Abstract
CD4+CD25+ regulatory T cells (Tregs) are essential negative regulators of immune responses. Here, we examined the signaling properties of human Tregs, using CD4+CD25+ Treg and CD4+CD25– control (Tcont) cell lines generated from cord blood. Treg cell lines were markedly hyporesponsive to stimulation with dendritic cells and with anti-CD3/CD28–coated beads. Hyporesponsiveness was reversed by exogenous interleukin-2 (IL-2). T-cell receptor (TCR)–CD3/CD28–mediated activation of Rap1 and Akt was retained in Tregs, but activation of Ras, mitogenactivated protein kinase 1/2 (MEK1/2), and extracellular signal-regulated kinase 1/2 (Erk1/2) was impaired. Tregs were blocked from cell cycle progression due to decrease of cyclin E and cyclin A and increase of p27kip1 (p27kip cyclin dependent kinase inhibitor). IL-2 induced sustained increase of cyclin E and cyclin A and prevented up-regulation of p27kip1. Tregs had high susceptibility to apoptosis that was reversed by IL-2, which correlated with activation of Erk1/2, up-regulation of Bcl-xL (B-cell CLL/lymphoma 2-like nuclear gene encoding mitochondrial protein, transcript variant 2), and phosphorylation of Bad (Bcl2 antagonist of cell death) at Ser112. Thus, Tregs share biochemical characteristics of anergy, including abortive activation of Ras-MEK-Erk, increased activation of Rap1, and increased expression of p27kip1. In addition, our results indicate that TCR–CD3/CD28–mediated and IL-2 receptor–mediated signals converge at the level of MEK-Erk kinases to regulate Treg survival and expansion and suggest that manipulation of the MEK-Erk axis may represent a novel strategy for Treg expansion for immunotherapy.
Introduction
CD4+CD25+ regulatory T cells (Tregs) are a naturally occurring subpopulation of T cells with immunosuppressive function. Tregs prevent autoimmunity but can also inhibit rejection of transplants, prevent the induction of antitumor responses, and regulate the immune response to infectious diseases.1 These natural properties make Tregs attractive tools for novel immunotherapeutic approaches. Extensive studies using mouse experimental models have provided compelling evidence that Tregs can induce transplantation tolerance, prevent graft-versus-host disease (GVHD), and control allergy and autoimmunity.2-4
A practical problem that prevents the clinical applicability of these experimental approaches is the difficulty of isolating sufficient numbers of Tregs from human blood. Development and expansion of Tregs in vivo require both T-cell receptor (TCR)–CD3/CD28–mediated and interleukin-2 (IL-2) receptor–mediated signals.5,6 Using these natural stimulatory requirements of Tregs, recent studies achieved in vitro generation of human Treg cell lines from cord blood CD4+CD25+ Tregs by culture with anti-CD3/CD28 monoclonal antibody (mAb)–coated beads and IL-2.7 These cell lines exhibit enhanced suppressor function compared with freshly isolated CD4+CD25+ Tregs and potentially provide an ideal tool for immunotherapy. Despite progress in the in vitro generation of CD4+CD25+ Treg cell lines, their replicative capacity is impaired. Thus, an approach to efficiently expand these in vitro–generated Tregs for in vivo immunotherapy is desirable. Identification of the molecular pathways that regulate cell cycle progression and viability may provide novel molecular targets for intervention in order to achieve large-scale expansion of Tregs in vitro and to obtain cell numbers sufficient for immunotherapy.
Mitogen-activated protein kinase–extracellular signal-regulated kinase (MEK-Erk), phosphatidylinositol-3-kinase (PI3K), and Akt/protein kinase B (hereafter referred to as Akt) play a critical role in T-cell survival, expansion, and differentiation.8,9 These pathways can be activated via TCR–CD3/CD28 costimulation, as well as via the IL-2 receptor.10,11 Both TCR/CD28–and IL-2 receptor–mediated signals are required for development and expansion of Tregs in mice.5,6 For these reasons, we investigated whether these signaling pathways are involved in TCR–CD3/CD28–mediated stimulation and in IL-2 receptor–mediated stimulation of human Tregs.
We used Treg cell lines and paired control (Tcont) cell lines generated, respectively, from CD4+CD25+ and CD4+CD25– cord blood cells.7 These cell lines provide a useful tool to examine the signaling properties of Tregs, studies that require high cell numbers that cannot be obtained using primary, naturally occurring human Tregs. Our studies showed that TCR–CD3/CD28–mediated activation of Rap1 and Akt was retained in Tregs, but activation of Ras, MEK1/2, and Erk1/2 was defective. Tregs entered the cell cycle but progression through the cell cycle was blocked due to rapid down-regulation of cyclin E and cyclin A, and increase of p27kip1. Addition of exogenous IL-2 prevented up-regulation of p27kip1 and induced a sustained increase of cyclin E and cyclin A. Tregs were prone to apoptosis; exogenous IL-2 prevented apoptosis and induced activation of Erk1/2 (at levels equivalent to those of Tconts), expression of Bcl-xL (B-cell CLL/lymphoma 2-like nuclear gene encoding mitochondrial protein, transcript variant 2), and phosphorylation of Bad (Bcl2 antagonist of cell death) at Ser112.
Thus, Tregs share many biochemical characteristics of anergy, including defective activation of Ras-MEK-Erk pathway, enhanced activation of Rap1, increased expression of p27kip1 (p27kip cyclin dependent kinase inhibitor), defective cell cycle progression, and high susceptibility to apoptosis. In addition, our results indicate that TCR–CD3/CD28–mediated and IL-2 receptor–mediated signals converge at the level of MEK-Erk kinases to determine the fate of Tregs toward survival and expansion.
Materials and methods
Generation and culture of Treg and Tcont cell lines
CD4+CD25+ and CD4+CD25– cells were isolated from umbilical cord blood mononuclear cells (CBMCs; Saint Louis Blood Bank, St Louis, MO) and Treg and Tcont cell lines were generated as described previously.7 Briefly, CD25+ cells were positively selected from CD34– CBMCs by CD25+ magnetic beads (Miltenyi-Biotec, Auburn, CA). CD4+CD25– cells were positively selected from exhaustively CD25-depleted CD34– CBMCs. Cells were cultured once with anti-CD3/CD28–coated dynal beads (University of Pennsylvania) at a ratio of 3 beads per cell. Media was RPMI 1640 plus 10% fetal calf serum (FCS) supplemented with glutamax, penicillin, and streptomycin (Gibco/Invitrogen, Carlsbad, CA). Cells were split as needed and fed media containing 50 units IL-2 (Chiron, Emeryville, CA) every 3 to 4 days. Cell lines became quiescent after 3 weeks, and were used for functional and molecular experiments at 3 to 4 weeks. All CD25+-derived lines were shown to be potently suppressive by allogeneic dendritic cell (DC) mixed lymphocyte reaction (MLR) assay.6 Prior to use in stimulation experiments, cell lines were dynal-bead depleted and rested overnight in culture medium. Cell lines were subsequently cultured as indicated to examine proliferation, IL-2 production, cell cycle progression, and susceptibility to apoptosis.
Proliferation and cytokine analysis
Cells were cultured at a concentration of 50 000 cells/well in 96-well flat-bottom plates (Costar, Cambridge, MA) with anti-CD3/CD28–coated dynal beads (University of Pennsylvania). Beads were used at 2 beads/cell for short-term stimulations. At the indicated times, cultures were pulsed with 1 μCi (0.037 MBq) 3H-thymidine for the last 16 hours of the culture. Supernatants harvested at the indicated time intervals were analyzed for IL-2 concentration by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). DCs for allogeneic stimulation assays were derived from healthy adult volunteer blood donor buffy coats (Memorial Blood Centers, Minneapolis, MN). CD14+ cells were positively selected by CD14 microbeads (Miltenyi-Biotec) and cultured in X-Vivo-15 media (BioWhittaker, Walkersville, MD) with granulocyte-macrophage colonystimulating factor (GM-CSF, 50 ng/mL) and IL-4 (20 ng/mL) (R&D Systems) for 5 to 7 days.12 For stimulations, 50 000 cells from Treg or Tcont cell lines were cultured with 10 000 DCs in 96-well round-bottom plates. Where indicated, IL-2 was added at 50 U/mL. For pharmacologic stimulation, phorbol ester (PMA) was used at 1 ng/mL and ionomycin, at 500 ng/mL (Sigma, St Louis, MO).
Cell activation, Western blotting, and glutathione-S–transferase (GST) pull-down assay
For activation experiments, Treg and Tcont cell lines were rapidly mixed with anti-CD3/CD28–coated dynal beads and incubated at 37°C for the indicated time intervals. Cells were lysed12 and equal amounts of protein were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot. Blots were stripped and reprobed as indicated. Antibodies specific for phosphorylated Akt, phosphorylated MEK1/2, Erk1/2, and phosphorylated Bad (Ser112) were from Cell Signaling Technologies (Beverly, MA). Antibodies for phosphorylated Erk1/2, phosphorylated Bad (Ser136), cyclin D2, cyclin E, cyclin A, cdk2, cdk4, and p27 were from Santa Cruz Biotechnology (Santa Cruz, CA). Retinoblastoma (Rb)–specific antibody was from Pharmingen (San Diego, CA). Antibody for Bcl-2 (B-cell CLL/lymphoma 2) was from DakoCytomation (Glostrup, Denmark); Bcl-xL was from Biosource International (Camarillo, CA). To detect Rap1GTP and RasGTP, cell lysates were incubated with GST-RalGDS-RBD and GST-Raf1-RBD, respectively, coupled with glutathione Sepharose beads.13 Complexes were analyzed by SDS-PAGE and blotted with anti-Rap1 (Santa Cruz Biotechnology) or anti-Ras (Oncogene, San Diego, CA) antibodies.
Assessment of cell viability
Analysis of viability was performed using an annexin V–based apoptosis detection kit (R&D Systems).
Results
Cord blood Treg cell lines exhibit impaired proliferation and IL-2 production
First we examined the functional properties of Treg and Tcont cell lines. After stimulation by anti-CD3/CD28–coated beads, assessment of proliferation was determined by 3H-thymidine incorporation. A significant degree of 3H-thymidine incorporation was detected in both Treg and Tcont cell lines at 24 hours of culture (Figure 1A). However, in subsequent days, only Tconts retained their ability for DNA synthesis, whereas proliferation of Tregs rapidly declined (Figure 1A). Assessment of IL-2 production revealed that, similarly to their proliferation capacity, Tconts produced significant levels of IL-2 at 24 hours of culture (Figure 1B). High levels of IL-2 were detectable in the culture supernatants up to 5 days of culture. In contrast, low levels of IL-2 were detected only on the first day of Treg culture and rapidly declined thereafter (Figure 1B). This pattern of proliferation and IL-2 production of Treg and Tcont cell lines from cord blood was similar to that observed when Treg and Tcont cell lines generated from peripheral blood were analyzed14 and was obtained with multiple lines generated from various individuals (data not shown). Of importance, addition of IL-2 in Tregs stimulated with anti-CD3/CD28–coated beads resulted in augmented and sustained proliferation (Figure 1C).
To determine whether Treg cell lines did not respond to TCR–CD3/CD28–mediated signals because they have requirements for additional costimulatory or accessory signals besides CD28 costimulation, we used allogeneic DCs as stimulators. Similarly to stimulation with anti-CD3/CD28–coated beads, when stimulated with allogeneic DCs, only Tconts exhibited sustained proliferation, whereas Treg cell lines were markedly hyporesponsive (Figure 1D). Similarly to TCR–CD3/CD28–coated beads, stimulation with allogeneic DCs and IL-2 recovered hyporesponsiveness of Treg cell lines and enabled a vigorous proliferative response (Figure 1E). However, Treg cell lines are incapable of responding to IL-2 receptor–mediated signals alone, in contrast to Tcont cell lines that proliferated readily to IL-2 alone (Figure 1F). Taken together, these results indicate that Tregs are incapable of responding to stimulation even in the presence of multiple costimulatory and accessory signals provided by DCs. Strikingly, Treg cell lines could respond quite well to antibody (anti-CD3/CD28–coated beads)–or DC-mediated stimulation when exogenous IL-2 was provided simultaneously. Thus, Treg cell lines require concomitant activation of antigen receptor and IL-2 receptor–mediated signaling pathways in order to proliferate.
Treg cell lines generated from cord blood exhibit impaired proliferation and IL-2 production in response to stimulation by TCR and costimulation. (A) Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads (3 + 28) for indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (B) Secretion of IL-2 was analyzed by ELISA. (C) Supplementation with IL-2 (50 U/mL) induces proliferation of Treg cell lines stimulated with anti-CD3/CD28 beads. Treg and Tcont cell lines were cultured as in panel A, but IL-2 (50 U/mL) was added during culture. (D) Tregs are markedly hyporesponsive to stimulation with allogeneic DCs compared with Tcont cell lines. Tcont and Treg cell lines were stimulated with allogeneic DCs as described in “Materials and methods” for the indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (E) Treg hyporesponsiveness mitigated by IL-2 supplementation. Treg and Tcont cell lines were cultured as in panel D, but IL-2 (50 U/mL) was added during culture. (F) Treg cell lines do not respond to IL-2 alone as do Tcont cell lines. Treg and Tcont cell lines were cultured in various concentrations of IL-2 (5-500 U/mL) and proliferation was assessed by thymidine incorporation, with peak responses shown after 2 days of culture. Results are all representative of 3 to 4 independent culture experiments. cpm indicates counts per minute.
Treg cell lines generated from cord blood exhibit impaired proliferation and IL-2 production in response to stimulation by TCR and costimulation. (A) Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads (3 + 28) for indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (B) Secretion of IL-2 was analyzed by ELISA. (C) Supplementation with IL-2 (50 U/mL) induces proliferation of Treg cell lines stimulated with anti-CD3/CD28 beads. Treg and Tcont cell lines were cultured as in panel A, but IL-2 (50 U/mL) was added during culture. (D) Tregs are markedly hyporesponsive to stimulation with allogeneic DCs compared with Tcont cell lines. Tcont and Treg cell lines were stimulated with allogeneic DCs as described in “Materials and methods” for the indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (E) Treg hyporesponsiveness mitigated by IL-2 supplementation. Treg and Tcont cell lines were cultured as in panel D, but IL-2 (50 U/mL) was added during culture. (F) Treg cell lines do not respond to IL-2 alone as do Tcont cell lines. Treg and Tcont cell lines were cultured in various concentrations of IL-2 (5-500 U/mL) and proliferation was assessed by thymidine incorporation, with peak responses shown after 2 days of culture. Results are all representative of 3 to 4 independent culture experiments. cpm indicates counts per minute.
Of interest, when treated with pharmacologic stimulators phorbol ester and the calcium ionophore ionomycin (PMA+IM), which overcome the TCR proximal signaling events, Tregs were capable of proliferating and producing IL-2 (data not shown). These results indicate that Tregs do not have an irreversible intrinsic inability to produce IL-2 and suggest that altered TCR–CD3/CD28–mediated signaling events may result in the inability of Tregs to produce IL-2 when stimulated via their antigen receptor in the presence of costimulation. These functional properties of Tregs highly resemble those of anergic CD4+ T cells that are incapable of responding to antigen-specific stimulation via their TCR but can respond to stimuli that bypass TCR-proximal signaling.15
Activation of Ras-MEK-Erk is impaired but activation of PI3K-Akt and Rap1 is retained in human Tregs
PI3K regulates IL-2 transcription via its downstream target Akt, and activated Akt can substitute for CD28 costimulation for the induction of IL-2 gene transcription.16 These observations suggest that defective TCR–CD3/CD28–mediated production of IL-2 in Tregs may be related to an impaired ability of these cells to activate Akt. Such a hypothesis was also supported by our previous observation that in primary human T lymphocytes the PI3K-Akt pathway is required for IL-2 production and cell cycle progression.11 Of interest, activation of Akt was detectable at comparable levels in TCR–CD3/CD28–stimulated Treg and Tcont cell lines (Figure 2A). In primary human T cells, both PI3K-Akt and MEK1/2-Erk1/2 pathways are indispensable for IL-2 production and cell cycle progression. Proliferation and IL-2 production are impaired when MEK1/2-Erk1/2 signaling is inhibited even if PI3K-Akt pathway remains intact.11 Moreover, MEK1/2-Erk1/2 activation is defective in anergic T cells.17 For this reason, we examined activation of the MEK1/2-Erk1/2 signaling pathway in TCR–CD3/CD28–stimulated Tregs and Tconts. Strikingly, activation of MEK1/2 and the MEK1/2 downstream targets, Erk1/2, were impaired in Tregs (Figure 2B).
Since activation of MEK1/2-Erk1/2 is directly downstream of Ras, we examined whether defective MEK1/2-Erk1/2 activation in Tregs might correlate with defective activation of Ras. As shown in Figure 2C, activation of Ras was also impaired in Tregs compared with Tconts. In contrast, activation of Rap1, another small guanosine triphosphatase (GTPase) of the Ras superfamily, was retained in Tregs; in fact, activated Rap1 was increased in Tregs compared with Tconts (Figure 2D).
Distinct TCR–CD3/CD28 signaling in Treg cell lines. Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads for the indicated time intervals. Cell lysates were analyzed by SDS-PAGE and examined by immunoblot for expression of (A) p-Akt and Akt; (B) p-MEK1/2, p-Erk1/2, and Erk1/2; and by pull-down assay for activation of (C) Ras and (D) Rap1. Expression of total Ras and Rap1 was determined by immunoblot with the specific antibodies in whole-cell lysates. Results are representative of 4 independent experiments conducted with 4 different pairs of Treg and Tcont cell lines. ON indicates overnight culture.
Distinct TCR–CD3/CD28 signaling in Treg cell lines. Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads for the indicated time intervals. Cell lysates were analyzed by SDS-PAGE and examined by immunoblot for expression of (A) p-Akt and Akt; (B) p-MEK1/2, p-Erk1/2, and Erk1/2; and by pull-down assay for activation of (C) Ras and (D) Rap1. Expression of total Ras and Rap1 was determined by immunoblot with the specific antibodies in whole-cell lysates. Results are representative of 4 independent experiments conducted with 4 different pairs of Treg and Tcont cell lines. ON indicates overnight culture.
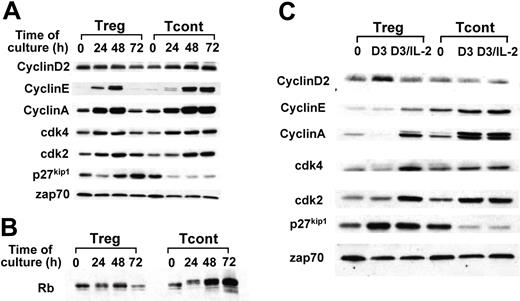
Cell cycle progression in response to TCR–CD3/CD28 is blocked in Treg cell lines and can be rescued by exogenous IL-2. Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads for the indicated time intervals; cell lysates were prepared and analyzed by (A-B) SDS-PAGE and immunoblot with indicated antibodies. (C) The same cell lines were stimulated with anti-CD3/CD28–coated dynal beads for 72 hours in total (indicated as D3) or for 56 hours, after which IL-2 (50 U/mL) was added and the culture was continued until 72 hours in total (indicated as D3/IL-2). Cell lysates were analyzed by SDS-PAGE and immunoblot with indicated antibodies. Results are representative of 3 independent experiments. zap70 indicates zeta chain associated protein kinase.
Cell cycle progression in response to TCR–CD3/CD28 is blocked in Treg cell lines and can be rescued by exogenous IL-2. Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads for the indicated time intervals; cell lysates were prepared and analyzed by (A-B) SDS-PAGE and immunoblot with indicated antibodies. (C) The same cell lines were stimulated with anti-CD3/CD28–coated dynal beads for 72 hours in total (indicated as D3) or for 56 hours, after which IL-2 (50 U/mL) was added and the culture was continued until 72 hours in total (indicated as D3/IL-2). Cell lysates were analyzed by SDS-PAGE and immunoblot with indicated antibodies. Results are representative of 3 independent experiments. zap70 indicates zeta chain associated protein kinase.
Cell cycle progression is blocked in Tregs
The above results suggested that signals controlling cell cycle progression were differentially activated in Tregs and Tconts. Analysis of the cell cycle regulatory molecules showed that both cell types were capable of expressing cyclin D2, indicating that they could enter the G1 phase. However, a significant difference was detected in the expression pattern of cyclin E, which is induced at the late G1 phase at the stage of transition through the G1 restriction point. Although in Tregs, induction of cyclin E was detected at 24 and 48 hours, expression of cyclin E was not sustained at 72 hours. Similarly, cyclin A was up-regulated at 24 and furthermore at 48 hours but was significantly decreased at 72 hours of culture (Figure 3A, lanes 1-4). In contrast, in Tconts, cyclin E and cyclin A were up-regulated at 24 hours and remained at high levels at 72 hours of culture (Figure 3, lanes 5-8).
Conversely, Tconts had a significant decrease of p27kip1 cdk inhibitor after culture (Figure 3, lane 5-8), whereas Tregs not only did not exhibit decrease of p27kip1, but they had a gradual increase of p27kip1 that exceeded the levels of background expression by 72 hours of culture (Figure 3, lanes 1-4). One interpretation of these results is that Tregs pass through the G1 restriction point and synthesize cyclin E, enter the S phase and synthesize cyclin A, but exit S phase and return to the G1 phase of the cell cycle, where they do not express cyclin E or cyclin A but express high levels of p27kip1. Consistent with this, phosphorylation of Rb was detected at 24 and 48 of culture, but declined and returned to the unphosphorylated form at 72 hours of culture. In contrast, in Tconts, which express high levels of cyclin E and cyclin A, a high degree of Rb phosphorylation was detectable at 72 hours of culture (Figure 3B).
IL-2 is a critical factor for expansion of Tregs in vitro and in vivo.5,18,19 Because our Treg cell lines were incapable of sustained IL-2 secretion, we examined whether their inability to progress through the cell cycle might be reversed by addition of exogenous IL-2. Indeed, addition of IL-2 in Tregs prevented up-regulation of p27kip1 and induced sustained expression of cyclin E and cyclin A at day 3 of culture (Figure 3C, lanes 2-3). In contrast, addition of IL-2 in Tconts did not alter the expression of cyclins, cdks, or p27kip1 on day 3 of culture, consistent with the hypothesis that endogenously produced IL-2 was sufficient to induce these events (Figure 3C, lanes 5-6).
Tregs become susceptible to activation-induced apoptosis and are rescued by IL-2
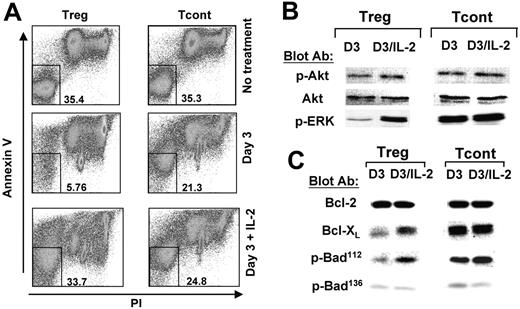
Besides promoting cell expansion, IL-2 has been shown to have a critical role in promoting viability of Tregs.5,20 For this reason, we examined whether Treg cell lines that do not produce IL-2 were susceptible to activation-induced apoptosis. As determined by annexin V/propidium iodide (PI) staining, Treg but not Tcont cell lines became highly susceptible to apoptosis after 72 hours of culture with anti-CD3/CD28–coated beads (Figure 4A). Addition of exogenous IL-2 significantly prevented apoptosis and promoted cell viability (Figure 4A).
IL-2 induces activation of Erk, and up-regulates expression of Bcl-xL and phosphorylation of Bad on Ser112 in Tregs
Our studies showed that Tregs required concomitant TCR/CD28- and IL-2 receptor–mediated signals in order to proliferate. Moreover, we determined that TCR/CD28-mediated stimulation induced successful activation of the PI3K target Akt but impaired activation of the MEK1/2 target Erk1/2. For these reasons, we investigated the role of IL-2 in activation of PI3K-Akt and MEK-Erk pathways in Tregs. As shown in Figure 4B, prior to addition of exogenous IL-2, Tregs had a significant degree of Akt phosphorylation that was comparable with that observed in Tconts (Figure 4B, lanes 1,3). Addition of IL-2 slightly augmented activation of Akt in Tregs (Figure 4B, lanes 1-2). IL-2 induced significant activation Erk1/2 in Tregs (Figure 4B, lanes 1-2), whereas it had no effect on activation of Erk1/2 in Tconts (Figure 4B, lanes 3-4). These results indicate that TCR–CD3/CD28–mediated signals in Tregs were sufficient to induce activation of Akt to levels comparable with those in Tconts, and further, that Akt phosphorylation may be independent of IL-2 signals. However, optimal activation of Erk1/2 is IL-2 dependent, as high-level activation of Erk1/2 was achieved only in Tconts that produced large amounts of endogenous IL-2. In contrast, exogenous IL-2 was required to induce this level of Erk1/2 activation in Tregs.
In T lymphocytes, IL-2 also promotes viability by regulating expression of Bcl-2 family members.21 Therefore, an obvious question was whether IL-2 regulated expression of Bcl-2 family members in Tregs. Among the 20 members of the Bcl-2 family, Bcl-2 and Bcl-xL have the most critical role in promoting viability in T lymphocytes.22 Bcl-2 was expressed at comparable levels in TCR–CD3/CD28–cultured Tregs and Tconts, and addition of IL-2 did not affect its expression (Figure 4C). In contrast, Bcl-xL was selectively up-regulated by IL-2 in Tregs (Figure 4C, lanes 1-2), whereas in Tconts it was expressed at high levels prior and after addition of IL-2 (Figure 4C, lanes 3-4).
IL-2/IL-2 receptor signaling prevents Tregs from undergoing activationinduced cell death. Treg and Tcont cell lines were rested or stimulated with anti-CD3/CD28–coated dynal beads for 3 days, or with anti-CD3/CD28–coated dynal beads and IL-2 as described in Figure 3. (A) Viability was analyzed at day 3 by annexin V and PI staining. Numbers represent percent of cells double negative for annexin V and PI. (B-C) Cell lysates from the same samples were analyzed by SDS-PAGE and immunoblot with indicated antibodies. Results are representative of 3 independent experiments.
IL-2/IL-2 receptor signaling prevents Tregs from undergoing activationinduced cell death. Treg and Tcont cell lines were rested or stimulated with anti-CD3/CD28–coated dynal beads for 3 days, or with anti-CD3/CD28–coated dynal beads and IL-2 as described in Figure 3. (A) Viability was analyzed at day 3 by annexin V and PI staining. Numbers represent percent of cells double negative for annexin V and PI. (B-C) Cell lysates from the same samples were analyzed by SDS-PAGE and immunoblot with indicated antibodies. Results are representative of 3 independent experiments.
Bad is a BH3-only proapoptotic member of the Bcl-2 family that couples death signals to mitochondria and promotes apoptosis by quelling the protective action of Bcl-2 and/or Bcl-xL.23 Phosphorylation of Bad inactivates its proapoptotic function in a mechanism involving binding to 14-3-3 scaffold proteins that results in sequestering of Bad and dissociation from the mitochondrial Bcl-2 and/or Bcl-xL. Phosphorylation of Bad at Ser112 is dependent upon activation of Erk1/2, while phosphorylation at Ser136 is dependent upon Akt.24,25 As shown in Figure 4C, addition of IL-2 in Tregs up-regulated phosphorylation of Bad on Ser.112 In contrast, phosphorylation of Bad on Ser136 was comparable prior to and after addition of IL-2 in Tregs (Figure 4C). These results are consistent with our findings that addition of exogenous IL-2 induced only a slight change in the levels of Akt phosphorylation but a dramatic increase in the levels of Erk1/2 phosphorylation in Tregs (Figure 4B). These results indicate that Erk1/2 mitogen-activated protein (MAP) kinases have a critical role in promoting IL-2–mediated viability of Tregs, and one mechanism involved in this effect is mediated by phosphorylation of Bad.
Discussion
Our present results indicated that activation of Akt was not sufficient and did not have a central role in promotion of viability and cell cycle progression in Tregs. This conclusion is supported by various findings: First, activation of PI3K/Akt is induced by TCR–CD3/CD28 stimulation at comparable levels in Treg and Tcont cell lines, but only Tconts are protected from apoptosis and progress in the cell cycle. Second, addition of exogenous IL-2 during TCR–CD3/CD28 stimulation, which significantly promoted viability and expansion of Tregs, had no significant effect on Akt phosphorylation. Third, addition of IL-2 during TCR–CD3/CD28 stimulation of Tregs did not lead to induction of Bad phosphorylation on Ser136, a site that is phosphorylated by Akt.25 Fourth, addition of IL-2 did not up-regulate expression of bcl-2, a function known to be mediated by Akt,26 but resulted in significant up-regulation of Bcl-xL. Our results demonstrating the minimal role of Akt in promoting Treg viability and expansion suggest that rapamycin therapy for tolerance induction during therapeutic immunosuppression may have a particular advantage, because it may not compromise viability or expansion of Tregs.
The studies presented here showed that Treg cell lines retain multiple features of anergic cells, including defective activation of Ras-MEK-Erk1/2, enhanced activation of Rap1, up-regulation of p27kip1, blockade of cell cycle progression, and high susceptibility to apoptosis.12,17,27,28 Although previous studies have reported that Tregs may have functional features of anergic T cells,18 our present work provides for the first time experimental evidence that cell lines generated from natural cord blood Tregs share molecular and biochemical properties of T-cell anergy and suggest that Tregs may be considered naturally occurring anergic cells. These cell lines retain their anergic properties even after expansion for 3 to 4 weeks and require sustained supplementation of exogenous IL-2 to remain viable. Of interest, although IL-2 induced remarkable up-regulation of cyclin E and cyclin A in Tregs, it induced only a slight down-regulation of p27kip1 expression (Figure 3C, lanes 2-3). The observation that IL-2 did not down-regulate p27kip1 in human Tregs is consistent with a previous report in which the effects of IL-2 in mouse Tregs were analyzed.29 Thus, in contrast to other T cells in which IL-2 results in a striking, rapid down-regulation of p27kip1,30 Tregs do not down-regulate p27kip1 in response to IL-2 receptor–mediated signals. This observation suggests that sustained high expression of p27kip1 may be a molecular hallmark of Tregs and may have a role in their biologic function.
A major difference between conventional anergic T cells and our Treg cell lines should be noted: In vitro culture of conventional anergic T cells in IL-2 reverses their molecular and functional anergic phenotype. In contrast, after prolonged in vitro culture in IL-2, Tregs retain their anergy-specific molecular phenotype and their unresponsiveness and suppressive properties. Although the reason of this discrepancy is currently unclear, one can envision that in vitro–generated, conventional anergic cells can only transiently and reversibly recapitulate the properties of Tregs, which are programmed to retain the molecular and functional state of unresponsiveness and suppression function as part of their biologic role.
Our present results are in agreement with earlier observations, which have shown that mouse CD4+CD25+ Tregs were incapable of activating Akt after IL-2 receptor ligation but were capable of activating this substrate following TCR–CD3/CD28 ligation.29 However, those studies did not examine the activation of MEK-Erk pathway. As shown here, TCR–CD3/CD28 signals led to activation of Akt but not Erk1/2, and did not allow for protection from apoptosis and cell cycle progression of Tregs. The addition of IL-2 did not have any effect on Akt activation, but induced activation of Erk1/2, up-regulation of Bcl-xL, and phosphorylation of Bad on Ser112, and coincided with protection from apoptosis and cell cycle progression. Because simultaneous activation of PI3K-Akt and MEK-Erk pathways are required for viability and cell cycle progression of T lymphocytes,11 our results provide a molecular explanation why both TCR–CD28–mediated and IL-2 receptor–mediated signals are required for viability and expansion of Tregs.5,6
Finally, our results indicate that TCR–CD3/CD28–mediated and IL-2 receptor–mediated signals converge at the level of MEK-Erk kinases to determine the fate of Tregs toward cell viability and expansion and suggest that MEK-Erk may represent a novel target for in vitro intervention in order to achieve large-scale expansion of Tregs for immunotherapy.
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-04-1531.
Supported by National Institutes of Health (NIH) grants AI 43552, AI 46548, CA105216, AI34495, HL56067, and HL63452; the Leukemia and Lymphoma Society Translational Award (6220-04); and the National Marrow Donor Program (contract no. 231-02-0007).
L.L. and W.R.G. contributed equally to this work.
L.L. conducted the biochemistry and cell signaling experiments and contributed in the preparation of the paper; W.R.G. supervised S.B.P. and Y.G. with the generation of cell lines, conducted the functional experiments, and analyzed the data, and contributed to the preparation of the paper; S.B.P. generated cell lines, conducted the functional experiments, and analyzed the data; Y.G. conduced short-term and long-term stimulation of the cell lines and prepared cell extracts; C.H.J. provided anti-CD3/CD28–coated beads and contributed to the preparation of the paper; B.R.B. supervised S.B.P. and contributed to the preparation of the paper; V.A.B. coordinated the project and was responsible for the preparation of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Treg cell lines generated from cord blood exhibit impaired proliferation and IL-2 production in response to stimulation by TCR and costimulation. (A) Treg and Tcont cell lines were stimulated with anti-CD3/CD28–coated dynal beads (3 + 28) for indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (B) Secretion of IL-2 was analyzed by ELISA. (C) Supplementation with IL-2 (50 U/mL) induces proliferation of Treg cell lines stimulated with anti-CD3/CD28 beads. Treg and Tcont cell lines were cultured as in panel A, but IL-2 (50 U/mL) was added during culture. (D) Tregs are markedly hyporesponsive to stimulation with allogeneic DCs compared with Tcont cell lines. Tcont and Treg cell lines were stimulated with allogeneic DCs as described in “Materials and methods” for the indicated time intervals. Proliferation was assessed by [3H] thymidine incorporation. (E) Treg hyporesponsiveness mitigated by IL-2 supplementation. Treg and Tcont cell lines were cultured as in panel D, but IL-2 (50 U/mL) was added during culture. (F) Treg cell lines do not respond to IL-2 alone as do Tcont cell lines. Treg and Tcont cell lines were cultured in various concentrations of IL-2 (5-500 U/mL) and proliferation was assessed by thymidine incorporation, with peak responses shown after 2 days of culture. Results are all representative of 3 to 4 independent culture experiments. cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2005-04-1531/6/m_zh80210585860001.jpeg?Expires=1765149360&Signature=xHxK7LOyypz3GlVLWL07jVXiiGBPQsgrZQLrXVXiC2YgqnVpSVidazGzGBrrf0NOmWnfBN~X~LOqpTjhZTGr73wbovMnaYZl2X-5cX413HeJlejDHRF~RZJFZWVs-1QoCpCGMksDlGl~4tYyEWufd5qjXl2wx1t1YujabzVnWWmXzQM-lNgX4sx8-zv2z3U7mOa7-KJ3dC7ENxTeLntuXmAAVmTTZ2oJTA~5ezsVQOW2Kb0AmGXVBE1j7ThwLVSk1h0c6LGpaLlKZWRu~1suy0~2El2zV7~wYsVJpUY8-Gk~6L6Dhaxjgfh-a~zyVxLfs26qITNUP-D0ksCBt5pr6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal