membrane and was constitutively phosphorylated on tyrosine in leukemia cells, and also in normal hematopoietic cells following mitogenic stimulation. Based on these results, we propose that aberrantly increased expression of Shp2 may contribute, collaboratively with other factors, to leukemogenesis.
Introduction
The Src homology 2 (SH2) domain containing phosphotyrosine phosphatase 2 (Shp2), a ubiquitously expressed enzyme, plays a crucial role in normal hematopoietic cell development.1-3 In vitro hematopoietic differentiation assay showed a severe suppression of erythroid/myeloid progenitor cell development from homozygous mutant (Shp2Δ46-110) embryonic stem (ES) cells.1 Consistently, neither erythroid nor myeloid progenitor cells of Shp2Δ46-110 origin were detectable in the fetal liver or bone marrow of chimeric animals that were derived from aggregation of mutant ES cells and wild-type embryos, although a significant contribution of mutant cells was observed in a few other organs or tissue of the chimeras.2 Subsequent experiments using the Rag-2 (recombination activating protein-2)–deficient blastocyst complementation assay demonstrated a function of Shp2 for lymphopoiesis in a cell-autonomous manner, and differentiation of lymphoid cell lineages in Shp2–/–/Rag-2–/– chimeric mice was blocked before pro-T- and pro-B-cell stages.4 Together, these observations suggest a stringent requirement for a functional Shp2 in normal hematopoietic cell development in mammals.
Upon stimulation of factor-dependent cell lines with interleukin-6 (IL-6), leukemia inhibitory factor (LIF), IL-3/granulocyte macrophage–colony-stimulating factor (GM-CSF), or erythropoietin (Epo), Shp2 rapidly becomes tyrosine-phosphorylated.5,6 Cells expressing a mutant gp130 that results in elimination of tyrosine phosphorylation fail to proliferate upon ligand stimulation.7 Shp2 appears to play a positive role in activation of Akt and Erk signaling pathways, which promote cell proliferation/survival and block cell apoptosis, critical events in tumorigenesis.8-10 Functional analysis suggested that Shp2 may act in both catalytic-dependent and -independent manners in mediating IL-3–stimulated proliferation and survival of hematopoietic cells.11 Several studies have also implicated Shp2 involvement in BCR/ABL-mediated signaling,12,13 which has proven to play a key role in leukemogenesis of Philadelphia chromosome–positive (Ph+) leukemia.14,15
More recently, several groups have reported detection of somatic mutations in the gene PTPN11 that encodes human Shp2 in juvenile and childhood leukemias.16-18 Mutations in PTPN11 were first identified in patients with Noonan syndrome (NS),19 a developmental disorder characterized by cardiac and skeletal defects and also associated with hematologic disorders, including juvenile myelomonocytic leukemia (JMML). The same group detected germ line mutations in PTPN11 in patients with Noonan syndrome and JMML, as well as somatic PTPN11 mutations in 34% nonsyndromic JMML and also in a small percentage of patients with myelodysplastic syndrome (MDS) and de novo acute myeloid leukemia (AML).16 Another group found missense mutations in PTPN11 in 16 of 49 JMML samples from patients without NS, with much lower frequency in other myeloid malignancies.18 A knock-in mouse model was established that expresses a Noonan syndrome–associated mutant D61G. Whereas homozygous D61G mutants are embryonic lethal, heterozygotes recapitulate many aspects of human Noonan syndrome, such as short stature and craniofacial abnormalities.20 In particular, PTPN11D61G/+ mutant mice develop mild myeloproliferative disorders and splenomegaly.20 Shp2 mutants specifically associated with sporadic leukemias are potent in transforming bone marrow cells in mice and Drosophila.21
Here, we demonstrate that the Shp2 enzyme is overexpressed in primary leukemia cells and also in leukemia cell lines, although no dominant active mutations were detected in the PTPN11 gene in 44 cases of adult leukemia. Suppression of Shp2 expression induced apoptosis and growth inhibition of leukemic clonogenic cells. Our results thus provide novel insight into the potential role of Shp2 in leukemogenesis, and also suggest that suppression of excessive Shp2 expression or activity may be a novel therapeutic strategy for patients with adult leukemia.
Patients, materials, and methods
Primary leukemia samples and human leukemia cell lines
A total of 52 adult cases (patient age range, 16-69 years) of various leukemias, including 20 AML (4 M1, 6 M2, 2 M3, 1 M4, 7 M5), 18 chronic myeloid leukemia (CML), 12 acute lymphoblastic leukemia (ALL), 1 chronic lymphocytic leukemia (CLL), and 1 acute mixed lineage leukemia based on the French-American-British (FAB) classification, were analyzed in this study. Primary leukemia cell specimens were obtained from patients before any treatment, and normal hematopoietic tissues (including bone marrows and peripheral blood mononuclear cells [PBMNCs]) were obtained from healthy donors, after informed consent. Mononuclear cells (MNCs) were purified by Ficoll-Hypaque density-gradient centrifugation. The remaining erythrocytes in the neutrophil pellet were removed by hydrolysis. MNCs and neutrophils were counted, and viability was determined by trypan blue exclusion. Additionally, cytospins were performed for morphologic analysis of the cells. Purified MNCs and neutrophils were used immediately for isolation of protein and RNA. A panel of human leukemia cell lines was used that included Ku812 (CML), HL-60 (AML-M2), NB4 (AML-M3), U937, U937 (AML-M5), K562 (AML-M6), and Jurkat (ALL). All these leukemia cell lines were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. This study was conducted under the approval of the institutional review board of Zhejiang University.
Mutation screening of Shp2 in leukemia samples
To detect any possible mutations in PTPN11 in leukemia specimens, we directly sequenced the cDNA sequence of the entire coding region of Shp2 in primary leukemia cell samples from 44 adult leukemia cases using reverse transcriptase–polymerase chain reaction (RT-PCR) and PE 377 DNA sequencer.
Immunofluorescent staining and immunoblotting analysis
Hematopoietic cells were fixed in freshly prepared 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 minutes at room temperature and then permeabilized with 0.2% Triton X-100 in PBS for 10 minutes. After several washes with PBS, nonspecific binding of antibodies was blocked for 30 minutes at 37°C with PBS, 2% bovine serum albumin (BSA), 5% normal goat serum (NGS). Slides were then incubated for overnight at 4°C with primary antibodies, then washed 3 times in PBS and reacted with fluorescein isothiocyanate (FITC)–conjugated anti–rabbit immunoglobulin G (IgG; Pierce, Rockford, IL) and Rhodamine-conjugated anti–mouse IgG (Pierce), diluted in PBS, 2% BSA, 5% NGS for 1 hour at 37°C. Samples were subsequently washed 3 times in PBS, and mounted in 20 mM Tris-HCl, pH 8.2, 90% glycerol. Slides were observed and photographed using a Zeiss Axiophot epifluorscence microscope (Oberkochen, Germany).
For immunoblot analysis, cell specimens were washed twice with PBS buffer and total cellular protein was extracted (1 × 106 cells/mL) using Mammalian Protein Extraction Reagent (M-PER; Pierce), according to the instruction manual. Membrane and cytosolic fractions of hematopoietic cells were prepared as previously described,22 and nucleus fraction was prepared according to a published method.23 Cell extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. (SDS-PAGE; 12% polyacrylamide gels), and then transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA) and reacted with primary antibodies overnight at 4°C. After washing 3 times with PBS–Tween 20 (PBS-T), the membranes were probed with a horseradish peroxidase–conjugated secondary antibody for 2 hours at room temperature, and reacted with SuperSignal West Pico Chemiluminescent Substrate (Pierce). Polyclonal anti-Shp2 antibody (raised a GST [glutathione S-transferase] fusion protein containing Syp residues 2-216) was described previously.24 Anti-phosphotyrosine antibody p-Tyr (PY99) was from Santa Cruz Biotechnology (Santa Cruz, CA); anti–β-actin antibody and horseradish peroxidase–conjugated secondary antibody were from Sigma (St Louis, MO).
Assessment of Shp2 mRNA level
Briefly, cellular total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), and then 1 μg RNA was reverse-transcribed with oligo dT15 by Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Madison, WI) at 37°C for 1 hour. Thermal cycle conditions for Shp2 were as follows: 95°C for 4 minutes and cycling for 40 cycles between 95°C for 30 seconds and 57°C for 20 seconds, 72°C for 1 minute 30 seconds, 72°C for 5 minutes. Amplified product for Shp2 is 1782 bp in size. β-actin cDNA was used as internal control. Thermal cycle conditions were as follows: 95°C for 4 minutes and cycling for 26 cycles between 95°C for 20 seconds and 57°C for 15 seconds, 72°C for 1 minute 45 seconds, 72°C for 5 minutes. Amplified product for actin was 750 bp in size. Results were collected and analyzed by an ABI Prism 377 Sequence Detection System (PE Applied Biosystems, Foster City, CA). The primer sequences for Shp2 were as follows: Shp2 forward: 5′-ATG ACA TCG CGG AGA TGG TT-3′; reverse primer: 5′-TCA TCT GAA ACT TTT CTG CTG T-3′. PCR primers for β-actin were as follows: forward primer: 5′-CTT CAA CAC CCC AGC CAT GTA-3′, reverse primer: 5′-TAG AAG CAT TTG CGG TGG ACG-3′.
Analysis of leukemia cell differentiation and proliferation
For induction of cellular differentiation, NB4 leukemia cells (0.5 × 106 cells/mL) were cultured in the presence of all-trans retinoic acid (ATRA) at 1 μM for up to 96 hours or Shp2-specific antisense oligos at 2 μM for up to 72 hours. Cells were harvested at different time points; live cells were counted by trypan blue exclusion, and the morphologic characteristics were evaluated under a light microscope after staining with Wright-Giemsa staining. Differentiation status and cell cycles of NB4 cells were assessed by measuring expression of CD11b antigen and cell cycles using flow cytometry (FCM).
PBMNC samples were cultured in RPMI-1640 with 10% FBS, and stimulated with phytohemagglutinin (PHA) at 10 μg/mL for 72 hours. The cultured cells were collected at different time points for analyses of expression levels of Shp2 protein, distribution of Shp2 in cells, and Ki-67 antigen, which is used as a marker of cell proliferation.
Growth inhibition assay
Sense S-oligonucleotides were synthesized to nucleotides 1-18 of the Shp2 mRNA (5′-atgacatcgcggagatgg-3′) and antisense S-oligonucleotides were synthesized to the complementary strand (5′-ccatctccgcgatgtcat-3′). Leukemic cells were plated at 0.2 × 103 cells per well in a 24-well culture plate in 0.5% of soft agar. After 24 hours, cells were incubated with Shp2 sense or antisense S-oligonucleotides and the FuGENE 6 Transfection Reagent (Roche, Indianapolis, IN) at different concentrations according to the instruction manual. After incubation for 7 days, colonies (> 40 cells) were counted using an inverted microscope. Each experiment was performed at least 3 times. The results were expressed as the mean percentage of clonal growth in plates.
One day before transfection, 1 × 105 leukemia cells/well were plated in 6-well tissue-culture plates. Leukemia cells were incubated with Shp2 sense or antisense S-oligonucleotides and the FuGENE 6 Transfection Reagent (Roche) at different concentrations according to the instruction manual. After culturing for 72 hours, leukemia cells were collected for analysis of apoptosis using annexin v with FCM, Shp2 expression with Western blot using a Shp2 antibody.
Statistical analysis
Results were expressed as means plus or minus the standard deviation (SD). All statistical analyses were made with a 2-sided Student t test. P values below .05 were considered to be statistically significant.
Results
There is no prevalence of mutations in PTPN11 in patients with adult leukemia
Somatic PTPN11 mutations were found in approximately 35% of JMML cases, 10% of childhood myelodysplasic syndromes, and at a lower incidence in other childhood hematopoietic disorders.16-18,25 We performed mutational analysis on PTPN11 for a total of 44 patients with adult leukemia (aged from 17 to 88 years), including 16 cases of primary AML (4 M1, 3 M2, 2 M3, 1 M4, 6 M5), 15 cases of primary CML (12 CML at chronic phase, 3 CML at blast crisis), and 13 cases of primary ALL. In these 44 adult leukemia samples, we did not detect any of the reported gain of function mutations in PTPN11, but found one nucleotide change: A1205C, predicting K402T, in one adult AML-M5 case (data not shown). In addition, we identified a silent nucleotide change in one adult patient with CML at blast crisis (data not shown). These data are consistent with 2 recent reports describing the uncommon PTPN11 mutations in adult leukemia samples.26,27 Johan and colleagues26 screened 64 cases of AML and only found a single missense mutation, and Watkins et al27 failed to detect any PTPN11 mutations in 96 cases of adult MDS, MDS-AML, and primary AML.
Altered expression, subcellular localization, tyrosine phosphorylation of Shp2 in human leukemia cell lines. (A) Cell lysates (20 μg total proteins) were analyzed by Western blot with antibodies against Shp2 or β-actin, as a control for equal sample loading. (B) For normal hematopoietic cell samples (PBMNCs), 30 μg total proteins were loaded for Western blot analysis for Shp2 and β-actin, while 5 μg total proteins from NB4 leukemia cell lysate was used as a positive control for the 2 Shp2 bands and for comparison of the relative Shp2 contents. (C) The histogram shows p-Shp2 protein levels normalized against the actin expression in leukemia cell lines and normal PBMNC samples. Data are representative of 3 independent experiments. (D) Leukemic cells were lysed in hypotonic buffer, and lysates were used to prepare total cellular protein (T), cytosolic (C), membrane-soluble (M), and nucleus (N) fractions as described in the text. An aliquot of each fraction (20 μg total proteins) was subjected to immunoblot analysis for Shp2. (E) Proteins were immunoprecipitated from each fraction with an anti-Shp2 antibody and subjected to immunoblot analysis with an antiphosphotyrosine (pTyr) antibody. IP indicates immunoprecipitation. Data are representative of 2 independent experiments.
Altered expression, subcellular localization, tyrosine phosphorylation of Shp2 in human leukemia cell lines. (A) Cell lysates (20 μg total proteins) were analyzed by Western blot with antibodies against Shp2 or β-actin, as a control for equal sample loading. (B) For normal hematopoietic cell samples (PBMNCs), 30 μg total proteins were loaded for Western blot analysis for Shp2 and β-actin, while 5 μg total proteins from NB4 leukemia cell lysate was used as a positive control for the 2 Shp2 bands and for comparison of the relative Shp2 contents. (C) The histogram shows p-Shp2 protein levels normalized against the actin expression in leukemia cell lines and normal PBMNC samples. Data are representative of 3 independent experiments. (D) Leukemic cells were lysed in hypotonic buffer, and lysates were used to prepare total cellular protein (T), cytosolic (C), membrane-soluble (M), and nucleus (N) fractions as described in the text. An aliquot of each fraction (20 μg total proteins) was subjected to immunoblot analysis for Shp2. (E) Proteins were immunoprecipitated from each fraction with an anti-Shp2 antibody and subjected to immunoblot analysis with an antiphosphotyrosine (pTyr) antibody. IP indicates immunoprecipitation. Data are representative of 2 independent experiments.
Shp2 is highly expressed in most leukemia cases
We next investigated whether the Shp2 protein expression is changed in various human leukemia cell lines or primary leukemia cells. Using a specific anti-Shp2 antibody for immunoblot analysis, we detected 2 bands for Shp2 in several human leukemia cell lines and normal hematopoietic mononuclear cells (Figure 1). Interestingly, the upper band was preferentially detected in human leukemia cell lines, whereas the lower band was observed in both leukemic and normal hematopoietic samples. We investigated the subcellular localization of the 2 Shp2 isoforms in leukemia cells. Solubilized-membrane, cytosolic, and nuclear fractions were prepared from leukemia blasts and subjected to Western blotting with the anti-Shp2 antibody. As shown in Figure 1D, Shp2 in the upper band was distributed to the membrane and nuclear fractions, whereas the lower one was present in the cytosolic fraction. Furthermore, we demonstrated that the upper band represented tyrosine-phosphorylated Shp2 (p-Shp2) using an anti-phosphotyrosine antibody for Western blotting following immunoprecipitation with an anti-Shp2 antibody (Figure 1E).
We screened a panel of primary leukemia samples, and increased levels of p-Shp2 protein were detected in nearly all of these samples from various subtypes of leukemias, as compared with normal hematopoietic cells (Figure 2). The mean ratios of p-Shp2 to β-actin in primary leukemia samples (n = 36), normal bone marrow (BM; n = 8), and peripheral blood (PB) hematopoietic samples (n = 12) were 1.23 ± 0.74, 0.163 ± 0.088, and 0.034 ± 0.045, respectively. There was no significant difference in expression levels of p-Shp2 protein among various subtypes of leukemia (Figure 2B). Interestingly, p-Shp2 was barely detectable in 60% (6/10) of PBMNC samples and all terminally differentiated neutrophil samples tested (n = 12, data not shown), suggesting that Shp2 is not tyrosine-phosphorylated and normally located in the cytosol in resting and terminally differentiated hematopoietic cells.
The dramatically increased levels of Shp2 in leukemia samples might be due to increased expression and/or decreased degradation of the protein. We assessed Shp2 mRNA expression levels in primary leukemia specimens using semiquantitative RT-PCR with specific oligonucleotide primers. Consistent with the protein expression profile of Shp2, high levels of Shp2 mRNA were detected in all (26/26) primary leukemia specimens tested, and the mean ratio of Shp2 to β-actin was 1.242 ± 1.057. However, Shp2 mRNA was detected only in 40% (10/25) of normal PBMNC samples at low levels under the same condition and its mean ratio of Shp2 to β-actin was 0.046 ± 0.076 (Figure 2). In addition, Shp2 mRNA was barely detectable in terminally differentiated neutrophils. These results suggest that elevated levels of Shp2 protein in leukemia cells are likely due to enhanced Shp2 gene expression.
Increased Shp2 expression in primary leukemia cells. (A) A representative Western blot result of partial leukemia cases. (B) Statistical analysis of all cases tested. For each sample, 20 μg cellular proteins was analyzed by Western blot for Shp2 and β-actin. Results were expressed as the ratios of Shp2 to β-actin, and the mean value in each group was indicated. (C) Total RNA was isolated from fresh leukemia cell samples from patients at diagnosis, and analyzed by RT-PCR using Shp2-specific primers described in the text. β-actin cDNA primers were used as internal control. PCR products were electrophoresed on 1% agarose gel stained with ethidium bromide. (D) Results were expressed as the ratios of Shp2 to β-actin. Horizontal lines in panels B and D represent means.
Increased Shp2 expression in primary leukemia cells. (A) A representative Western blot result of partial leukemia cases. (B) Statistical analysis of all cases tested. For each sample, 20 μg cellular proteins was analyzed by Western blot for Shp2 and β-actin. Results were expressed as the ratios of Shp2 to β-actin, and the mean value in each group was indicated. (C) Total RNA was isolated from fresh leukemia cell samples from patients at diagnosis, and analyzed by RT-PCR using Shp2-specific primers described in the text. β-actin cDNA primers were used as internal control. PCR products were electrophoresed on 1% agarose gel stained with ethidium bromide. (D) Results were expressed as the ratios of Shp2 to β-actin. Horizontal lines in panels B and D represent means.
Altered Shp2 expression and localization is associated with cell proliferation
To investigate whether there is a correlation between membrane localization/tyrosine phosphorylation of Shp2 and cell proliferation, we examined Shp2 expression and subcellular localization in hematopoietic cells under resting and rapidly proliferating status. Unstimulated and PHA-stimulated PBMNC samples from healthy donors were used as resting and proliferating hematopoietic cell samples, respectively. Approximately 95% of cells in unstimulated PBMNC samples were quiescent, and up to 50% of cells underwent proliferation in PBMNC samples 72 hours after stimulation with PHA at 10 μg/mL (data not shown). As shown in Figure 3, the level of membrane/nuclear Shp2 protein only accounted for about 20% of total Shp2 protein, and 80% of Shp2 protein was in the cytosol in resting hematopoietic cells. However, once the resting cells at G0/G1 phase entered into proliferating phase, membrane/nuclear Shp2 protein markedly increased to approximately 80% of the total, with a concomitant decrease of cytosolic Shp2 protein to about 20%, suggesting that Shp2 is redistributed to the plasma membrane and nucleus from the cytosol in proliferating cells.
To determine the distribution pattern and expression level of Shp2 protein at different cell-cycle stages of leukemia cells, we performed double immunofluorescence labeling of leukemia cells with anti-Shp2 antibody and monoclonal antibody Ki-67. The Ki-67 antigen has been widely used as a marker of cell proliferation because of its presence in proliferating cells in all phases of the cell division cycle and absence in nonproliferating cells.28 In addition, the Ki-67 antigen has also been used to distinguish different cell-cycle phases based on its distribution pattern in various cell-cycle phases.29 Interestingly, Shp2 protein expression level greatly differed in the cells at different phases of the cell cycle. In nonproliferating cells, Shp2 protein is diffusively distributed in the entire cytoplasm at low level (Figure 4A). However, once cells enter proliferating status, Shp2 protein expression level dramatically increased with cell-cycle progression, peaked in the mitotic cells at prophase, and then decreased after metaphase (Figure 4B). In proliferating cells at S/G2 phase, membrane-associated Shp2 was detected at a high level, but no nuclear Shp2 was observed.
Mitogenic stimulation of Shp2 phosphorylation and relocation. Normal resting hematopoietic cells were stimulated with PHA and collected at different time points for analysis of Shp2 protein levels by Western blotting. The histogram shows p-Shp2 (▦) versus Shp2 (□) contents in samples enriched with resting or proliferating hematopoietic cells. Data are representative of 3 independent experiments.
Mitogenic stimulation of Shp2 phosphorylation and relocation. Normal resting hematopoietic cells were stimulated with PHA and collected at different time points for analysis of Shp2 protein levels by Western blotting. The histogram shows p-Shp2 (▦) versus Shp2 (□) contents in samples enriched with resting or proliferating hematopoietic cells. Data are representative of 3 independent experiments.
Overexpression of Shp2 correlates with the hyperproliferative phenotype of patients with leukemia
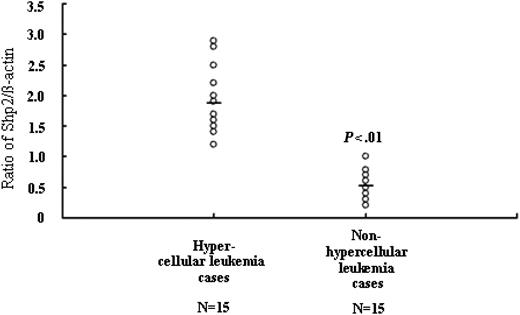
To determine whether overexpression of p-Shp2 in leukemia samples is associated with the hyperproliferative phenotype of leukemia, we analyzed the expression levels of p-Shp2 in relation with the bone marrow cellularity in various leukemia cases. As shown in Figure 5, p-Shp2 protein expression level in patients with leukemia with hyperplastic bone marrow were much higher than those with hypoplastic bone marrow, suggesting that the expression levels of p-Shp2 protein positively correlated with the malignant proliferative potential of the bone marrow in patients with leukemia (Figure 5).
Subcellular localization of Shp2 in leukemia cells during cell cycle. (A) Cells at interphase. (B) Cells at mitosis. Double immunofluorescence labeling of Shp2 with Ki-67 antigen was performed in quiescent and proliferating cells. Cells were fixed, permeabilized, and stained with anti-Shp2 (green) and anti–Ki-67 antigen (red) antibodies. The scale bar represents 10 μm. Similar results were also found in lymphoblastic leukemic cells and normal hematopoietic progenitor cells (data not shown). Images were viewed at 400 ×/0.75 numerical aperture (NA), with a Zeiss Axioskop II microscope, Images were captured with a SPOT 1.3.0 CCD camera (Diagnostic Instruments, Detroit, MI) and transferred to Adobe Photoshop 6.0 (Adobe, San Jose, CA).
Subcellular localization of Shp2 in leukemia cells during cell cycle. (A) Cells at interphase. (B) Cells at mitosis. Double immunofluorescence labeling of Shp2 with Ki-67 antigen was performed in quiescent and proliferating cells. Cells were fixed, permeabilized, and stained with anti-Shp2 (green) and anti–Ki-67 antigen (red) antibodies. The scale bar represents 10 μm. Similar results were also found in lymphoblastic leukemic cells and normal hematopoietic progenitor cells (data not shown). Images were viewed at 400 ×/0.75 numerical aperture (NA), with a Zeiss Axioskop II microscope, Images were captured with a SPOT 1.3.0 CCD camera (Diagnostic Instruments, Detroit, MI) and transferred to Adobe Photoshop 6.0 (Adobe, San Jose, CA).
Relationship between p-Shp2 levels and bone marrow cellularity in leukemia. For each sample, 20 μg cellular proteins was analyzed by Western blot with anti-Shp2 and β-actin antibodies. Results were expressed as Shp2/β-actin ratios, and the mean values in hypercellular leukemia and nonhypercellular leukemia cases were 1.873 ± 0.527 and 0.525 ± 0.262, respectively.
Relationship between p-Shp2 levels and bone marrow cellularity in leukemia. For each sample, 20 μg cellular proteins was analyzed by Western blot with anti-Shp2 and β-actin antibodies. Results were expressed as Shp2/β-actin ratios, and the mean values in hypercellular leukemia and nonhypercellular leukemia cases were 1.873 ± 0.527 and 0.525 ± 0.262, respectively.
Shp2 is down-regulated during myeloid differentiation
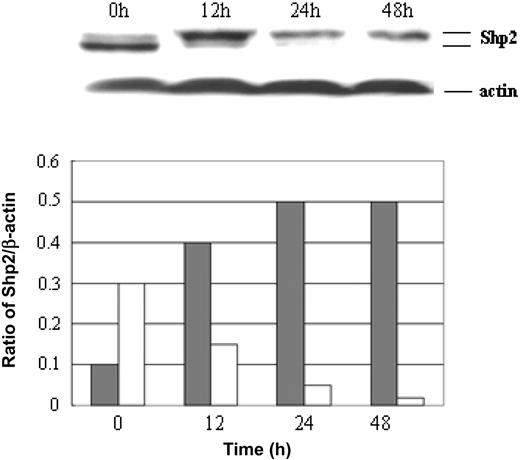
The low levels of Shp2 mRNA and p-Shp2 protein in terminally differentiated neutrophils and their high expression levels in leukemic blasts suggest that p-Shp2 protein is possibly involved in differentiation regulation of leukemia cells. To address this issue, we first examined the relationship between Shp2 protein expression and differentiation degree of leukemia cells using the NB4 leukemia cell line with ATRA, which is widely used for blood differentiation assay. NB4 cells were treated with ATRAat 1 μM for up to 96 hours or Shp2 antisense (AS) for 72 hours at 2 μM. The Shp2 protein level, cell-cycle phases, the expression of cluster differentiation antigen CD11b, and cell morphology were evaluated at different time points. The p-Shp2 protein level as well as proliferating cells (S-phase cells) were significantly decreased (Figure 6A), with differentiation progression as indicated by the increase of CD11b antigen (Figure 6B). As shown in Figure 6C, the expression levels of both Shp2 protein and Ki-67 antigen were high in undifferentiated leukemia cells (Figure 6Ci-iii), but markedly decreased after induction with ATRA for 72 hours (Figure 6Cv-vii). Meanwhile, undifferentiated leukemia blast cells (Figure 6Civ) were developed into differentiated myeloid cells (Figure 6Cviii). Similarly, treatment of NB4 cells with antisense Shp2 AS oligo induced differentiation and suppressed cell proliferation (Figure 6C-D), and a significant increase of CD11b expression was also detected in NB4 cells after treatment with Shp2 AS for 48 to 72 hours at 2 μM (Figure 6E). These data suggest that p-Shp2 protein expression may be involved in differentiation of hematopoietic cells.
Suppression of Shp2 expression induces apoptosis and growth inhibition of leukemia clonogenic cells
To evaluate whether Shp2 plays a critical role in proliferation of leukemic hematopoiesis as in normal hematopoiesis, we examined the effects of blocking Shp2 expression with Shp2 antisense oligonucleotides on leukemia cell clone growth. K562 leukemia cells, which exhibit a high level of Shp2 protein, were treated with Shp2 antisense oligonucleotides and sense oligonucleotides at different concentrations. As shown in Figure 7A, the growth of leukemia clones was severely suppressed after exposure to Shp2 antisense oligonucleotides for 7 days. In addition, we observed increased apoptosis of leukemia cells along with decreased Shp2 protein content after Shp2 antisense treatment (Figure 7B). Similar results were also observed in other human leukemia cell lines (data not shown), suggesting a positive role of Shp2 in leukemic cell survival and proliferation.
Shp2 expression inversely correlates with differentiation of hematopoietic cells. (A) Leukemic cells were treated with ATRA at 1 μM and collected at different time points to determine the percentage of cells in the S phase (top panel) and the Shp2 protein amounts by Western blot (bottom panel). (B) CD11b expression levels by FCM analysis. (C) In top panel, leukemic cells were treated with ATRA at 1 μM, and collected at 72 hours for analysis of Shp2 expression using double immunofluorescence labeling with anti-Shp2 (green) and Ki-67 (red) antibodies. Shown are leukemia cells before and after induction with ATRA. For morphologic analysis, cells untreated or treated with ATRA for 72 hours were collected and stained with Wright-Giemsa staining. The bottom panel shows NB4 cells treated with Shp2 AS (2 μM) for 0 and 72 hours. Images were viewed at 100 ×/0.3 NA with a Zeiss Axiovert S100 microscope. Images were captured with a SPOT 1.3.0 CCD camera and transferred to Adobe Photoshop 6.0. (D) NB4 cells were treated with Shp2 AS at 3 μM and collected at different time points for analysis of cell cycles with FCM. Error bars represent standard error. (E) CD11b expression levels on NB4 cells were determined by FCM analysis following treatment with Shp2 AS (2 μM) for 72 hours. The scale bar represents 10 μm. Data are representative of 3 independent experiments.
Shp2 expression inversely correlates with differentiation of hematopoietic cells. (A) Leukemic cells were treated with ATRA at 1 μM and collected at different time points to determine the percentage of cells in the S phase (top panel) and the Shp2 protein amounts by Western blot (bottom panel). (B) CD11b expression levels by FCM analysis. (C) In top panel, leukemic cells were treated with ATRA at 1 μM, and collected at 72 hours for analysis of Shp2 expression using double immunofluorescence labeling with anti-Shp2 (green) and Ki-67 (red) antibodies. Shown are leukemia cells before and after induction with ATRA. For morphologic analysis, cells untreated or treated with ATRA for 72 hours were collected and stained with Wright-Giemsa staining. The bottom panel shows NB4 cells treated with Shp2 AS (2 μM) for 0 and 72 hours. Images were viewed at 100 ×/0.3 NA with a Zeiss Axiovert S100 microscope. Images were captured with a SPOT 1.3.0 CCD camera and transferred to Adobe Photoshop 6.0. (D) NB4 cells were treated with Shp2 AS at 3 μM and collected at different time points for analysis of cell cycles with FCM. Error bars represent standard error. (E) CD11b expression levels on NB4 cells were determined by FCM analysis following treatment with Shp2 AS (2 μM) for 72 hours. The scale bar represents 10 μm. Data are representative of 3 independent experiments.
Discussion
Numerous studies have shown that Shp2 plays a critical role in a variety of growth factor and cytokine signaling involved in hematopoiesis.30,31 Most recently, several laboratories found gain-of-function mutations for Shp2 in juvenile and childhood leukemias.16-18 We investigated the prevalence of mutations in PTPN11 in leukemic cells from adult patients in China. However, we identified no mutation in PTPN11 among all tested leukemia patients, suggesting that PTPN11 mutations are indeed uncommon for adult patients with leukemia, consistent with recent reports.26,27
Shp2 antisense oligonucleotides induce growth inhibition and apoptosis of leukemic clonogenic growth. (A) Leukemic cells were plated in 24-well culture plates in 0.5% soft agar with different concentrations of Shp2-specific antisense oligonucleotide (▪) for 7 days, and then colonies (> 40 cells) were counted using an inverted microscope. Each experiment was performed at least 3 times. Note: The sense oligonucleotide of Shp2 (□) was also found to have a modest growth inhibition on leukemic colonies at high concentrations. (B) Microphotographs of leukemic colonies after exposure to antisense (i, iii) and sense (ii, iv) oligonucleotides at 2 μM for 72 hours. Images were viewed at 400 ×/0.75 NA, with a Zeiss Axioskop II microscope. Images were captured with a SPOT 1.3.0 CCD camera and transferred to Adobe Photoshop 6.0. (C) Leukemia cells were cultured in RPMI-1640 with 10% serum, treated with different doses (0 μM-4 μM) of Shp2-specific antisense oligonucleotides for 72 hours, and then collected for analysis of Shp2 expression by Western blot, and apoptosis with FCM. Data are representative of 3 independent experiments with standard error.
Shp2 antisense oligonucleotides induce growth inhibition and apoptosis of leukemic clonogenic growth. (A) Leukemic cells were plated in 24-well culture plates in 0.5% soft agar with different concentrations of Shp2-specific antisense oligonucleotide (▪) for 7 days, and then colonies (> 40 cells) were counted using an inverted microscope. Each experiment was performed at least 3 times. Note: The sense oligonucleotide of Shp2 (□) was also found to have a modest growth inhibition on leukemic colonies at high concentrations. (B) Microphotographs of leukemic colonies after exposure to antisense (i, iii) and sense (ii, iv) oligonucleotides at 2 μM for 72 hours. Images were viewed at 400 ×/0.75 NA, with a Zeiss Axioskop II microscope. Images were captured with a SPOT 1.3.0 CCD camera and transferred to Adobe Photoshop 6.0. (C) Leukemia cells were cultured in RPMI-1640 with 10% serum, treated with different doses (0 μM-4 μM) of Shp2-specific antisense oligonucleotides for 72 hours, and then collected for analysis of Shp2 expression by Western blot, and apoptosis with FCM. Data are representative of 3 independent experiments with standard error.
In this study, we demonstrate that Shp2 is highly expressed in most primary leukemia cells and also in established leukemia cell lines. More importantly, Shp2 was found constitutively phosphorylated on tyrosyl residues and the p-Shp2 was preferentially localized to the plasma membrane. RT-PCR analysis demonstrated that the Shp2 mRNA levels were significantly increased in many of the primary leukemia specimens tested. Both the expression levels of Shp2 mRNA and p-Shp2 protein amounts in patients with leukemia with hyperplastic bone marrow were much higher than in those with hypoplastic bone marrow, suggesting a positive correlation between expression/activation of the Shp2 enzyme with the hyperproliferative phenotype of leukemia.
Gilliland and colleagues32 have proposed that development of AML involves cooperating mutations in transcription factors and in cytoplasmic signaling proteins. It is also possible that expression of dominant active mutants of receptor tyrosine kinases such as Flt3 or c-Kit may lead to overactivation of Shp2 in leukemogenesis. Thus, it will be important to determine what causes the preferential plasma localization and constitutive tyrosine phosphorylation of Shp2 in the adult leukemia cells. In previous studies, gain-of-function mutations in PTPN11 were found mutually exclusive to mutations in Ras or NF1.17 This observation suggests that these proteins operate in the same Ras signaling pathway and that one mutation for a component in the pathway is sufficient to cause malignant cell proliferation. It is not known yet whether Ras or NF1 mutations exist in leukemia cells with overexpressed Shp2.
To verify whether Shp2 plays a positive role in the proliferation of leukemic hematopoiesis, we examined the effect of a Shp2 antisense oligonucleotide on clonogenic cell growth of leukemia. Our data suggest that leukemia clonogenic cell growth was significantly reduced along with increased apoptosis of leukemia cells following down-regulation of Shp2 expression. Interestingly, we also observed that Shp2 was barely detectable in terminally differentiated and quiescent hematopoietic cells, but was abundantly expressed in proliferating cells. Therefore, we propose that the phospho-Shp2 protein is likely involved in differentiation regulation of hematopoietic cells. Consistently, the phospho-Shp2 protein level as well as proliferating cells were significantly decreased with differentiation progression of NB4 leukemia cells after treatment with ATRA, monitored by increase of CD11b antigen and morphologic features of myelomonocytic differentiation. Similarly, down-regulation of Shp2 expression in NB4 leukemia cells also promoted cell differentiation. In summary, our new findings reported here demonstrate that overexpression and constitutive activation of Shp2 protein is a common phenotype in various types of human leukemia, and is closely associated with the proliferative capacity of leukemic blasts. These results not only provide new insights into the role of Shp2 in leukemogenesis, but also may have important implications for considering the use of Shp2 as a novel target for leukemia therapeutics alone or in combination with conventional agents. During the revision of this manuscript, 2 groups reported that expression of a dominant active E76K Shp2 mutant, but not the wild-type Shp2 protein, in murine hematopoietic cells induced augmented proliferative response to GM-CSF and IL-3.33,34 Thus, it appears that overexpression of Shp2 may not be sufficient, but other factors are required for leukemogenesis.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2004-10-4057.
Supported in part by National Natural Science Foundation (NNSFC) grant 30 270 572 (R.Z.X) and National Institutes of Health grants CA78 606 and GM53 660 (G.S.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jing Jie for her support of this project.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal