Abstract
Platelet aggregation is a dynamic entity, capable of directing its own growth and stability via the activation of signaling cascades that lead to the expression and secretion of various secondary agonists. Here we show that the signaling pathways triggered during platelet aggregation include an intrinsic pro-thrombotic activity mediated by 2 homophilic adhesion molecules, CD84 and CD150 (SLAM [signaling lymphocyte activation molecule]), which are tyrosine phosphorylated in a platelet aggregation–dependent fashion. The 2 CD84/SLAM adapter proteins, SAP (SLAM-associated protein) and EAT-2 (EWS-activated transcript-2), were found in platelets; only SAP, however, was found to immunoprecipitate with tyrosine-phosphorylated SLAM. The immobilized extracellular domain of CD84 promoted microaggregate formation, while SAP-deficient platelets demonstrated defective spreading on immobilized CD84, demonstrating a functional role in platelets for SLAM family interactions. Finally, analysis of SLAM-deficient mice revealed an overall defect in platelet aggregation in vitro and a delayed arterial thrombotic process in vivo. The data indicate that signaling of the adhesion molecules in the SLAM family, activated by proximity during aggregation, further stabilize platelet-platelet interactions in thrombosis.
Introduction
Platelet aggregates provide the structural framework for the occlusive events causing acute myocardial infarction and thrombotic stroke. Because of this critical role in human pathology, it has become increasingly clear that understanding the mechanisms regulating both thrombus growth and stability will provide insights not only to an important biologic question, but also to new strategies regulating platelet thrombosis. The initial adhesion of platelets to the injured vessel wall is primarily mediated by binding of platelet membrane glycoprotein (GP) Ib-IX-V to von Willebrand factor and of GPVI and integrin α2β1 to collagen. Following platelet adhesion, binding of agonists such as thrombin, adenosine diphosphate (ADP), and thromboxane A2 induce signaling events that activate the “inside-out” receptor function of platelet integrin αIIbβ3, allowing it to bind soluble fibrinogen, resulting in platelet cross-linking. Interestingly, the stability of platelet-platelet contacts appears to be secondary to αIIbβ3-mediated interactions. Several secondary reactions have been identified, including αIIbβ3 “outside-in” signaling, tyrosine phosphorylation of numerous proteins, cytoskeletal rearrangements, certain secretory reactions,1 and signaling via novel platelet proteins. These include CD40L, a tumor necrosis factor (TNF) family member, Gas6, a ligand for the Axl/Sky family of receptor tyrosine kinases, which are both involved in stabilization of platelet-rich thrombi2,3 ; and the Eph kinases and ephrins, specifically EphA4 and ephrinB1, which on the surface of platelets enhance the binding of αIIbβ3 to immobilized fibrinogen in the presence of physiologic agonists.4,5
The SLAM (signaling lymphocyte activation molecule) family of homophilic adhesion receptors is a subset of the CD2 cell-surface receptor Ig superfamily and is defined by binding to the cytoplasmic adapters SAP and EAT-2, which recognize at least 2 ITSM (Immunoreceptor Tyrosine-based Switch Motifs) sequence motifs: TxYxxV/I (where x is any amino acid) in their cytoplasmic domains.6 SLAM proteins have been shown to be tyrosine phosphorylated during leukocyte activation7 and recruit the SH2 domain–containing adapter proteins SAP or EAT-2 to mediate diverse functional activities. SAP has been shown to be an adapter protein involved in SLAM family signaling, which recruits a src-like kinase to the receptor.8,9 SAP/EAT-2 binding homophilic adhesion molecules of the family include SLAM (CD150, IPO-3), CD84 (Ly9B), Ly108 (NTB-A, SF2000), CRACC (CD2-like receptor-activating cytotoxic cells)/CS-1, and 2B4 (CD244, NAIL). Although CD84 was previously shown to be expressed at relatively higher levels on platelets than on T or B cells,10 a role for SLAM family members in platelet biology has not been described.
Here we show, using a proteomics approach, that CD84 becomes tyrosine phosphorylated during platelet aggregation. Of the 2 cytoplasmic tyrosines that become phosphorylated, 1 exists in an ITSM motif, a putative recognition motif for the SAP/EAT-2 adapter proteins. Cross-linking of surface CD84 also induced CD84 tyrosine phosphorylation: immobilized CD84 promoted microaggregate formation in a SAP-dependent manner. We also show that SLAM phosphorylation in platelets was aggregation dependent and that SLAM-deficient platelets did not aggregate to the maximum level observed with wt platelets. In vivo, SLAM–/– mice displayed delayed thrombus formation without effecting tail bleeding times. Based on these observations, we propose the involvement of the SLAM family of receptors as secondary mediators of aggregation. The SLAM family proteins in platelets provides an unexpected mechanism that synergizes with integrins to mediate platelet thrombosis.
Materials and methods
Purification of platelet RNA and expression of SLAM family members in platelets
Approval was obtained from Millenium Pharmaceutical's Western Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Platelets from a healthy volunteer were collected by apheresis and included a filtration step that removed lymphocyte contaminants. These instruments typically give a platelet concentrate with less than 0.01% leukocyte contamination. We confirmed this by polymerase chain reaction (PCR) analysis of RNA prepared from these platelet concentrates and found leukocyte RNA contaminants were undetectable in our preparation. This procedure yielded approximately 250 mL platelets (∼1 × 109 platelets/mL). Platelets were diluted 1:4 with CGS (13 mM sodium citrate, 30 mM glucose, 0.12 M sodium chloride) containing 0.1 μg/mL PGI2. Platelets were pelleted by spinning for 20 minutes at 2000g in a Beckman Coulter Allegra 6 centrifuge at room temperature. The supernatant was removed, and the platelet pellet was resuspended in RNA-STAT-60 (Tel-Test) using 1 mL RNA-STAT-60 per 2.9 × 108 platelets, and immediately vortexed to facilitate platelet lysis. The platelet lysate was further processed according to RNA-STAT-60 protocol specifications. Total RNA was then treated with RNA Easy (Qiagen, Valencia, CA) for further purification. Five micrograms of DNAse-treated platelet RNA was converted to cRNA, fragmented, and hybridized to Millennium Pharmaceuticals (South San Francisco, CA) cardiovascular custom array per the manufacturers' suggested protocol (Affymetrix, Santa Clara, CA). Probe sets for the following genes were designed based on their published sequences: CD84 (NM_003874), SLAM (NM_003037), SAP (NM_002351), CD48 (AL121985), CD58 (NM_001779), BLAME (NM_020125), and 2B4 (NM_016382). Data were analyzed with MASv5 (Affymetrix) and Rosetta Resolver (Rosetta Biosoftware, Seattle, WA) and expressed as a relative number to the expression level measured in peripheral blood lymphocytes. Using Superscript II reverse transcriptase, 5 μg human platelet and spleen (BD Pharmingen, San Diego, CA) RNA were transcribed to cDNA and SAP and GAPDH were amplified using Platinum Pfx DNA polymerase. The resulting products were resolved on an agarose gel.
Platelet preparation for aggregation and spreading
Blood was drawn from C57Bl/6 (Charles River Laboratory, Germantown, MD), C57Bl/6 SAP–/–11 (backcrossed to C57Bl/6 mice 6 times), C57Bl/6 SLAM–/–12 (backcrossed to C57Bl/6 mice 7 times), and C57Bl/6 diYF1 mice. Platelets from human and mouse blood were prepared as previously described.13 Both human and murine washed platelets were resuspended in Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer with 1 mM CaCl2 and MgCl2 at a cell density of 1 to 3 × 108 cells/mL.
Platelet aggregation
Aggregations were measured in a Chronolog lumi-aggregometer. Aggregations of washed human platelets (3 × 108 cells/mL) were initiated with 0.5 U thrombin (Haematologic Technologies, Essex Junction, VT), 4 to 10 μg/mL collagen (Chronolog, Haverton, PA), or 5 μM-human TRAP (thrombin receptor activating peptide, SFLLRN, SynPep) and inhibited in the presence of 2 μM eptifibatide (Integrilin, Millennium Pharmaceuticals). Aggregation of murine platelet-rich plasma (PRP) was initiated with 1 to 2.5 mM mTRAP (murine thrombin receptor activating peptide, AYPGKF, SynPep), 1 to 10 μg/mL collagen or 0.5 to 10 μM ADP, and inhibited in the presence of 5 or 10 μM ML00341819.14
Platelet lysate preparation
Platelets were lysed in an equal volume of 2 × lysis buffer (20 mM Tris[tris(hydroxymethyl)aminomethane]-HCl, pH 8, 2% Triton X-100, 4 mM EDTA [ethylenediaminetetraacetic acid], 250 mM NaCl, 20% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 mM Na2VO4, 2 μg/mL leupeptin, and 2 μg/mL aprotinin), and incubated on ice for 30 minutes. Samples were sonicated for 40 seconds and the insoluble fraction was removed by centrifugation at 14 000g for 20 minutes at 4°C.
Phosphopeptide identification by in-gel digestion and mass spectrometry
The solubilized TRAP aggregated platelet proteins were incubated with α-phosphotyrosine IgG beads (PY99, Santa Cruz Biotechnology, Santa Cruz, CA) overnight. The phosphoproteins were eluted with 100 mM phenylphosphate, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by Coomassie staining. All Coomassie-stained bands were excised and subjected to in-gel proteolytic digestion with trypsin and analyzed by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS). Data-dependent LC/MS/MS was performed using electrospray ionization on an LCQ Deca XP ion trap mass spectrometer (Thermo Finnigan, San Jose, CA). An aliquot of each digest mixture was introduced to the mass spectrometer by reversed-phase chromatographic separation with a 75-μm ID capillary column flowing at a rate of approximately 350 nL/min and eluted using a 30-minute acetonitrile/0.1% formic acid gradient. Chromatographic separation yielded approximately 30-second peak widths, and mass spectra were acquired in 9-second cycles. Each cycle was of the form: 1 full MS scan followed by 4 MS/MS scans on the most abundant precursor ions, subject to dynamic exclusion for a period of 1.5 minutes. The identity of each peptide sequenced was determined by interpreting the MS/MS spectra using SpectrumMill software (Agilent Technologies, South San Francisco, CA).
Flow cytometry analysis
Human washed platelets (1 × 106) were left resting or activated with 5 μM TRAP for 5 minutes at 37°C without stirring. Fixed unpermeabilized platelets were stained with α-CD84-FITC (ID labs), α-CD62P-PE (ID labs), or isotype control FITC or phycoerythrin (PE) Igs for 1 hour at room temperature. Platelets were analyzed on a FACSort flow cytometer (Becton Dickinson Immunocytometry, San Jose, CA).
Immunoprecipitation and cross-linking of surface CD84
Immunoprecipitations were performed with protein G beads and 2.5 μg/mL α-CD84 mouse IgG, α-SLAM (9D1) rabbit IgG, or isotype controls overnight at 4°C. Immunoblotting of the complexes were performed with α-CD84 (ID labs), α-pTyr-4G10 (Upstate Cell Signaling Solutions, Charlottesville, VA), and α-pTyr-PY20 (Santa Cruz Biotechnology) mouse IgGs, or α-SLAM (Y2.1), α-murine-SAP, and α-murine-EAT2 rabbit (a kind gift from Dr Christopher Denny, University of California, Los Angeles) IgGs. The methods used to attempt to coimmunoprecipitate EAT-2 with SLAM from platelets was the same as described to coimmunoprecipitate SLAM and EAT-2 from lymphocytes.15 For cross-linking experiments, 3 × 108 platelets/mL were incubated with 5 μg/mL α-CD84 IgG for 30 minutes at room temperature, followed by cross-linking the bound IgG with α-mouse secondary antibody for 10 minutes at 37°C. After cross-linking, the reactions were stopped by the addition of ice-cold 2 × lysis buffer. Immunoprecipitation of CD84 was performed as described above under “Platelet lysate preparation.”
CD84 extracellular domain Glu-fusion protein generation
An expression construct containing the cDNA corresponding to the peptide epitope EYMPME (Glu-Glu tag) and the extracellular domain of CD84 (CD84-Ec) was cloned into the mammalian expression vector pSecTag2/Hygro A (Invitrogen, Carlsbad, CA) by PCR. Supernatants from 293T cells were purified over a Glu-antibody column (Covance Research Products, Denver, PA).16 Purity of the protein was assessed by SDS-PAGE and Coomassie staining.
Platelet spreading
For spreading experiments, glass coverslips were incubated with 50 μg/mL CD84-Ec for 2 hours at 37°C. Following washing, the coverslips were blocked with 0.5% fatty acid–free bovine serum albumin (BSA) (Sigma, St Louis, MO)/phosphate-buffered saline (PBS) for 1 hour at room temperature. Washed human (0.5-1 × 108 cells/mL) platelets were adhered to the coated coverslips for 30 minutes at 37°C. Similarly, washed murine platelets (0.5-1 × 108 cells/mL) were adhered in the absence or presence of 1 μM ADP added immediately prior to plating. When required, platelets were treated with the P2Y1 antagonist (100 μM MRS2179) for 10 minutes prior to plating. Nonadhered platelets were removed by washing, and the adhered platelets were fixed, permeabilized, and stained with tetramethyl-rhodamine-5(and 6)-isothiocyanate (TRITC)–conjugated phalloidin. Platelets were viewed on a Nikon Eclipse E1000 microscope with 40 ×/0.95 NA or 100 ×/1.4 NA objectives (Nikon, Tokyo, Japan). Images were collected with a Hamamatsu camera (Hamamatsu City, Japan), and imaging was performed using Simple PCI (Technical Instruments, Cranberry Township, PA). Photo images displayed are representative of 3 different images from at least 3 experiments.
Arterial thrombosis
Ferric chloride injury model was performed as previously described13 (Figure 6). Briefly, anaesthetized mice were injected with rhodamine 6G to fluorescently label platelets in vivo. Mesenteric arteries were injured with 10% ferric chloride, and the thrombotic process was recorded in real time for 40 minutes or until vessel occlusion occurred.
Statistical analyses
All comparisons were done using unpaired Student t test. P values below .05 were considered significant.
Results
Identification of platelet CD84
To identify novel membrane receptors that become tyrosine phosphorylated during platelet aggregation, we used a proteomics approach. In preliminary experiments, we confirmed that many platelet proteins become tyrosine phosphorylated during aggregation. Washed platelets were induced to aggregate by TRAP-induced stimulation, and the platelet aggregates were detergent solubilized and the tyrosine-phosphorylated proteins were captured on an α-phosphotyrosine affinity column (PY99). Bound proteins were eluted with phenylphosphate, subject to SDS-PAGE, and 8 bands were detected after Coomassie staining (data not shown). The identities of these eluted proteins were obtained by in-gel tryptic digestion and sequencing by LC/MS/MS. While most proteins were known signaling proteins in platelets, the band that migrated at approximately 70 kDa contained 8 peptides corresponding to a total of 33% of the amino acid sequence of CD84 (Table 1), a membrane protein not known to signal by platelet aggregation. The subcellular distribution of CD84 in human platelets was determined by FACS (Figure 1A). CD84 was observed on the surface of unstimulated platelets with little change in expression upon agonist stimulation (Figure 1A, upper panel), while P-selectin (CD62P) showed a marked increase in expression (Figure 1A, lower panel). These data indicate that CD84 pre-exists on the surface of unactivated platelets and becomes tyrosine phosphorylated during platelet aggregation.
CD84 tryptic peptides detected by LC/MS/MS
Peptide no. . | Peptide sequence . |
|---|---|
| 1 | 288 (R)lyDEILQSK(V) 297 |
| 2 | 297 (K)VLPSKEEPVNTVySEVQFADK(M) 319 |
| 3 | 108 (K)ADINTQADPYTTTK(R) 123 |
| 4 | 321 (K)ASTQDSKPPGTSSYEIVI(-) 339 |
| 5 | 84 (R)IHALGPNYNLVISDLR(M) 101 |
| 6 | 50 (K)IIAWTSK(T) 57 |
| 7 | 57 (K)TSVAYVTPGDSETAPVVTVTHR(N) 80 |
| 8 | 123 (R)YNLQIYR(R) 131 |
Peptide no. . | Peptide sequence . |
|---|---|
| 1 | 288 (R)lyDEILQSK(V) 297 |
| 2 | 297 (K)VLPSKEEPVNTVySEVQFADK(M) 319 |
| 3 | 108 (K)ADINTQADPYTTTK(R) 123 |
| 4 | 321 (K)ASTQDSKPPGTSSYEIVI(-) 339 |
| 5 | 84 (R)IHALGPNYNLVISDLR(M) 101 |
| 6 | 50 (K)IIAWTSK(T) 57 |
| 7 | 57 (K)TSVAYVTPGDSETAPVVTVTHR(N) 80 |
| 8 | 123 (R)YNLQIYR(R) 131 |
Lowercase represents the site of phosphorylation established by LS/MS/MS. Superscript numbers indicate amino acid numbers; parentheses, first; -, last amino acid.
Aggregation- and immuneclustering-induced tyrosine phosphorylation of CD84
CD84 contains 2 extracellular IgG motifs and 4 cytoplasmic tyrosines: 273Y, 275Y, 290Y, and 310Y. Two of these are embedded within ITSM (TxYxxV/I where x is any amino acid) motifs, 271TI273YTYI276 and 308TV310YSEV313. The LC/MS/MS data presented in Table 1 indicated that 290Y and 310Y were phosphorylated in platelet aggregates, demonstrating that one of the CD84 ITSM motifs becomes tyrosine phosphorylated as a consequence of platelet aggregation. Confirmation that platelet aggregation induced CD84 tyrosine phosphorylation was obtained by Western blotting of CD84-immunocomplexes isolated from resting or aggregated platelets with an α-phosphotyrosine–specific antibody (Figure 1B, upper panel). Tyrosine phosphorylation of CD84 was a consequence of platelet aggregation. The aggregation dependency for CD84 tyrosine phosphorylation was established by the effects of the platelet αIIbβ3-specific antagonist eptifibatide, which prevents platelet aggregation by blocking the binding of fibrinogen to activated αIIbβ3 but does not block platelet activation17 (Figure 1B). Eptifibatide was found to markedly reduce the tyrosine phosphorylation of CD84. We typically observed that a significant (∼30%-50%) pool of tyrosine-phosphorylated CD84 was rendered Triton X-100 insoluble during platelet aggregation (Figure 1B, lower panel). Similar observations have been made for αIIbβ3 and other signaling proteins phosphorylated during aggregation,18 suggesting that protein-protein interactions induced by tyrosine phosphorylation of CD84 was responsible for the inaccessibility of CD84 Triton X-100 solubilization.
Expression and phosphorylation of CD84 in platelets. (A) Top panel: FACS analysis of α-CD84 IgG-FITC stained resting (broken line) or Thrombin Receptor Activating Peptide (TRAP)–activated (solid line) platelets. Dotted line indicates staining with nonspecific IgG. Bottom panel: platelet activation confirmed by α-CD62P IgG-PE staining of resting (solid line) and activated (broken line) platelets. (B) Western blotting of immunoprecipitates from resting (lane 1) or agonist-induced platelet aggregates formed in the absence (lanes 2-4) or presence (lanes 5-7) of 2 μM eptifibatide. (C) Western blotting of immunoprecipitates from washed human platelets activated with either α-CD84-IgG (lanes 1, 3, and 4) or nonspecific IgG (lane 2) compared with platelet aggregation induced with 5 μM TRAP (lane 5). Antibody-induced signaling induced with 15 μg/mL α-mouse whole-IgG (lanes 1 and 2) or α-mouse Fab′2 (lane 3). For both B and C, lysed platelets were immunoprecipitated with α-CD84-IgG and immunoblotted with α-phosphotyrosine antibodies PY20 and 4G10 (top panel) or α-CD84 (bottom panel). Data are representative of experiments repeated at least 3 to 5 times.
Expression and phosphorylation of CD84 in platelets. (A) Top panel: FACS analysis of α-CD84 IgG-FITC stained resting (broken line) or Thrombin Receptor Activating Peptide (TRAP)–activated (solid line) platelets. Dotted line indicates staining with nonspecific IgG. Bottom panel: platelet activation confirmed by α-CD62P IgG-PE staining of resting (solid line) and activated (broken line) platelets. (B) Western blotting of immunoprecipitates from resting (lane 1) or agonist-induced platelet aggregates formed in the absence (lanes 2-4) or presence (lanes 5-7) of 2 μM eptifibatide. (C) Western blotting of immunoprecipitates from washed human platelets activated with either α-CD84-IgG (lanes 1, 3, and 4) or nonspecific IgG (lane 2) compared with platelet aggregation induced with 5 μM TRAP (lane 5). Antibody-induced signaling induced with 15 μg/mL α-mouse whole-IgG (lanes 1 and 2) or α-mouse Fab′2 (lane 3). For both B and C, lysed platelets were immunoprecipitated with α-CD84-IgG and immunoblotted with α-phosphotyrosine antibodies PY20 and 4G10 (top panel) or α-CD84 (bottom panel). Data are representative of experiments repeated at least 3 to 5 times.
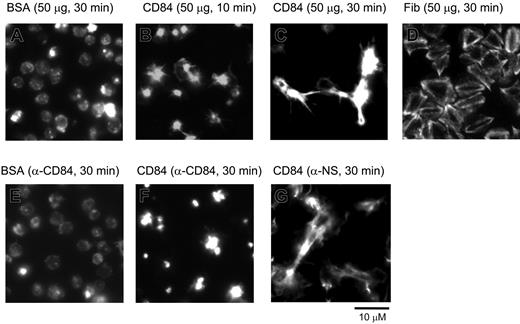
Spreading of human platelets on the immobilized extracellular domain of CD84. Washed human platelets seeded on coverslips coated with various proteins at a concentration of 50 μg/mL. Platelets were preincubated with either 5 μg/mL α-CD84 (E and F) or isotype control Fab fragment (G) prior to seeding. Pictures are representatives of experiments repeated at least 3 times in duplicate.
Spreading of human platelets on the immobilized extracellular domain of CD84. Washed human platelets seeded on coverslips coated with various proteins at a concentration of 50 μg/mL. Platelets were preincubated with either 5 μg/mL α-CD84 (E and F) or isotype control Fab fragment (G) prior to seeding. Pictures are representatives of experiments repeated at least 3 times in duplicate.
CD84 signaling in intact platelets was further investigated by measuring CD84 tyrosine phosphorylation following receptor ligation with an α-CD84-IgG or nonspecific IgG. Cross-linking of bound α-CD84 IgG, but not nonspecific IgG, together with either whole α-mouse IgG or its Fab′2 fragment, resulted in CD84 tyrosine phosphorylation (Figure 1C, upper panel). As α-CD84 IgG by itself was unable to induce phosphorylation, antibody-induced CD84 tyrosine phosphorylation was likely to have occurred specifically through ligation and clustering of surface CD84 and not the Fc portion of the activating IgGs.
CD84 extracellular domain induces platelet spreading through homophilic interactions
It has previously been established that CD84 is an adhesion receptor and functions by homophilic interactions and by clustering.19 These data include the demonstration that a recombinant protein expressing the extracellular domain of CD84 binds to platelets, and this binding is blocked by an α-CD84 blocking monoclonal antibody (mAb); Fab fragments of the α-CD84 blocking mAb block the binding of the recombinant CD84 protein to CD84-transfected cells,19 and ligation of lymphocytic surface expressed CD84 results in enhanced interferon (IFN)–γ secretion19 and proliferation.20 Since CD84 signals occurred during platelet aggregation and by clustering, we speculated that homophilic interactions occurred between the CD84 ectodomains. To address this possibility, a construct encoding the 2 extracellular IgG domains of CD84 fused to a Glu-tag (CD84-Ec) was expressed in mammalian cells and studied for its platelet stimulatory activity (Figure 2). Human washed platelets seeded on immobilized CD84-Ec displayed a time-dependent morphologic change typical of platelet stimulation followed by platelet-platelet contact, contraction, and filopodia extension. By contrast, platelets seeded on immobilized BSA for the same duration displayed a round inactive morphology. The morphologic changes elicited by CD84 were markedly different from that observed with immobilized fibrinogen (Figure 2D), an αIIbβ3 ligand that functions as a weak platelet agonist and fails to induce aggregates.21 In addition, an α-CD84-Fab, but not isotype-control Fab fragment, blocked CD84-Ec–induced morphologic changes (Figure 2E-F). The data suggest that homophilic interactions of CD84 induce platelet stimulations.
Identification of additional SLAM family members and adapter proteins in human platelets
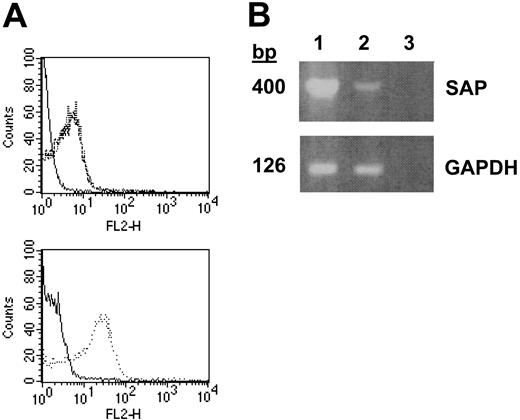
To determine if other SLAM family members and the SLAM adapter protein SAP were expressed in platelets, we performed high throughput molecular profiling of RNA isolated from double-phoresed human platelets using custom arrays, representing 25 000 genes. The sequences for the genes represented on the custom arrays were derived both from sequencing of platelet and human umbilical vein endothelial cell (HUVEC) cDNA libraries as well as all the nonoverlapping human genes present in the public gene database. Analysis of the data obtained from these custom assays revealed that RNA from platelets hybridized to probes for the following members of the SLAM family: CD84, SLAM, CD48 and 2B4, with no signal detected for 2 members of the CD2 family of cell-surface receptors that are expressed in leukocytes: CD58 or BLAME (B-lymphocyte activator macrophage expressed). Comparing the relative expression of these SLAM family members in platelets to peripheral blood leukocytes (PBLs) showed that CD84 was expressed at a relative level of 1.16, SLAM at 0.25, CD48 at 0.78, and 2B4 at 0.11. A strong signal for the SLAM adapter protein SAP also was detected (relative expression to PBLs was 0.38). Since in platelets SLAM message was as abundant as CD84 and since α-SLAM antibodies were available, we directly determined whether SLAM existed in platelets. Indeed, using FACS analysis (Figure 3A), SLAM expression was found to be similar to CD84 in that it was readily detected on the surface of unstimulated platelets, and its expression was not increased upon agonist stimulation. Although the expression of SAP also was observed by reverse transcriptase–polymerase chain reaction (RT-PCR) of RNA from platelets (Figure 3B), the direct demonstration of SAP in human platelets could not be confirmed due to the lack of an α-human SAP antibody.
Expression of SLAM and SAP in human platelets. (A) Top panel: FACS analysis of resting platelets stained with nonspecific (solid line) or α-SLAM (broken line) IgG-PE and TRAP-activated platelets stained with α-SLAM IgG-PE (dotted line). Bottom panel: platelet activation confirmed by α-CD62P IgG-PE staining of resting (solid line) and activated (dotted line) platelets. (B) RT-PCR of SAP and GAPDH from platelet (lane 1) and spleen (lane 2) cDNA with dH2O as a control (lane 3). Data are representative of experiments repeated at least 3 to 5 times.
Expression of SLAM and SAP in human platelets. (A) Top panel: FACS analysis of resting platelets stained with nonspecific (solid line) or α-SLAM (broken line) IgG-PE and TRAP-activated platelets stained with α-SLAM IgG-PE (dotted line). Bottom panel: platelet activation confirmed by α-CD62P IgG-PE staining of resting (solid line) and activated (dotted line) platelets. (B) RT-PCR of SAP and GAPDH from platelet (lane 1) and spleen (lane 2) cDNA with dH2O as a control (lane 3). Data are representative of experiments repeated at least 3 to 5 times.
Expression and phosphorylation of SLAM, SAP, and EAT-2 in murine platelets. (A) Left panel: representative washed mouse platelet aggregation measurement showing inhibition of 4 μg/mL collagen-induced aggregation by the specific murine αIIbβ3 inhibitor ML00341819. Right panel: Western blot of resting or 4 μg/mL collagen-induced aggregated samples in the absence (lane 2) or presence of 5 μM (lane 3) or 10 μM (lane 4) ML00341819. Lysed platelets were immunoprecipitated with α-SLAM (9D-1) IgG. (B) Western blotting of washed wt, SAP–/–, and SLAM–/– mouse platelets resting (R) or aggregated (A) with 0.5 U/mL thrombin. Lysed platelets were immunoprecipitated with either nonspecific (lane 1) or α-SLAM (9D-1) IgGs (lanes 2-7). For both A and B, immunoblotting was performed with α-phosphotyrosine PY20 and 4G10, α-SLAM, α-SAP, α-EAT2 antibodies as indicated. Data shown are representative of experiments performed 3 times.
Expression and phosphorylation of SLAM, SAP, and EAT-2 in murine platelets. (A) Left panel: representative washed mouse platelet aggregation measurement showing inhibition of 4 μg/mL collagen-induced aggregation by the specific murine αIIbβ3 inhibitor ML00341819. Right panel: Western blot of resting or 4 μg/mL collagen-induced aggregated samples in the absence (lane 2) or presence of 5 μM (lane 3) or 10 μM (lane 4) ML00341819. Lysed platelets were immunoprecipitated with α-SLAM (9D-1) IgG. (B) Western blotting of washed wt, SAP–/–, and SLAM–/– mouse platelets resting (R) or aggregated (A) with 0.5 U/mL thrombin. Lysed platelets were immunoprecipitated with either nonspecific (lane 1) or α-SLAM (9D-1) IgGs (lanes 2-7). For both A and B, immunoblotting was performed with α-phosphotyrosine PY20 and 4G10, α-SLAM, α-SAP, α-EAT2 antibodies as indicated. Data shown are representative of experiments performed 3 times.
Expression of SLAM, SAP, and EAT-2 and aggregation-induced tyrosine phosphorylation of SLAM in murine platelets
In an effort to study SLAM family function and SLAM family signaling in platelets, we turned to a model where both immunoreagents and mouse models were available. The expression of SLAM, SAP, and EAT-2 in mouse platelets was confirmed by Western blotting (Figure 4A-B). To determine if SLAM was also tyrosine phosphorylated upon aggregation, SLAM immunoprecipitates isolated from resting and activated wt (wild-type) murine platelets were probed with an α-phosphotyrosine–specific antibody (Figure 4A). As observed for CD84 in human platelets, aggregation induced robust tyrosine phosphorylation of SLAM, and aggregation dependency of this tyrosine phosphorylation was confirmed by its inhibition in the presence of the mouse platelet αIIbβ3-specific antagonist ML0034181914 (Figure 4A). Aggregated SLAM–/– platelets did not display a tyrosine-phosphorylated band at the molecular weight of SLAM (Figure 4B, top panel). SAP was detected in immunoprecipitates of SLAM from aggregated platelets (Figure 4, third panel). Intriguingly, aggregation-induced SLAM tyrosine phosphorylation also occurred in SAP–/– platelets (Figure 4B, second panel). This observation is of interest as it indicates a difference in regulation of platelet SLAM phosphorylation compared to that seen in thymocytes, where SLAM tyrosine phosphorylation is entirely SAP dependent.8,9 Even though EAT-2 was not present on our custom arrays, an antibody against murine EAT-2 revealed its presence in murine platelets. Unlike SAP, however, it did not coimmunoprecipitate with phosphorylated SLAM (Figure 4B).
Contribution of SLAM and SAP to stabilization of platelet aggregates in vitro and thrombus formation in vivo
SLAM–/– and SAP–/– platelets were analyzed by agonist-induced aggregation reactions (Figure 5). Compared to wt platelets, the aggregation response of SLAM–/– platelets was blunted when stimulated with low (1 mM, data not shown) or high (2.5 mM) doses of mTRAP (Figure 5).22 A similar defect was observed when platelets were stimulated with collagen; however, response to ADP was normal. SAP–/– platelet aggregations were not impaired at low (data not shown) or high concentrations of mTRAP, collagen, or ADP (Figure 5iv-5vi).
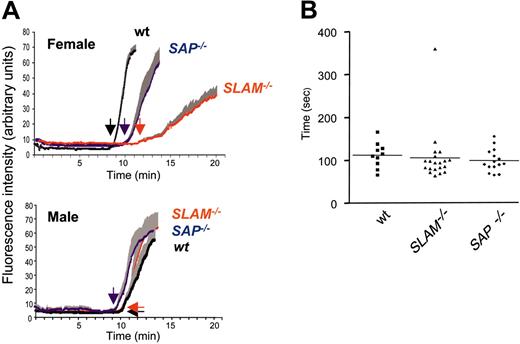
The roles for SLAM and SAP were further examined in vivo using a thrombosis model (Figure 6A) and by tail-bleeding measurements (Figure 6B). In vivo platelet function was assessed by real-time continuous analysis of the thrombotic profile post ferric-chloride injury of the mesenteric arteries of 3-week-old mice (Figure 6A). Female SLAM–/– mice revealed a delay in the time for appearance of first thrombus and occlusion of the vessel (time to occlusion (± SEM, n = 7) was 11.78 ± 0.48 minutes (female wt) and 21.73 ± 2.4 (female SLAM–/–, P vs wt < .01). Compared to wt mice, there also was an increase in embolization of small thrombi during the thrombotic process. Unlike the in vitro aggregation phenotype, the thrombosis defect was sex specific and was apparent only in females. Factors within the vasculature of male mice most likely differ from that of female mice to compensate for this difference. Both SLAM–/– and SAP–/– mice demonstrated normal tail-bleeding times (Figure 6B).
In vitro agonist-induced aggregation measurements of platelets from wt, SLAM–/–, and SAP–/– mice. Representative aggregation measurements in PRP obtained from wt (black), SLAM–/– (A-C), or SAP–/– (D-F), male (red) or female (blue) mice in response to 2.5 mM murine TRAP (A and D), 10 μg/mL collagen (B and E), and 10 μM ADP (C and F). Traces from wt male and female aggregatory responses were superimposable. Tracings represent aggregation profiles generated from blood pulled from 3 mice repeated at least 5 times.
In vitro agonist-induced aggregation measurements of platelets from wt, SLAM–/–, and SAP–/– mice. Representative aggregation measurements in PRP obtained from wt (black), SLAM–/– (A-C), or SAP–/– (D-F), male (red) or female (blue) mice in response to 2.5 mM murine TRAP (A and D), 10 μg/mL collagen (B and E), and 10 μM ADP (C and F). Traces from wt male and female aggregatory responses were superimposable. Tracings represent aggregation profiles generated from blood pulled from 3 mice repeated at least 5 times.
In vivo thrombus formation and bleeding times in wt, SLAM–/–, and SAP–/– mice. (A) Intravital microscopy of thrombus formation after FeCl3 injury of the mesenteric artery in young C57Bl/6 wt (black), SLAM–/– (blue), and SAP–/– (red) mice was determined as described.13 The deposition of rhodamine 6G–labeled platelets at site of vessel wall injury (thrombotic profile) was determined by intravital microscopy of the mesenteric artery of 3-week-old mice; one vessel was studied per mouse. The increase in fluorescence intensity relates in a linear fashion to the number of platelets.%13 Tracings represent the means of the thrombotic profiles obtained from female (upper panel) and male (lower panel) mice (n = 7, ± SEM). Arrows indicate the time of appearance of the first thrombus. (B) Timing of cessation of tail bleeding in wt (n = 10), SLAM–/– (n = 20), and SAP–/– (n = 16) mice performed as previously described.1 No sex differences were observed in any group. Means depicted by (horizontal lines).
In vivo thrombus formation and bleeding times in wt, SLAM–/–, and SAP–/– mice. (A) Intravital microscopy of thrombus formation after FeCl3 injury of the mesenteric artery in young C57Bl/6 wt (black), SLAM–/– (blue), and SAP–/– (red) mice was determined as described.13 The deposition of rhodamine 6G–labeled platelets at site of vessel wall injury (thrombotic profile) was determined by intravital microscopy of the mesenteric artery of 3-week-old mice; one vessel was studied per mouse. The increase in fluorescence intensity relates in a linear fashion to the number of platelets.%13 Tracings represent the means of the thrombotic profiles obtained from female (upper panel) and male (lower panel) mice (n = 7, ± SEM). Arrows indicate the time of appearance of the first thrombus. (B) Timing of cessation of tail bleeding in wt (n = 10), SLAM–/– (n = 20), and SAP–/– (n = 16) mice performed as previously described.1 No sex differences were observed in any group. Means depicted by (horizontal lines).
CD84-induced spreading is dependent on the adapter molecule SAP
Previous studies in leukocytes have shown that CD84 signaling partly depends on the binding of SAP and EAT-2 to the tyrosinephosphorylated ITSM motif. Since this motif becomes tyrosine phosphorylated by aggregation, we next asked whether CD84 signaling depended on those proteins. Because the adapter molecule SAP is expressed in murine platelets (Figure 4), we determined whether a SAP-dependent signaling pathway was involved in CD84-induced spreading using platelets from SAP–/– mice. In the setting where platelet adhesion was stimulated by CD84-Ec, SAP–/– platelets displayed reduced spreading compared to wt in the absence or presence of ADP. On the other hand, SAP–/– platelets showed normal spreading on immobilized fibrinogen (Figure 7). To confirm that αIIbβ3 outside-in signaling is not involved in the CD84-Ec–induced response, we studied platelets from the diYF mouse, which harbors a mutated version of the β3 integrin, where the cytoplasmic tyrosines are substituted with phenylalanines.1 While the diYF platelets show a spreading defect on fibrinogen-coated surfaces (data not shown), no defect was observed with respect to area spread above baseline (± SD) on CD84-Ec–coated surfaces in the absence (wt platelets 5.6 ± 1.6 μm,2 diYF platelets 4.6 ± 2.1 μm2) or presence of 1 μM ADP (wt platelets 7.5 ± 2.8 μm,2 diYF platelets 7.3 ± 2.9 μm2). Thus, a SAP-dependent pathway is involved in CD84-induced signaling in platelets that does not require signaling through the cytoplasmic domain of αIIbβ3. We also tested the hypothesis that CD84 may act as an adhesion receptor for SLAM. SLAM–/– platelets adhered with the same efficacy to immobilized CD84-Ec as did wt platelets (spreading above baseline (± SD) was 4.5 ± 1.5 μm2 for wt platelets, 4.3 ± 0.9 μm2 for SLAM–/– platelets; in the presence of 1 μM ADP spreading above baseline (± SD) was 7.8 ± 1 μm2 for wt platelets and 8.3 ± 2.3 μm2 for SLAM–/– platelets), implying that CD84 is not a coreceptor for SLAM.
Discussion
It is well established that the platelet integrin αIIbβ3 is responsible for the primary interaction of platelets during thrombosis and hemostasis and that soluble stimuli (eg, CD40L, thromboxane A2, ADP, Gas6) are released to support this process. Published data, including those from our laboratory, have been based on the assumption that aggregate-induced signaling is induced by “outside-in” signaling through αIIbβ3. The signaling reactions though additional receptors induced by platelet-platelet contact, particularly under conditions induced by arterial shear, have not been previously described. The data presented in this paper have led us to the identification of additional aggregation-induced signaling receptors that are induced by platelet-platelet contact. We show that the 2 members of the SLAM family of homophilic adhesion receptors, which operate at the interface of antigen-presenting and lymphoid cells in immune responses, are not only expressed on the platelet surface but also tyrosine phosphorylated in an aggregation-dependent manner, contributing to cohesion of platelet-platelet contacts, and that ligation of these receptors will induce platelet activation.
Previous studies have shown that SLAM on the surface of T cells interacts with SLAM on the surface of antigen-presenting cells, thereby inducing interleukin-2 (IL-2) and IFN-γ production. This event is dependent on the recruitment of the adapter protein SAP to tyrosine-phosphorylated SLAM as demonstrated by the lack of SLAM tyrosine phosphorylation in stimulated SAP–/– thymocytes or peripheral blood T cells.8 In this scenario it has been suggested that SLAM functions as a co-receptor to mediate T-cell receptor signaling. Similarly, in T and B cells, CD84 is tyrosine phosphorylated following receptor ligation induced by an agonistic mAb,23 and CD84-CD84 homophilic interactions result in CD84-induced IFN-γ secretion and enhanced proliferation.19 However, these CD84-mediated events are not all reliant on SAP, as T cells from X-linked lymphoproliferative syndrome patients, where the SAP gene is mutated, respond the same way as T cells from unaffected individuals to anti-CD3 stimulation.20
Spreading of wt and SAP–/– platelets on the immobilized extracellular domain of CD84. Graphic representation of wt (n = 5) and SAP–/– (n = 5) washed mouse platelet spreading on the indicated surfaces in the presence or absence of added 1 μM ADP or 100 μM MRS2179. **, P < .01; ***P < .005.
Spreading of wt and SAP–/– platelets on the immobilized extracellular domain of CD84. Graphic representation of wt (n = 5) and SAP–/– (n = 5) washed mouse platelet spreading on the indicated surfaces in the presence or absence of added 1 μM ADP or 100 μM MRS2179. **, P < .01; ***P < .005.
In platelets, both SLAM and CD84 are tyrosine phosphorylated in an aggregation-dependent manner; however, there seem to be differences in SLAM/CD84 signaling in platelets compared to lymphocytes. First, SLAM tyrosine phosphorylation in murine platelets does not require SAP, a conclusion based on the similar SLAM tyrosine phosphorylation levels observed upon aggregation of wt and SAP–/– platelets (Figure 4B). Secondly, EAT-2, which also binds to the same SLAM tyrosine-phosphorylated residues as SAP in lymphocytes, is present in murine platelets and does not bind to platelet phosphorylated SLAM (Figure 4B). An additional difference is that tyrosine at position 290, which is outside the ITSM consensus sites on CD84 (Table 1), is tyrosine phosphorylated in platelets but has not been detected in lymphocytes. While we cannot rule out the dependency of platelet SLAM and CD84 signaling on SAP and EAT-2, clearly there are differences in signaling between lymphocytes and platelets.
Recently it has been reported24 that binding of the soluble ligand Gas6 to its receptors can activate downstream signaling pathways, leading to tyrosine phosphorylation of the β3 integrin, thereby enhancing outside-in signaling via αIIbβ3. From these findings, it was speculated that blocking this interaction may be a novel approach for the prevention of irreversible platelet aggregates. In contrast, SLAM family signaling during platelet aggregation is independent of β3 tyrosine phosphorylation as demonstrated by normal spreading of diYF platelets on immobilized CD84-Ec. Although it is possible that SLAM family proteins may have cis- or trans- interactions with αIIbβ3 during aggregation, it seems more probable that SLAM signaling is secondary to integrin-mediated platelet-platelet contact and that the emerging model of the immune synapse may be applicable to platelets. In this model, primary cell-cell interactions are mediated by integrins, but secondary signaling by additional receptors is initiated by cell proximity. In addition to the SLAM family, these could include other receptors and soluble factors such as the Eph kinases and ephrins, CD40L, and Gas6, which function secondary to platelet aggregation.2,3 We thus speculate that platelet-platelet proximity mediated by αIIbβ3-induced aggregation constitutes a prothrombotic environment, much like a pro-immune environment generated by the lymphocyte synapse induced during an inflammatory response, and that pharmaceutical targeting of SLAM family proteins represents a novel strategy for preventing the formation of irreversible platelet aggregates.
Prepublished online as Blood First Edition Paper, July 21, 2005; DOI 10.1182/blood-2005-01-0333.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Pablo Engle for helpful discussions in the preparation of the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal