CD146, also known as S-endo-1, P1H12, Mel-CAM, and MUC18, is a well-described adhesion marker of endothelial cells, which has also been identified on a limited number of other cell types, but not in fresh peripheral blood lymphocytes from healthy individuals.1,2 Due to its presumed specificity, the expression of CD146 has been used as the sole criterion to identify circulating endothelial cells (CECs), and has been widely used to isolate these cells from peripheral blood (reviewed in Blann et al3 and Khan et al4 ). In contrast, our flow cytometric studies of peripheral blood have routinely detected CD146+ leukocytes.
In fresh peripheral blood specimens from 10 healthy individuals stained with a panel of monoclonal antibodies to leukocyte antigens and analyzed by flow cytometry, CD45+ CD146+ cells were consistently identified as 1% or less of the mononuclear cells. Table 1 and Figure 1 illustrate the expression of CD146 found on lymphoid subsets. Phycoerythrin-conjugated CD146 (clone p1H12) obtained from Becton Dickinson Biosciences (San Jose, CA) and from Chemicon (Temecula, CA) yielded similar results.
Mean percentage of each lymphocyte subset that expresses cell surface CD146 (from peripheral blood of 10 healthy individuals)
Lymphocyte subset . | Mean ± SD, % . |
|---|---|
| CD3+ T cells | 2.09 ± 0.84 |
| CD3+ CD4+ T cells | 2.08 ± 0.73 |
| CD3+ CD8+ T cells | 2.50 ± 2.47 |
| CD19+ B cells | 0.74 ± 0.86 |
| CD3− NK cells | 0.11 ± 0.13 |
Lymphocyte subset . | Mean ± SD, % . |
|---|---|
| CD3+ T cells | 2.09 ± 0.84 |
| CD3+ CD4+ T cells | 2.08 ± 0.73 |
| CD3+ CD8+ T cells | 2.50 ± 2.47 |
| CD19+ B cells | 0.74 ± 0.86 |
| CD3− NK cells | 0.11 ± 0.13 |
The CD3, CD146+ cells also expressed CD2, CD5, and CD7, confirming that these cells were T lymphocytes. The use of pulse width measurements eliminated the possibility that these cells were doublets of CECs and T cells, and the use of a viability stain (7-aminoactinomycin D [7-AAD]), as well as isotype controls, excluded the possibility that this staining was due to nonspecific binding of the monoclonal antibodies. Washing peripheral blood mononuclear cells, as well as whole peripheral blood, with phosphate-buffered saline (PBS) twice prior to staining revealed no change in the percentage of T cells positive for CD146 or in the intensity of CD146 staining, thus eliminating the possibility that the lymphoid CD146 was due to binding of soluble CD146.
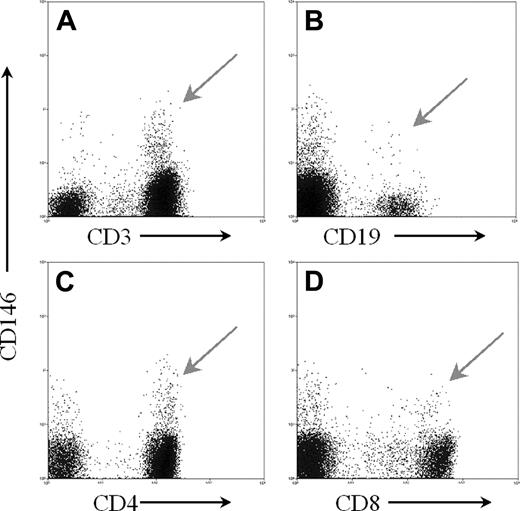
Freshly drawn peripheral blood stained with CD146 and antibodies to various markers for lymphocyte subsets. Using forward and side light scatter, a gate was set around the lymphoid region. A second gate was set to exclude any dead cells displaying 7-AAD staining, and a third gate based on pulse width was set to exclude doublets. All of these histograms were gated on these 3 gates. Additionally, the CD4 and CD8 histograms were also gated on the CD3+ cells. (A) CD3 versus CD146; (B) CD19 versus CD146; (C) CD4 versus CD146; and (D) CD8 versus CD146. Arrows indicate CD146+ lymphocyte subsets.
Freshly drawn peripheral blood stained with CD146 and antibodies to various markers for lymphocyte subsets. Using forward and side light scatter, a gate was set around the lymphoid region. A second gate was set to exclude any dead cells displaying 7-AAD staining, and a third gate based on pulse width was set to exclude doublets. All of these histograms were gated on these 3 gates. Additionally, the CD4 and CD8 histograms were also gated on the CD3+ cells. (A) CD3 versus CD146; (B) CD19 versus CD146; (C) CD4 versus CD146; and (D) CD8 versus CD146. Arrows indicate CD146+ lymphocyte subsets.
Minimal coexpression of CD146 with CD25, CD69, and human leukocyte antigen-D region related (HLA-DR) was observed on CD3+ cells in fresh peripheral blood. The CD146, CD3+ cells were αβ positive, γδ negative, and partially positive for expression of CD28. CD3+ T cells staining positive for CD146 were negative for the endothelial markers CD31, CD51/61, and von Willebrand factor. Antibodies to T-cell receptor (TCR) vβ regions revealed that CD3+CD146+ T cells were polyclonal in both fresh and stimulated blood.
Peripheral blood mononuclear cells were cultured in vitro in the presence of mitogens or interleukin-2 (IL-2) for up to 5 days. Both increased the number of T cells expressing CD146 as well as the mean intensity of the CD146 staining, consistent with observations of Pickl et al.5 CD146 expression was induced in CD146- T cells purified by flow cytometric cell sorting and then cultured in vitro with mitogens for 72 hours.
Fresh B cells were activated in vitro with CD40 ligand (CD40L) and IL-4. The activated B cells displayed increased numbers of CD146+ cells after 5 days of activation, and the intensity of CD146 staining increased compared with the fresh cells. CD146- B cells, sorted by flow cytometry from fresh peripheral blood, revealed de novo expression of CD146 after in vitro activation.
Preliminary data suggest that CD146+ T cells exhibit an increased ability to adhere to endothelial cells, as determined by in vitro binding assays. The current data indicate that CD146 is an intrinsic, inducible antigen on multiple lymphocyte subsets and may be involved in adherence to the endothelium. CD146 can no longer be regarded as an endothelial-specific marker, particularly in the context of CEC isolation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal