Abstract
Recently, somatic mutations of the nucleophosmin gene (NPM1), which alter the subcellular localization of the product, have been reported in acute myeloid leukemia (AML). We analyzed the clinical significance of NPM1 mutations in comparison with cytogenetics, FLT3, NRAS, and TP53 mutations, and a partial tandem duplication of the MLL gene (MLL-TD) in 257 patients with AML. We found NPM1 mutations, including 4 novel sequence variants, in 64 of 257 (24.9%) patients. NPM1 mutations were associated with normal karyotype and with internal tandem duplication (ITD) and D835 mutations in FLT3, but not with other mutations. In 190 patients without the M3 French-American-British (FAB) subtype who were treated with the protocol of the Japan Adult Leukemia Study Group, multivariate analyses showed that the NPM1 mutation was a favorable factor for achieving complete remission but was associated with a high relapse rate. Sequential analysis using 39 paired samples obtained at diagnosis and relapse showed that NPM1 mutations were lost at relapse in 2 of the 17 patients who had NPM1 mutations at diagnosis. These results suggest that the NPM1 mutation is not necessarily an early event during leukemogenesis or that leukemia clones with NPM1 mutations are sensitive to chemotherapy. (Blood. 2005;106:2854-2861)
Introduction
Acute myeloid leukemia (AML) is characterized by autonomous proliferation and impaired differentiation of hematopoietic progenitors but is a genetically and phenotypically heterogeneous disease. A number of genetic mutations, such as point mutations, gene rearrangements, and chromosomal translocations, which are involved in the pathogenesis of leukemia, have been documented. Recently, it was suggested that AML is the consequence of 2 broad complementation classes of mutations: those that confer a proliferative or a survival advantage to hematopoietic progenitors (class 1)—including activating mutations in tyrosine kinases such as BCR-ABL1, ETV6-PDGFRB, KIT, and FLT3 or their downstream effectors such as NRAS—and those that impair hematopoietic differentiation and confer properties of self-renewal (class 2)—including rearrangements or point mutations of core binding factor (CBF) genes and PML-RARA.1 Mutations in FLT3, NRAS, and KIT have been found in approximately 30% to 35%, 15% to 20%, and 5% to 10% of adult patients with AML, respectively, indicating that mutations in these 3 genes are the most frequent genetic alterations in AML.2-12 FLT3 and KIT mutations are often found in AML patients with PML-RARA and CBF gene translocations, respectively,13,14 whereas FLT3 mutations have been preferentially found in AML patients with normal karyotype. Because mutated FLT3 reportedly induces myeloproliferative disease (MPD), but not AML, in primary hematopoietic progenitors in the murine bone marrow transplantation model and MPD does not have serial transplantability, FLT3 mutations alone are not sufficient for the development of AML.15 Therefore, it has been suggested that additional mutations are involved in the pathogenesis of AML with FLT3 mutations.
Recently, Falini et al16 reported that the nucleophosmin gene (NPM1) is mutated in a high proportion of adults with AML, resulting in an aberrant cytoplasmic localization of the product (NPMc+). Of note is that NPMc+ is associated with a wide spectrum of morphologic subtypes of AML, a normal karyotype, and FLT3 mutations. Furthermore, NPMc+ AML is clinically associated with better responsiveness to induction chemotherapy, although its prognostic implications for long-term outcome remain unclear. Although the prevalence and significance of several genetic abnormalities in patients with AML have been reported to date, the most powerful prognostic factor in AML has been the karyotype of the leukemia cells.17 Three cytogenetic risk groups (favorable, intermediate, and poor) are widely accepted, but there is a practical limitation to the definition of cytogenetic risk, especially in patients in the intermediate group. Additional prognostic factors are, therefore, required. We and several groups9,10,12,18 have demonstrated that FLT3 mutations are a strong prognostic factor in AML, especially in patients with normal karyotype, but they do not affect responsiveness to induction chemotherapy. Therefore, NPM1 mutations seem to characterize a distinct disease entity not only of AML with normal karyotype but also of AML with FLT3 mutations.
NPM is a ubiquitously expressed phosphoprotein, continuously shuttles between the nucleus and the cytoplasm,19,20 and is involved in the oncogenesis of some types of leukemia and lymphoma because the NPM gene is a partner in several tumor-associated chromosomal translocations.21-23 However, it is also thought to have a tumor-suppressor function and to regulate the p53 pathway through its chaperoning activity.24-26 It is suggested that a loss of nuclear NPM function caused by mutation might impair the p53 pathway and that a lack of p53 might induce genetic instability27 ; hence, NPM1 mutations seem to cause AML cells to acquire additional genetic alterations.
In this study, we analyzed the prevalence and clinical characteristics of NPM1 mutations in comparison with cytogenetics, FLT3, NRAS, and TP53 mutations and a partial tandem duplication of the MLL gene (MLL-TD) in 257 patients with newly diagnosed de novo AML. The prognostic implications of NPM1 mutations were evaluated in 190 patients with AML, excluding those with the M3 FAB subtype, who were treated according to the protocol of the Japan Adult Leukemia Study Group (JALSG). Furthermore, to clarify the stability of NPM1 mutations and the potential effect on genetic instability in AML cells during disease progression, we compared the mutational status of these genes in 39 paired samples obtained at initial diagnosis and first relapse.
Patients, materials, and methods
Patients and samples
The diagnosis of AML was based on the French-American-British (FAB) classification. The study population included 257 patients with newly diagnosed de novo AML, as follows: 9 with the M0, 54 with the M1, 89 with the M2, 15 with the M3, 54 with the M4, 19 with the M5, 8 with the M6, and 9 with the M7 FAB subtype. In 39 of the 257 AML patients, paired samples obtained at diagnosis and first relapse were available. Furthermore, in 6 patients, samples obtained at diagnosis and complete remission (CR) were available. Bone marrow (BM) samples from patients with AML were subjected to Ficoll-Hypaque (Pharmacia LKB, Uppsala, Sweden) density gradient centrifugation. All samples taken at diagnosis or relapse were confirmed to contain more than 90% leukemia cells after enrichment by centrifugation. Informed consent was obtained from all patients to use their samples for banking and molecular analysis, and approval for these studies was obtained from the Nagoya University institutional review board.
Cytogenetic G-banding analysis was performed according to standard methods. In this study, cytogenetic risk groups were stratified according to the criteria adopted by the Medical Research Council.28
Screening for mutations of the FLT3, NRAS, and TP53 genes and of MLL-TD
High molecular weight DNA and total RNA were extracted from the samples using standard methods. FLT3 gene mutations of the ITD (FLT3/ITD) and activation loop (FLT3/D835Mt), NRAS gene mutations of codons 12, 13, and 61, and TP53 gene mutations of exons 5 to 8 were examined as reported and were confirmed by the sequencing procedure.4,29,30 MLL-TD was examined by reverse transcription-polymerase chain reaction (RT-PCR), as described previously.31
Screening for mutations of the NPM1 gene
For the screening of NPM1 mutations, we amplified genomic DNA corresponding to exon 12 of NPM1 by PCR using the primers NPM1-F, 5′-TTAACTCTCTGGTGGTAGAATGAA-3′ and NPM1-R, 5′-CAAGACTATTTGCCATTCCTAAC-3′, as previously reported.16 Amplified products were separated through agarose gel, purified using a QIAquick gel extraction kit (Qiagen Inc, Chatsworth, CA), and directly sequenced on a DNA sequencer (310; Applied Biosystems, Foster City, CA) using a BigDye terminator cycle sequencing kit (Applied Biosystems). If mutations were found by direct sequencing, the fragments were cloned into a pGEM-T Easy vector (Promega, Madison, WI), then transfected into the Escherichia coli strain DH5α. At least 4 recombinant colonies were selected, and plasmid DNA was prepared using a QIAprep Spin Miniprep Kit (Qiagen Inc) and sequenced.
Analysis of clinical characteristics
It was necessary to analyze the clinical characteristics in a well-documented group. Among the 257 patients analyzed, 15 had acute promyelocytic leukemia (APL). APL has been considered a separate disease entity among AML, and the introduction of all-trans retinoic acid (ATRA) has dramatically improved its clinical outcome.32 In addition, 52 patients with AML, excluding those with APL, were treated with independent regimens. We, therefore, analyzed the clinical characteristics of 190 patients with AML, excluding those with APL, who were treated with the AML87, AML89, and AML92 protocols of JALSG.33-35 (Each protocol is presented in Document S1; see the Supplemental Document link at the top of the online article, at the Blood website.)
Statistical analysis
Differences in continuous variables were analyzed using the Mann-Whitney U test for distribution between 2 groups. Analysis of frequencies was performed using the Fisher exact test for 2 × 2 tables or the Pearson χ2 test for larger tables. Multivariate analysis to identify risk factors for achieving CR was performed using the logistic regression model. Survival probabilities were estimated by the Kaplan-Meier method, and differences in survival distributions were evaluated using the log-rank test. Overall survival was defined as the time from the first day of therapy to death or last visit. Relapse-free survival was defined as the time from the first day of CR to relapse, death, or last visit. Patients undergoing hematopoietic stem cell transplantation were censored at the time of transplantation. The prognostic significance of the clinical variables was assessed using the Cox proportional hazards model. These statistical analyses were performed with StatView-J 5.0 (Abacus Concepts Inc, Berkeley, CA). For all analyses, the P values were 2-tailed, and P < .05 was considered statistically significant.
Results
NPM1 mutations were frequently found in AML
We first screened for mutations within exon 12 of the NPM1 gene through direct sequencing in 257 patients with AML, then confirmed each type of mutation by cloning. We found the NPM1 mutation in 64 of 257 (24.9%) patients (Table 1). Importantly, direct sequencing revealed that all AML cells with NPM1 mutations retained the wild-type allele. Given that all samples contained more than 90% AML cells, all mutations seemed to occur in only one allele. Previously, 6 kinds of mutants, designated mutations A to F, were identified. In this study, we found 49 mutations of type A, 7 mutations of type B, 4 mutations of type D, and 4 novel mutants that were designated mutations G to J (Figure 1). All novel mutations included distinct 4-bp insertions (mutation G, TTTG; mutation H, CTTG; mutation I, TAAG; mutation J, TATG) at position 960, resulting in the same frameshift as mutations A to D. Predicted mutant proteins of mutations G and H and of mutation J were the same as those of mutations A and B, respectively. The protein of mutation I contained a lysine residue at position 289, although the other residues were conserved.
Relationship among NPM1 mutation, FAB type, and genetic alterations in AML
. | . | NPM1 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | Total no. patients . | Mutation, no. (%)* . | Wild type, no. (%)† . | P . | ||||
| FAB subtype | .005 | |||||||
| M0 | 9 | 2 (22.2) | 7 (77.8) | |||||
| M1 | 54 | 17 (31.5) | 37 (68.5) | |||||
| M2 | 89 | 12 (13.5) | 77 (86.5) | |||||
| M3 | 15 | 0 (0) | 15 (100) | |||||
| M4 | 54 | 21 (38.9) | 33 (61.1) | |||||
| M5 | 19 | 9 (47.3) | 10 (52.7) | |||||
| M6 | 8 | 2 (25.0) | 6 (75.0) | |||||
| M7 | 9 | 1 (11.1) | 8 (88.9) | |||||
| Cytogenetics | <.001 | |||||||
| t(8;21) | 31 | 0 (0) | 31 (100) | |||||
| inv(16) | 8 | 1 (12.5) | 7 (87.5) | |||||
| t(15;17) | 15 | 0 (0) | 15 (100) | |||||
| t(9;22) | 3 | 1 (33.3) | 2 (66.7) | |||||
| del(5) | 7 | 0 (0) | 7 (100) | |||||
| del(7) | 5 | 0 (0) | 5 (100) | |||||
| Others | 43 | 5 (11.6) | 38 (88.4) | |||||
| Normal | 97 | 46 (47.4) | 51 (52.6) | |||||
| Unknown | 48 | 11 (22.9) | 37 (77.1) | |||||
| FLT3 | ||||||||
| Mutations, total | 67 | 39 (58.2) | 28 (41.8) | <.001 | ||||
| ITD | 58 | 35 (60.3) | 23 (39.7) | <.001‡ | ||||
| D835 | 9 | 4 (44.4) | 5 (55.6) | .028‡ | ||||
| Wild type | 190 | 25 (13.2) | 165 (86.8) | |||||
| TP53 | NS | |||||||
| Mutation | 16 | 2 (12.5) | 14 (87.5) | |||||
| Wild type | 227 | 58 (25.6) | 169 (74.4) | |||||
| Not done | 14 | 5 (35.7) | 10 (64.3) | |||||
| NRAS | NS | |||||||
| Mutation | 34 | 9 (26.5) | 25 (73.5) | |||||
| Wild type | 202 | 48 (23.8) | 154 (76.2) | |||||
| Not done | 21 | 7 (33.3) | 14 (66.7) | |||||
| MLL-TD | NS | |||||||
| Mutation | 17 | 2 (11.8) | 15 (88.2) | |||||
| Wild type | 130 | 33 (25.4) | 97 (74.6) | |||||
| Not done | 110 | 29 (26.4) | 81 (73.6) | |||||
| Numbers and percentages of 257 patients with AML are shown by FAB type; cytogenetics; and FLT3, TP53, NRAS, and MLL-TD mutations, according to NPM1 mutation. | ||||||||
| NS indicates not significant. | ||||||||
. | . | NPM1 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|
. | Total no. patients . | Mutation, no. (%)* . | Wild type, no. (%)† . | P . | ||||
| FAB subtype | .005 | |||||||
| M0 | 9 | 2 (22.2) | 7 (77.8) | |||||
| M1 | 54 | 17 (31.5) | 37 (68.5) | |||||
| M2 | 89 | 12 (13.5) | 77 (86.5) | |||||
| M3 | 15 | 0 (0) | 15 (100) | |||||
| M4 | 54 | 21 (38.9) | 33 (61.1) | |||||
| M5 | 19 | 9 (47.3) | 10 (52.7) | |||||
| M6 | 8 | 2 (25.0) | 6 (75.0) | |||||
| M7 | 9 | 1 (11.1) | 8 (88.9) | |||||
| Cytogenetics | <.001 | |||||||
| t(8;21) | 31 | 0 (0) | 31 (100) | |||||
| inv(16) | 8 | 1 (12.5) | 7 (87.5) | |||||
| t(15;17) | 15 | 0 (0) | 15 (100) | |||||
| t(9;22) | 3 | 1 (33.3) | 2 (66.7) | |||||
| del(5) | 7 | 0 (0) | 7 (100) | |||||
| del(7) | 5 | 0 (0) | 5 (100) | |||||
| Others | 43 | 5 (11.6) | 38 (88.4) | |||||
| Normal | 97 | 46 (47.4) | 51 (52.6) | |||||
| Unknown | 48 | 11 (22.9) | 37 (77.1) | |||||
| FLT3 | ||||||||
| Mutations, total | 67 | 39 (58.2) | 28 (41.8) | <.001 | ||||
| ITD | 58 | 35 (60.3) | 23 (39.7) | <.001‡ | ||||
| D835 | 9 | 4 (44.4) | 5 (55.6) | .028‡ | ||||
| Wild type | 190 | 25 (13.2) | 165 (86.8) | |||||
| TP53 | NS | |||||||
| Mutation | 16 | 2 (12.5) | 14 (87.5) | |||||
| Wild type | 227 | 58 (25.6) | 169 (74.4) | |||||
| Not done | 14 | 5 (35.7) | 10 (64.3) | |||||
| NRAS | NS | |||||||
| Mutation | 34 | 9 (26.5) | 25 (73.5) | |||||
| Wild type | 202 | 48 (23.8) | 154 (76.2) | |||||
| Not done | 21 | 7 (33.3) | 14 (66.7) | |||||
| MLL-TD | NS | |||||||
| Mutation | 17 | 2 (11.8) | 15 (88.2) | |||||
| Wild type | 130 | 33 (25.4) | 97 (74.6) | |||||
| Not done | 110 | 29 (26.4) | 81 (73.6) | |||||
| Numbers and percentages of 257 patients with AML are shown by FAB type; cytogenetics; and FLT3, TP53, NRAS, and MLL-TD mutations, according to NPM1 mutation. | ||||||||
| NS indicates not significant. | ||||||||
n = 64; 24.9%.
n = 193; 75.1%.
These variables were compared with those of the wild type.
In 2 patients whose leukemia cells had NPM1 mutations at diagnosis, the mutations were lost at CR, indicating that these were somatic mutations.
Morphologic and genotypic characteristics of AML with NPM1 mutations
NPM1 mutations were found in patients with AML of all FAB subtypes except M3 (Table 1). Cytogenetic data were available for 209 patients. Consistent with findings of a previous report,16 the NPM1 mutation was preferentially found in patients with normal karyotype (46 of 97; 47.4%), but it was not found in patients with t(8;21), t(15;17), del(5), or del(7). In total, there was a significant difference in the frequency of the NPM1 mutation among patients with (7 of 112; 6.3%) and without (46 of 97; 47.4%) cytogenetic abnormalities (P < .001). Moreover, an NPM1 mutation was found in each of 8 and 3 patients with inv(16) and t(9;22), respectively.
FLT3/ITD and FLT3/D835Mt were found in 58 (22.6%) and 9 (3.5%) of 257 patients, respectively (Table 1). Both FLT3 mutations were significantly associated with NPM1 mutations: 35 of 58 (60.3%) FLT3/ITD (P < .001) and 4 of 9 (44.4%) FLT3/D835Mt (P = .028) were found in the patients with NPM1 mutations. TP53, NRAS, and MLL-TD mutations were found in 16 of 243 (6.6%), 34 of 236 (14.4%), and 17 of 147 (11.6%) patients, respectively, although there was no significant correlation between these mutations and the NPM1 mutation (Table 1).
Clinical characteristics and prognoses of AML patients with or without NPM1 mutations
Among the 257 patients with AML, 190 patients (excluding those with M3 who were treated with the AML87, AML89, and AML92 protocols of the JALSG) were evaluated for clinical characteristics and initial response to therapy (Table 2). Of these patients, 49 (25.8%) had NPM1 mutations. The presence of NPM1 mutations was related neither to sex nor to the occurrence of hepatosplenomegaly or extramedullary involvement. Patients with NPM1 mutations (median, 58 years; range, 15-77 years) were significantly older than those without mutations (median, 47 years; range, 15-85 years) (P = .003). White blood cell (WBC) counts and peripheral blood blast cells were significantly higher in the NPM1 mutation group than in the wild-type group (P = .002 and P = .029, respectively). According to the FAB classification, the NPM1 mutation was infrequent in the M2 subtype (P = .004). According to the cytogenetic risk groups, the NPM1 mutation was preferentially found in the intermediate risk group (P < .001). NPM1 mutations were associated with FLT3/ITD (P < .001) and FLT3/D835Mt (P = .018), but not with TP53 and NRAS mutations. Because we could analyze MLL-TD in only 118 of the 190 patients, we excluded MLL-TD from the variables for statistical analysis.
Characteristics of 190 patients with AML, excluding patients with the M3 subtype, treated according to JALSG protocols
. | . | NPM1 . | . | . | |
|---|---|---|---|---|---|
. | Total N = 190 . | Mutation, n = 49 . | Wild type, n = 141 . | P . | |
| Age, y | 50 (15-85) | 58 (15-77) | 47 (15-85) | .003 | |
| WBC count, × 109/L | 24.5 (0.9-372) | 52.2 (1.0-372) | 23.3 (0.9-337.6) | .002 | |
| PB blast count, × 109/L | 14.9 (0.017-357) | 29.6 (0.093-357) | 12.6 (0.017-324) | .029 | |
| FAB subtype | .004 | ||||
| M0 | 3 | 1 | 2 | ||
| M1 | 44 | 12 | 32 | ||
| M2 | 78 | 10 | 68 | ||
| M4 | 46 | 16 | 30 | ||
| M5 | 14 | 8 | 6 | ||
| M6 | 4 | 1 | 3 | ||
| M7 | 1 | 1 | 0 | ||
| Cytogenetics | <.001 | ||||
| Favorable | 33 | 1 | 32 | ||
| t(8;21) | 27 | 0 | 27 | ||
| inv(16) | 6 | 1 | 5 | ||
| Intermediate | 119 | 42 | 77 | ||
| Normal | 79 | 37 | 42 | ||
| Others | 40 | 5 | 35 | ||
| Poor | 14 | 1 | 13 | ||
| t(9;22) | 3 | 1 | 2 | ||
| del(5) or del(7) | 11 | 0 | 11 | ||
| Unknown | 24 | 5 | 19 | ||
| Outcome | .025 | ||||
| CR | 139 | 42 | 97 | ||
| Failure | 51 | 7 | 44 | ||
| FLT3 | |||||
| Mutation, total | 51 | 31 | 20 | <.001 | |
| ITD mutation | 43 | 27 | 16 | <.001* | |
| D835 mutation | 8 | 4 | 4 | .018* | |
| Wild type | 139 | 18 | 121 | ||
| P53 | NS | ||||
| Mutation | 8 | 1 | 7 | ||
| Wild type | 182 | 48 | 134 | ||
| NRAS | NS | ||||
| Mutation | 26 | 8 | 18 | ||
| Wild type | 164 | 41 | 123 | ||
. | . | NPM1 . | . | . | |
|---|---|---|---|---|---|
. | Total N = 190 . | Mutation, n = 49 . | Wild type, n = 141 . | P . | |
| Age, y | 50 (15-85) | 58 (15-77) | 47 (15-85) | .003 | |
| WBC count, × 109/L | 24.5 (0.9-372) | 52.2 (1.0-372) | 23.3 (0.9-337.6) | .002 | |
| PB blast count, × 109/L | 14.9 (0.017-357) | 29.6 (0.093-357) | 12.6 (0.017-324) | .029 | |
| FAB subtype | .004 | ||||
| M0 | 3 | 1 | 2 | ||
| M1 | 44 | 12 | 32 | ||
| M2 | 78 | 10 | 68 | ||
| M4 | 46 | 16 | 30 | ||
| M5 | 14 | 8 | 6 | ||
| M6 | 4 | 1 | 3 | ||
| M7 | 1 | 1 | 0 | ||
| Cytogenetics | <.001 | ||||
| Favorable | 33 | 1 | 32 | ||
| t(8;21) | 27 | 0 | 27 | ||
| inv(16) | 6 | 1 | 5 | ||
| Intermediate | 119 | 42 | 77 | ||
| Normal | 79 | 37 | 42 | ||
| Others | 40 | 5 | 35 | ||
| Poor | 14 | 1 | 13 | ||
| t(9;22) | 3 | 1 | 2 | ||
| del(5) or del(7) | 11 | 0 | 11 | ||
| Unknown | 24 | 5 | 19 | ||
| Outcome | .025 | ||||
| CR | 139 | 42 | 97 | ||
| Failure | 51 | 7 | 44 | ||
| FLT3 | |||||
| Mutation, total | 51 | 31 | 20 | <.001 | |
| ITD mutation | 43 | 27 | 16 | <.001* | |
| D835 mutation | 8 | 4 | 4 | .018* | |
| Wild type | 139 | 18 | 121 | ||
| P53 | NS | ||||
| Mutation | 8 | 1 | 7 | ||
| Wild type | 182 | 48 | 134 | ||
| NRAS | NS | ||||
| Mutation | 26 | 8 | 18 | ||
| Wild type | 164 | 41 | 123 | ||
Clinical characteristics of the 190 patients with AML, excluding those with the M3 subtype, who were treated according to the JALSG protocols, are shown. Median values (with range in parentheses) are indicated for age, WBC count, and PB blast count. Numbers of patients are shown by FAB type, cytogenetics, outcome, and gene mutation. The favorable risk group was defined by t(8;21) or inv(16); the poor risk group by t(9;22), del(5), or del(7); and the intermediate risk group by normal or other karyotypes.
PB indicates peripheral blood; NS, not significant.
Compared with wild-type variables.
Of the 190 patients, 139 (73.2%) achieved CR after induction chemotherapy. The CR rate was significantly higher in the patients with NPM1 mutations (42 of 49; 85.7%) than without them (97 of 141; 68.8%) (P = .025). In addition, Fisher exact test showed that FAB subtypes other than M2, cytogenetic findings other than good risk, and the presence of NRAS and TP53 mutations were unfavorable factors for achieving CR (P < .001, P = .002, P = .030, and P = .034, respectively). Age (older than 60), WBC count (more than 100 × 109/L), and presence of the FLT3 mutation were not associated with the CR rate. Multivariate logistic regression analysis showed that wild-type NPM1 (P < .001), FAB subtypes other than M2 (P = .008), and cytogenetic findings other than good risk (P = .039) were independent unfavorable factors for achieving CR (Table 3).
Logistic regression analysis results of the effect of unfavorable factors for CR in 190 patients, excluding those with the M3 subtype, treated according to JALSG protocols
Variable . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| NPM1, wild type | <.001 | 8.434 | 2.562-27.77 |
| FAB, other than M2 | .008 | 3.247 | 1.353-7.813 |
| Cytogenetic, other than good risk | .039 | 5.240 | 1.091-25.16 |
| NRAS, mutation | .056 | 2.667 | 0.978-7.299 |
| TP53, mutation | .058 | 6.024 | 0.941-38.46 |
| WBC count, more than 100 × 109/L | .090 | 2.427 | 0.872-6.757 |
| FLT3, mutation | .279 | 1.719 | 0.645-4.583 |
| Age, older than 60 y | .960 | 2.427 | 0.446-2.340 |
Variable . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| NPM1, wild type | <.001 | 8.434 | 2.562-27.77 |
| FAB, other than M2 | .008 | 3.247 | 1.353-7.813 |
| Cytogenetic, other than good risk | .039 | 5.240 | 1.091-25.16 |
| NRAS, mutation | .056 | 2.667 | 0.978-7.299 |
| TP53, mutation | .058 | 6.024 | 0.941-38.46 |
| WBC count, more than 100 × 109/L | .090 | 2.427 | 0.872-6.757 |
| FLT3, mutation | .279 | 1.719 | 0.645-4.583 |
| Age, older than 60 y | .960 | 2.427 | 0.446-2.340 |
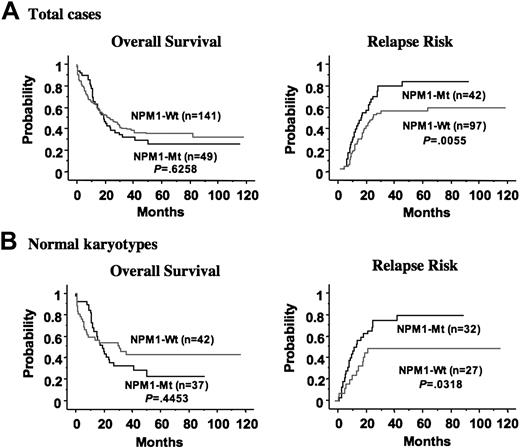
Kaplan-Meier analyses according to NPM1 mutation are shown in Figure 2A. Univariate analysis showed that the poor prognostic factors for overall survival were age 60 or older (P = .001), mutation of TP53 (P < .001), FAB subtypes other than M2 (P = .005), FLT3/ITD (P = .010), high WBC count (more than 100 × 109/L) (P = .019), and cytogenetic findings (poor vs others) (P = .024). Multivariate Cox regression analysis with stepwise selection showed that the mutation of TP53 (odds ratio, 4.002 [95% confidence interval (CI), 1.876-8.538]; P < .001), age 60 or older (odds ratio, 1.651 [95% CI, 1.131-2.410]; P = .009), and FAB subtypes other than M2 (odds ratio, 1.643 [95% CI, 1.127-2.396]; P = .010) were independent poor prognostic factors for overall survival (Table 4). When adjusted with age 60 years or older, FLT3/ITD, FAB subtype other than M2, high WBC count (more than 100 × 109/L), and mutation of NRAS, mutation of NPM1 was an adverse prognostic factor (odds ratio, 1.949 [95% CI, 1.164-3.268]; P = .011). Although careful assessment and further investigation are needed, the mutation of NPM1 may act as a prognostic factor for overall survival.
Unfavorable prognostic factors for overall survival in 190 patients, excluding those with the M3 subtype, treated according to JALSG protocols
Prognostic factors . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| TP53 mutation | .003 | 4.002 | 1.876-8.538 |
| Age, older than 60 y | .009 | 1.651 | 1.131-2.410 |
| FAB, other than M2 | .010 | 1.643 | 1.127-2.396 |
Prognostic factors . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| TP53 mutation | .003 | 4.002 | 1.876-8.538 |
| Age, older than 60 y | .009 | 1.651 | 1.131-2.410 |
| FAB, other than M2 | .010 | 1.643 | 1.127-2.396 |
Because only 1 of 139 patients who achieved CR died during CR, we compared the probability of relapse between patients with and without NPM1 mutations (Figure 2A). Univariate analysis showed that the unfavorable factors for relapse were mutation of NPM1 (P = .006), FLT3/ITD (P = .007), cytogenetic findings (poor vs others) (P = .007), high WBC count (more than 100 × 109/L) (P = .016), FAB subtypes other than M2 (P = .024), and age 60 or older (P = .024). Multivariate Cox regression analysis with stepwise selection identified that cytogenetic findings (poor vs others) (odds ratio, 3.876 [95% CI, 1.718-8.772]; P = .001) and mutation of NPM1 (odds ratio, 2.106 [95% CI, 1.324-3.350]; P = .002) were independent unfavorable factors for relapse (Table 5).
Unfavorable prognostic factors for relapse in 139 patients with AML, excluding those with the M3 subtype, who achieved CR
Prognostic factors . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| Poor cytogenetics | .001 | 3.876 | 1.718-8.772 |
| NPM1 mutation | .002 | 2.106 | 1.324-3.350 |
Prognostic factors . | P . | Odds ratio . | 95% CI . |
|---|---|---|---|
| Poor cytogenetics | .001 | 3.876 | 1.718-8.772 |
| NPM1 mutation | .002 | 2.106 | 1.324-3.350 |
Mutations in NPM1 exon 12. (A) The mutated nucleotide and the predicted amino acid sequence in NPM1 exon 12 found in the present study are shown in comparison with the wild-type sequence. Type of mutation (mutations A, B, D) is designated according to a previous report. Four novel mutant variants are designated mutations G, H, I, and J. Red and blue indicate nucleotide insertions and termination codons, respectively. Green indicates the conserved residues in mutant NPM. Purple indicates putative residues of a consensus nuclear export signal (Lx[1-3]Vx[2-3]VxL; x indicates any residue). (B) Sequence results after cloning. Boxes indicate inserted 4-bp nucleotides. Arrowhead indicates the position of the insert at nucleotide 960 of the NPM1 gene.
Mutations in NPM1 exon 12. (A) The mutated nucleotide and the predicted amino acid sequence in NPM1 exon 12 found in the present study are shown in comparison with the wild-type sequence. Type of mutation (mutations A, B, D) is designated according to a previous report. Four novel mutant variants are designated mutations G, H, I, and J. Red and blue indicate nucleotide insertions and termination codons, respectively. Green indicates the conserved residues in mutant NPM. Purple indicates putative residues of a consensus nuclear export signal (Lx[1-3]Vx[2-3]VxL; x indicates any residue). (B) Sequence results after cloning. Boxes indicate inserted 4-bp nucleotides. Arrowhead indicates the position of the insert at nucleotide 960 of the NPM1 gene.
In addition, we analyzed the prognostic value of NPM1 mutations in 79 patients with normal karyotype. NPM1 mutations were found in 37 of 79 (46.8%) patients. Of these 79 patients, 59 (74.7%) achieved CR after induction chemotherapy. The CR rate was significantly higher in the patients with NPM1 mutations (32 of 37; 86.4%) than in those without (27 of 42; 64.3%) (P = .037 by Fisher exact test). Multivariate logistic regression analysis, including wild-type NPM1, FAB subtypes (other than M2), presence of FLT3, NRAS, and TP53 mutations, age (older than 60 years), and WBC count (more than 100 × 109/L) showed that wild-type NPM1 was the only independent unfavorable factor for achieving CR (odds ratio, 4.908 [95% CI, 1.011-23.824]; P = .048). Kaplan-Meier curves according to NPM1 mutation in this group are shown in Figure 2B. Multivariate Cox regression analysis with stepwise selection showed that age 60 or older was the only poor prognostic factor for overall survival (odds ratio, 2.068 [95% CI, 1.175-3.641]; P = .012). Mutation of NPM1 was not a significant prognostic factor for overall survival regardless of whatever factors were used for adjustment by means of Cox analysis. The relapse risk was analyzed in 59 patients who achieved CR. Multivariate Cox regression analysis with stepwise selection identified that the NPM1 mutation was the only independent unfavorable factor for relapse (odds ratio, 2.096 [95% CI, 1.050-4.186]; P = .036). However, because the patient number of this group was small, larger-scale analysis was required.
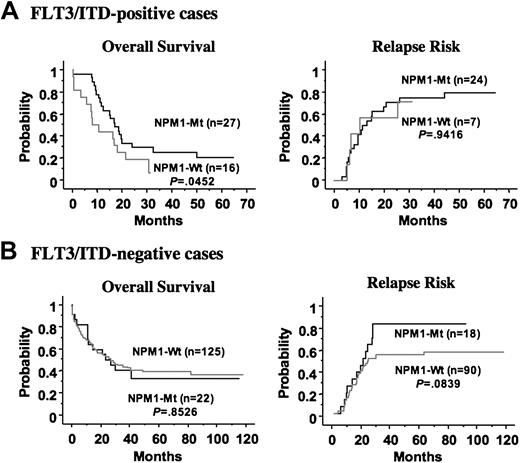
FLT3/ITD has been identified as an unfavorable prognostic factor in patients with AML, although the prognostic implications of FLT3/D835Mt remain unclear. Given that the NPM1 mutation was associated with FLT3/ITD, it was important to define the role of NPM1 mutations alone or in combination with FLT3/ITD for long-term prognosis. Therefore, we compared the clinical impact of NPM1 mutations in patients with and without FLT3/ITD. Interestingly, mutation of NPM1 was an independent favorable prognostic factor for CR in patients with FLT3/ITD (odds ratio, 20.8 [95% CI, 2.0-200]; P = .011 by multivariate logistic regression analysis), but it did not affect the CR rate in patients without FLT3/ITD. In addition, mutation of NPM1 was a favorable prognostic factor for overall survival in patients with FLT3/ITD (P = .045), but not in those without FLT3/ITD. However, mutation of NPM1 was not associated with the high relapse rate in patients with FLT3/ITD, whereas it tended to be a worse factor for relapse in those without FLT3/ITD (P = .084) (Figure 3).
Kaplan-Meier curves according to NPM1 mutation. (A) Overall survival and relapse risk in all patients. (B) Overall survival and relapse risk in patients with normal karyotype. Statistical difference was evaluated with the log-rank test.
Kaplan-Meier curves according to NPM1 mutation. (A) Overall survival and relapse risk in all patients. (B) Overall survival and relapse risk in patients with normal karyotype. Statistical difference was evaluated with the log-rank test.
Comparison of mutational status of the NPM1 gene at diagnosis and relapse
Mutational status of the NPM1 and FLT3 genes was compared between diagnosis and relapse in 39 AML patients. NRAS and TP53 mutations were also analyzed in 20 and 29 patients, respectively (Table 6). An NPM1 mutation was found in 17 of 39 patients at diagnosis. Of the 17 patients with NPM1 mutations at diagnosis, 15 carried the same mutation at relapse, although 2 patients (unique patient number [UPN] 16 and UPN 17) lost the mutation at relapse. In UPN 16, the karyotype of leukemia cells was normal (46XY) at diagnosis, although it changed to 46XYdel(20)(q1?) at relapse. In contrast, FLT3, NRAS, and TP53 were of the wild type at both stages. In UPN 17, leukemia cells showed normal karyotype (46XX), FLT3/ITD, and wild-type NRAS and TP53 mutations at diagnosis, and these were retained at relapse.
Comparisons of mutations between diagnosis and relapse
. | . | . | . | . | NPM1 . | . | FLT3 . | . | NRAS . | . | TP53 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Age, y . | Sex . | FAB . | CR, d . | D . | R . | D . | R . | D . | R . | D . | R . | 2nd CR . | ||||
| 1 | 56 | M | M4 | 255 | Mt | Mt | Mt | Mt | WT | WT | WT | WT | No | ||||
| 2 | 38 | F | M2 | 574 | Mt | Mt | Mt | Mt | WT | WT | WT | WT | Yes | ||||
| 3 | 62 | M | M1 | 328 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | Yes | ||||
| 4 | 55 | M | M5 | 408 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 5 | 15 | F | M2 | 181 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 6 | 62 | F | M1 | 521 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 7 | 50 | M | M2 | 264 | Mt | Mt | Mt | Mt | ND | ND | ND | ND | Yes | ||||
| 8 | 66 | M | M1 | 608 | Mt | Mt | Mt | WT | WT | WT | WT | WT | Yes | ||||
| 9 | 67 | F | M4 | 483 | Mt | Mt | WT | Mt | WT | WT | WT | WT | Yes | ||||
| 10 | 57 | F | M1 | 603 | Mt | Mt | WT | WT | Mt | WT | WT | WT | Yes | ||||
| 11 | 54 | M | M1 | 175 | Mt | Mt | WT | WT | WT | WT | WT | WT | No | ||||
| 12 | 53 | F | M1 | 214 | Mt | Mt | WT | WT | WT | WT | WT | WT | No | ||||
| 13 | 54 | M | M4 | 1026 | Mt | Mt | WT | WT | ND | ND | ND | ND | Yes | ||||
| 14 | 53 | M | M4 | 542 | Mt | Mt | WT | WT | ND | ND | ND | ND | No | ||||
| 15 | 70 | M | M1 | 773 | Mt | Mt | WT | WT | ND | ND | WT | WT | No | ||||
| 16 | 64 | M | M4 | 489 | Mt | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 17 | 44 | F | M0 | 147 | Mt | WT | Mt | Mt | WT | WT | Mt | Mt | No | ||||
| 18 | 59 | M | M1 | 222 | WT | WT | Mt | Mt | ND | ND | ND | ND | No | ||||
| 19 | 55 | M | M5 | 128 | WT | WT | Mt | Mt | ND | ND | ND | ND | Yes | ||||
| 20 | 16 | M | M4 | 308 | WT | WT | WT | Mt | Mt | Mt | WT | WT | No | ||||
| 21 | 34 | F | M4 | 370 | WT | WT | WT | Mt | Mt | Mt | WT | WT | No | ||||
| 22 | 56 | F | M4 | 161 | WT | WT | WT | Mt | WT | WT | Mt | WT | No | ||||
| 23 | 29 | M | M0 | 361 | WT | WT | WT | WT | Mt | Mt | WT | WT | No | ||||
| 24 | 26 | F | M4 | 430 | WT | WT | WT | WT | Mt | WT | WT | WT | Yes | ||||
| 25 | 48 | M | M2 | 525 | WT | WT | WT | WT | WT | Mt | WT | WT | Yes | ||||
| 26 | 74 | F | M1 | 147 | WT | WT | WT | WT | WT | WT | WT | Mt | Yes | ||||
| 27 | 44 | F | M1 | 137 | WT | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 28 | 39 | F | M2 | 200 | WT | WT | WT | WT | WT | WT | WT | WT | Yes | ||||
| 29 | 22 | M | M1 | 168 | WT | WT | WT | WT | WT | WT | WT | WT | Yes | ||||
| 30 | 68 | M | M2 | 208 | WT | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 31 | 23 | M | M5 | 133 | WT | WT | WT | WT | ND | ND | Mt | Mt | No | ||||
| 32 | 62 | M | M2 | 271 | WT | WT | WT | WT | ND | ND | WT | WT | No | ||||
| 33 | 55 | F | M2 | 281 | WT | WT | WT | WT | ND | ND | WT | WT | Yes | ||||
| 34 | 32 | M | M1 | 257 | WT | WT | WT | WT | ND | ND | ND | ND | No | ||||
| 35 | 60 | F | M5 | 429 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 36 | 47 | M | M2 | 435 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 37 | 19 | M | M2 | 389 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 38 | 58 | M | M3 | 412 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 39 | 44 | F | M4 | 266 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
. | . | . | . | . | NPM1 . | . | FLT3 . | . | NRAS . | . | TP53 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | Age, y . | Sex . | FAB . | CR, d . | D . | R . | D . | R . | D . | R . | D . | R . | 2nd CR . | ||||
| 1 | 56 | M | M4 | 255 | Mt | Mt | Mt | Mt | WT | WT | WT | WT | No | ||||
| 2 | 38 | F | M2 | 574 | Mt | Mt | Mt | Mt | WT | WT | WT | WT | Yes | ||||
| 3 | 62 | M | M1 | 328 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | Yes | ||||
| 4 | 55 | M | M5 | 408 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 5 | 15 | F | M2 | 181 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 6 | 62 | F | M1 | 521 | Mt | Mt | Mt | Mt | ND | ND | WT | WT | No | ||||
| 7 | 50 | M | M2 | 264 | Mt | Mt | Mt | Mt | ND | ND | ND | ND | Yes | ||||
| 8 | 66 | M | M1 | 608 | Mt | Mt | Mt | WT | WT | WT | WT | WT | Yes | ||||
| 9 | 67 | F | M4 | 483 | Mt | Mt | WT | Mt | WT | WT | WT | WT | Yes | ||||
| 10 | 57 | F | M1 | 603 | Mt | Mt | WT | WT | Mt | WT | WT | WT | Yes | ||||
| 11 | 54 | M | M1 | 175 | Mt | Mt | WT | WT | WT | WT | WT | WT | No | ||||
| 12 | 53 | F | M1 | 214 | Mt | Mt | WT | WT | WT | WT | WT | WT | No | ||||
| 13 | 54 | M | M4 | 1026 | Mt | Mt | WT | WT | ND | ND | ND | ND | Yes | ||||
| 14 | 53 | M | M4 | 542 | Mt | Mt | WT | WT | ND | ND | ND | ND | No | ||||
| 15 | 70 | M | M1 | 773 | Mt | Mt | WT | WT | ND | ND | WT | WT | No | ||||
| 16 | 64 | M | M4 | 489 | Mt | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 17 | 44 | F | M0 | 147 | Mt | WT | Mt | Mt | WT | WT | Mt | Mt | No | ||||
| 18 | 59 | M | M1 | 222 | WT | WT | Mt | Mt | ND | ND | ND | ND | No | ||||
| 19 | 55 | M | M5 | 128 | WT | WT | Mt | Mt | ND | ND | ND | ND | Yes | ||||
| 20 | 16 | M | M4 | 308 | WT | WT | WT | Mt | Mt | Mt | WT | WT | No | ||||
| 21 | 34 | F | M4 | 370 | WT | WT | WT | Mt | Mt | Mt | WT | WT | No | ||||
| 22 | 56 | F | M4 | 161 | WT | WT | WT | Mt | WT | WT | Mt | WT | No | ||||
| 23 | 29 | M | M0 | 361 | WT | WT | WT | WT | Mt | Mt | WT | WT | No | ||||
| 24 | 26 | F | M4 | 430 | WT | WT | WT | WT | Mt | WT | WT | WT | Yes | ||||
| 25 | 48 | M | M2 | 525 | WT | WT | WT | WT | WT | Mt | WT | WT | Yes | ||||
| 26 | 74 | F | M1 | 147 | WT | WT | WT | WT | WT | WT | WT | Mt | Yes | ||||
| 27 | 44 | F | M1 | 137 | WT | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 28 | 39 | F | M2 | 200 | WT | WT | WT | WT | WT | WT | WT | WT | Yes | ||||
| 29 | 22 | M | M1 | 168 | WT | WT | WT | WT | WT | WT | WT | WT | Yes | ||||
| 30 | 68 | M | M2 | 208 | WT | WT | WT | WT | WT | WT | WT | WT | No | ||||
| 31 | 23 | M | M5 | 133 | WT | WT | WT | WT | ND | ND | Mt | Mt | No | ||||
| 32 | 62 | M | M2 | 271 | WT | WT | WT | WT | ND | ND | WT | WT | No | ||||
| 33 | 55 | F | M2 | 281 | WT | WT | WT | WT | ND | ND | WT | WT | Yes | ||||
| 34 | 32 | M | M1 | 257 | WT | WT | WT | WT | ND | ND | ND | ND | No | ||||
| 35 | 60 | F | M5 | 429 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 36 | 47 | M | M2 | 435 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 37 | 19 | M | M2 | 389 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 38 | 58 | M | M3 | 412 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
| 39 | 44 | F | M4 | 266 | WT | WT | WT | WT | ND | ND | ND | ND | Yes | ||||
Mutational status of NPM1, FLT3, NRAS, and TP53 mutations was analyzed in 39 paired samples obtained at diagnosis and relapse. Italics in data field indicate a change in mutational status.
D indicates diagnosis; R, relapse; Mt, mutation; WT, wild type; ND not done.
Kaplan-Meier curves according to NPM1 mutation in the FLT3/ITD-positive and -negative patients. (A) In the FLT3/ITD-positive group, the NPM1 mutation was a favorable prognostic factor for overall survival, but did not affect the relapse risk. (B) In the FLT3/ITD-negative group, the NPM1 mutation did not affect overall survival. The NPM1 mutation tended to be a worse factor for relapse in this group, although it was not statistically significant. Statistical difference was evaluated with the log-rank test.
Kaplan-Meier curves according to NPM1 mutation in the FLT3/ITD-positive and -negative patients. (A) In the FLT3/ITD-positive group, the NPM1 mutation was a favorable prognostic factor for overall survival, but did not affect the relapse risk. (B) In the FLT3/ITD-negative group, the NPM1 mutation did not affect overall survival. The NPM1 mutation tended to be a worse factor for relapse in this group, although it was not statistically significant. Statistical difference was evaluated with the log-rank test.
An FLT3 mutation was found in 11 of 39 patients at diagnosis, and all mutations were FLT3/ITD. The FLT3 mutation was lost at relapse in 1 patient, but it emerged at relapse in 4 patients who did not have the FLT3 mutation at diagnosis. An NRAS mutation was found in 5 of 20 patients at diagnosis. The NRAS mutation was lost at relapse in 2 patients, but it emerged at relapse in 1 patient. A TP53 mutation was found in 3 of 28 patients at diagnosis. The TP53 mutation was lost at relapse in 1 patient, but it emerged at relapse in 1 patient. Taken together, in 11 of 39 (28.2%) patients, the gene status of NPM1, FLT3, NRAS, and TP53 was different at diagnosis than it was at relapse (Table 6). However, NPM1 gene status was relatively stable, in contrast to that of FLT3, and was not related to inducing a genotypic change in leukemia cells at relapse. In addition, age and CR duration were not associated with the genotypic change of leukemia cells at relapse. Importantly, the NPM1 mutation was not associated with achieving second CR after first CR.
Discussion
In this study, we found NPM1 mutations in 64 of 257 (24.9%) adult patients with de novo AML. Of the 64 NPM1 mutations, 60 were variants reported previously, and 4 were novel. All the novel variants had a 4-bp insertion at position 960, resulting in the same frameshift as previously reported. The C-terminal of NPM is important to the nuclear localization of NPM,36 and tryptophan residues at positions 288 and 290 are reportedly associated with the nucleolar localization of NPM.37 Interestingly, predicted mutant proteins contain a nuclear export signal (NES) motif at the C-terminal, which may be a reason for the cytoplasmic dislocation of mutant NPM.38,39 All novel variants found in the present study also contained the NES motif. Therefore, we believe that the generation of the NES motif by the insertion mutation is strongly associated with the cytoplasmic dislocation of mutant NPM.
FLT3, TP53, and NRAS mutations are the most frequent genetic alterations involved in the pathogenesis of AML. It was reported that the NPM1 mutation is closely associated with FLT3/ITD. We found that it is also associated with FLT3/D835Mt, but a large-scale study is necessary to confirm this association because of the small number of patients with FLT3/D835Mt in this study. It is noteworthy that the NPM1 mutation was not associated with TP53, NRAS, and MLL-TD mutations. In fact, the NPM1 mutation was found in only 2 of 16 patients with the TP53 mutation, although this was not statistically significant. Because the TP53 mutation is known to be present in patients with karyotypic abnormalities,40,41 the NPM1 mutation seems to be infrequent in this group. The NRAS mutation is negatively related to FLT3/ITD but is found in all patients with AML, regardless of karyotypic abnormalities.6,42 In contrast, a possible association between MLL-TD and FLT3/ITD was reported.43 Therefore, to identify factors associated with NPM1 mutations, we performed multivariate logistic regression analysis that included the presence or absence of FLT3, TP53, NRAS, and MLL-TD mutations and karyotypic abnormality in 119 patients in whom all these genetic alterations were determined. The analysis showed that the normal karyotype (P < .001; odds ratio, 26.0 [95% CI, 6.231-108.73]) and the FLT3 mutation (P < .001; odds ratio, 15.2 [95% CI, 3.690-62.50]) were independently associated with NPM1 mutation. These results suggested that the NPM1 mutation might be involved in the pathogenesis of AML with and without FLT3 mutations.
The most important finding in the present study is that in patients with AML, excluding those with the M3 subtype, the NPM1 mutation is a favorable factor for achieving CR after induction chemotherapy, but it implicates a high relapse rate. FLT3/ITD is clinically demonstrated to be a poor prognostic factor in patients with AML, especially those in the intermediate-risk group. FLT3/ITD confers autonomous proliferation to hematopoietic progenitors through its constitutive kinase activity, though alone it is not sufficient for the development of AML. In the murine APL model, the transduction of FLT3/ITD into PML-RARA transgenic bone marrow cells resulted in a shortened latency with increasing penetration of APL-like disease.44 The NPM1 mutation is essentially absent in patients with APL or CBF leukemia, in contrast to the high frequency of FLT3/ITD and KIT mutations in patients with APL and CBF leukemia, respectively. Only the mutation of NPM1 was an independent favorable prognostic factor for achieving CR and a marginally favorable prognostic factor for overall survival in patients with FLT3/ITD. However, it did not affect the CR rate, and its prognostic value for relapse was not significant in patients without FLT3/ITD. Mutant NPM, therefore, might be involved in the pathogenesis of AML by its conferring a differentiation block or properties of self-renewal, or both, to the hematopoietic progenitors in concert with mutant FLT3, and it might be associated with sensitivity to chemotherapy. More recently, it was reported that several genes putatively involved in the maintenance of a stem cell phenotype, such as HOX and JAG1 genes, were up-regulated in NPMc+ AML cells,45 supporting this hypothesis. However, the exact role of mutant NPM in the pathogenesis of AML, especially without FLT3/ITD, has not been fully resolved. Our sequential analysis using the paired samples obtained at diagnosis and relapse demonstrated that the NPM1 mutation is not related to acquiring additional genetic changes in FLT3, TP53, and NRAS genes. In addition, the NPM1 mutation was not a favorable factor for achieving second CR after relapse, suggesting that unknown genetic or epigenetic changes might additionally occur in AML cells with the NPM1 mutation during disease progression. It is notable that NPM1 mutations were lost at relapse in 2 of the 17 patients who had NPM1 mutations at initial diagnosis. Loss of the mutation at relapse has been repeatedly observed in the mutations of FLT3 and NRAS, and these mutations are thought of as late or secondary events in leukemogenesis. Loss of the NPM1 mutation at relapse suggests that it is not necessarily an earlier event and that it is not needed for continuance of the disease. However, because NPM is involved in ribosome assembly/transport, cytoplasmic/nuclear trafficking, regulation of DNA polymerase alpha activity, centrosome duplication, acute response of mammalian cells to environmental stress, and stabilization of the p53 pathway,25,26,46-48 it is possible that mutant NPM also has a multifunctional role in the pathogenesis of AML. Alternatively, mutant NPM might use its multiple functions according to the genetic state of leukemia progenitors. Further biologic studies are necessary to clarify how mutant NPM is involved in the pathogenesis of AML and what kinds of genetic or epigenetic alterations cooperate with mutant NPM for the development of AML.
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2005-04-1733.
Supported by Grants-in-Aid from the Ministry of Health, Labor, and Welfare and from the Scientific Research and the 21st Century COE Program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders” of the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
T.S., H.K., R.U., and T.N. designed the research protocol; T.S., H.K., K.O., A. Tomita, and S.Y. performed the genetic analysis; T.S., H.K., R.S., and T.N. analyzed the data; H.K., K.O., R.S., Y.K., S.M., N.A., K.K., F.Y., C.S., H.A., M.N., T.M., K.S., A. Takeshita, R.U., T.K., N.E., and T.N. collected samples and managed clinical data; and T.S., H.K., and T.N. wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This study was performed in cooperation with JALSG. We thank Manami Kira for secretarial assistance.

![Figure 1. Mutations in NPM1 exon 12. (A) The mutated nucleotide and the predicted amino acid sequence in NPM1 exon 12 found in the present study are shown in comparison with the wild-type sequence. Type of mutation (mutations A, B, D) is designated according to a previous report. Four novel mutant variants are designated mutations G, H, I, and J. Red and blue indicate nucleotide insertions and termination codons, respectively. Green indicates the conserved residues in mutant NPM. Purple indicates putative residues of a consensus nuclear export signal (Lx[1-3]Vx[2-3]VxL; x indicates any residue). (B) Sequence results after cloning. Boxes indicate inserted 4-bp nucleotides. Arrowhead indicates the position of the insert at nucleotide 960 of the NPM1 gene.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/8/10.1182_blood-2005-04-1733/6/m_zh80200585480001.jpeg?Expires=1769233588&Signature=Kl-imI-IwFoQglkHsSCMoVlIdq0UBqrmXHEVuqjFeNkAnB4dQ3uHN7iwoXIOyFchtWJ~M5rKxY~15aYJdhDf~v~zCRq586nsgi4~B479H~h4On5HCtq~YJSMCwFZz1r7I3zSrTPTZPVC77gwmh8EYVMaO6q~nL0dejyWoHdzDK8uEhObjissq1Xr5P8y7UJu1YFAPYVFgKMufYNfKLcp7dwDxIpw1rHalQVwzVgXI5eP6L0qz53WkflD~LXu~CzmNPRYdtxL6Nxu8d89mXlMiMFvbvhsAxG9Xay6TNAbHBTnJxQI6PUZ81tGzxlvunFUO1Vr8N3FWY0uXPg2Q4bq0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal