Abstract
Binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein (GP) Ib-IX-V complex initiates a signaling cascade that causes αIIbβ3 activation and platelet aggregation. Previous work demonstrated that botrocetin (bt)/VWF–mediated agglutination activates αIIbβ3 and elicits adenosine triphosphate (ATP) secretion in a thromboxane A2 (TxA2)– and Ca2+-dependent manner. This agglutination-elicited TxA2 production occurs in the absence of ATP secretion. However, the signaling components and signaling network or pathway activated by GPIb-mediated agglutination to cause TxA2 production have not been identified. Therefore, the focus of this study was to elucidate at least part of the signal transduction network or pathway activated by GPIb-mediated agglutination to cause TxA2 production. The phosphatidylinositol 3-kinase (PI3K) selective inhibitor wortmannin, and mouse platelets deficient in Lyn, Src, Syk, Src homology 2 (SH2) domain–containing leukocyte protein 76 (SLP-76), phospholipase Cγ2 (PLCγ2), linker for activation of T cells (LAT), or Fc receptor γ-chain (FcRγ-chain) were used for these studies. LAT and FcRγ-chain were found not to be required for agglutination-driven TxA2 production or activation of αIIbβ3, but were required for granule secretion and aggregation. The results also clearly demonstrate that bt/VWF-mediated agglutination-induced TxA2 production is dependent on signaling apparently initiated by Lyn, enhanced by Src, and propagated through Syk, SLP-76, PI3K, PLCγ2, and protein kinase C (PKC).
Introduction
Binding of von Willebrand factor (VWF) to the platelet membrane glycoprotein (GP) Ib-IX-V complex initiates signaling that results in αIIbβ3 activation and platelet aggregation.1-4 Interestingly, different modes of stimulation of the GPIb complex activate αIIbβ3 by apparently different mechanisms. For example, activation of αIIbβ3 in response to adhesion-independent shear stress–induced GPIb signaling (as in a cone and plate viscometer) requires Ca2+ influx and probably mobilization of internal Ca2+ stores, as well as adenosine diphosphate (ADP) secretion, but not thromboxane A2 (TxA2) production.3-6 In contrast, activation of αIIbβ3 in response to adhesion-dependent shear stress–induced GPIb signaling (flow) does not require Ca2+ influx (although Ca2+ influx potentiates the process),7 but does require mobilization of internal Ca2+ stores,2,7 and is not dependent on either ADP secretion or TxA2 production.2,7 Likewise, adhesion-dependent, shear stress–independent GPIb-induced activation of αIIbβ3 appears to require mobilization of internal stores, but not Ca2+ influx, ADP, or TxA2.7,8 In further contrast to these systems, αIIbβ3 activation in response to botrocetin (bt)–facilitated, GPIb/VWF-mediated agglutination is dependent on TxA2 and the agglutination-elicited TxA2 production is not dependent on Ca2+ influx or mobilization of internal Ca2+ stores.9 Despite the central role of agglutination-elicited TxA2 production in bt/VWF/GPIb-induced platelet activation,9 little is known about how this occurs. Consequently, this study was designed to elucidate at least part of the signal transduction pathway activated by GPIb-mediated agglutination to cause TxA2 production. Our results elucidate many of the details of this GPIb initiated signaling process.
Materials and methods
Materials
4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), wortmannin, was purchased from EMD Biosciences (San Diego, CA). Ro31-8220 was purchased from Biomol Research (Plymouth Meeting, PA). Apyrase and prostaglandin E1 (PGE1) were from Sigma-Aldrich (St Louis, MO). Complete Mini Protease Inhibitor Cocktail Tablets were from Roche Diagnostics (Indianapolis, IN). 4G10 was purchased from Upstate (Charlottesville, VA). Anti–phospho-Akt (Ser473) antibody was from Cell Signaling Technology (Beverly, MA). Protein A/G PLUS–Agarose, anti–phospholipase Cγ2 (PLCγ2) polyclonal antibody, and anti-Syk polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin antibody was from BMB (Indianapolis, IN). Peroxidase-conjugated donkey anti–mouse and donkey anti–rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). PepTag assay kit for the nonradioactive detection of phosphorylated protein kinase C (PKC) was purchased from Promega (Madison, WI). Human von Willebrand factor was from Haematologic Technologies (Essex Junction, VT). Botrocetin was prepared as previously described.10
Animals
Fc receptor γ-chain (FcRγ-chain–/–) and control mice were from Taconic (Germantown, NY). Mice deficient in Lyn,11 LAT (linker for activation of T cells),12 PLCγ2,13 and Src homology 2 (SH2) domain–containing leukocyte protein 76 (SLP-76)14 were generated as described. Wild-type C57/BL6 mice were used as the Lyn+/+ controls. Syk–/– chimeric mice were produced by fetal liver cell transplantation as described.15 Breeding stock to produce the Src-deficient mice were obtained from Jackson Laboratories (Bar Harbor, ME). Unless otherwise stated, wild-type littermate siblings were used as controls.
Platelet aggregation
Blood was collected from the abdominal aorta of isofluorane-anesthetized mice into syringes containing 100 μL/mL Whites (2.94% sodium citrate, 136 mM glucose, pH 6.4), 0.1 μg/mL PGE1, and 1 U/mL apyrase, as anticoagulant.16 Washed platelets were prepared from platelet-rich plasma (PRP) by differential centrifugation of the PRP containing 5 mM EDTA (ethylenediaminetetraacetic acid) at 1100g for 10 minutes. Platelets were resuspended in modified Tyrode solution (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)), pH 7.4. Aggregation was measured in a lumi-aggregometer (Chrono-Log, Havertown, PA) using washed platelets (300 μL) adjusted to approximately 106 platelets/μL. Inhibitors were incubated with the platelets for 3 minutes prior to stimulation.
Measurement of ATP secretion
Adenosine triphosphate (ATP) secretion was measured using CHORONOLUME reagent (Chrono-Log) according to the manufacturer's protocol. ATP secretion data were obtained from at least 3 tests. Bars in each bar graph represent the means ± SD.
Measurement of TxA2
After a 7-minute aggregation period, platelets were removed by centrifugation in the presence of 5 mM EDTA. The platelet-free supernatant fraction was diluted 1:50 with the assay buffer supplied in the TxB2 enzyme immunoassay (EIA) kit (Assay Designs, Ann Arbor, MI). TxB2, a stable metabolite of TxA2, was measured using the manufacturer's protocol. TxB2 production data were obtained from at least 3 tests. The terms TxA2 and TxB2 are used interchangeably throughout this article. Bars in each bar graph represent the means ± SD.
Immunoprecipitation and Western blotting
For detection of tyrosine phosphorylated PLCγ2, Syk, and SLP-76, aggregated platelet samples were added to the same volume of lysis buffer (100 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.4, 2% Nonidet P-40 [NP-40], 300 mM NaCl, 2 mM EDTA, 2 mM PMSF [phenylmethylsulfonyl fluoride], 2 μg/mL of aprotinin, leupeptin, and pepstatin, 2 mM Na3VO4, 2 mM NaF, and a Complete Mini Protease Inhibitor Cocktail Tablet). The samples were incubated on ice for 30 minutes, then 4 μg/mL 4G10 was added and the samples incubated overnight at 4°C. Then, 50 μL Protein A/G PLUS–Agarose was added to each sample prior to incubatation for 2 hours at 4°C. The beads were harvested by centrifugation at 2000g for 2 minutes and the beads were washed 3 times with 500 μL lysis buffer and twice with phosphate-buffered saline (PBS) solution. Proteins were boiled in sample buffer and resolved on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred to nitrocellulose. Western blots were performed using anti-PLCγ2, anti-Syk, or anti–SLP-76 antibody at a 1/1000 dilution, followed by incubation with horseradish peroxidase–conjugated donkey anti–rabbit antibody at a 1/5000 dilution. Blots were developed using Supersignal chemiluminescent substrate (Pierce, Rockford, IL).
For detection of phospho-Akt, samples of aggregated platelets were washed and suspended in EDTA-HEPES-saline (EHS) buffer (10 mM HEPES,150 mM NaCl,1 mMEDTA, pH 7.4) and solubilized in one-third volume of Laemmli reducing sample buffer (24.6 mg/mL dithriothreitol [DTT] added immediately prior to use), boiled for 5 minutes and loaded onto a 10% SDS polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane, and treated with anti–phospho-Akt antibody, followed by a secondary peroxidase–conjugated antibody, and developed using chemiluminescence for phospho-Akt detection. After stripping, the membranes were incubated with antiactin antibody to confirm that a similar amount of protein was present in each lane.
Measurement of PKC activation
Samples of aggregated platelets were transferred to centrifuge tubes and then centrifuged at 1200g for 2 minutes. Each pellet was dissolved with 150 μL lysis buffer (20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 250 mM sucrose, 100 μM leupeptin, and 50 mg/mL PMSF). PKC activity was assayed using the PepTag nonradioactive detection kit. Reaction mixtures were prepared containing 5 μL PepTag PKC reaction 5 × buffer, 2 μg PepTag C1 peptide, 1 μL peptide protection solution, 5 μL protein sample, and 4 μL water. Four microliters of 2.5 μg/mL PKC were substituted for the sample proteins as the positive control. For the negative control, protein samples were replaced by water. The reaction mixtures were incubated in a 30°C water bath for 1 hour. The reaction was stopped by placing the tubes in a boiling water bath for 10 minutes. Samples containing 1 μL 80% glycerol were loaded onto a 0.8% agarose gel using a solution of 50 mM Tris-HCl, pH 8.0, as the running buffer, and then run at 100V for 25 minutes to separate the phosphorylated and unphosphorylated PepTag peptides. Visualization of results was accomplished by exposing the gel to UV light using a BIO-RAD Gel Doc 2000 (Bio-Rad Laboratories, Hercules, CA).
Results
Evaluation of the role(s) of Lyn and Src in TxA2 production elicited bt/VWF-mediated agglutination
Both immunoprecipitation and inhibitor studies provide evidence that Src family kinase function is an early step in GPIb-elicited signaling that results in αIIbβ3 activation.8,17-19 For example, Src and Lyn have been shown to be associated with GPIb17,18 and the Src family kinase selective inhibitors, PP1 and PP2, prevent GPIb-induced platelet and αIIbβ3 activation.8,18 Consequently, Lyn–/– and Src–/– platelets were stimulated with bt/VWF to investigate the role(s) of these Src family kinases in signaling induced by GPIb-mediated platelet agglutination. The Lyn deficient platelets agglutinated, but did not produce TxA2, secrete ATP, or aggregate (Figure 1A-B). The Src–/– platelets agglutinated and aggregated in response to bt/VWF, but TxA2 production and ATP secretion were diminished about 50% compared with littermate control platelets (Figure 1A-B).
The biphasic aggregation-like response of bt/VWF-stimulated wild-type platelets is composed of agglutination and agglutination-dependent aggregation.9 As shown in Figure 1, aggregation, but not agglutination, is inhibited by EDTA. Aggregation is inhibited by EDTA because EDTA chelates the Ca2+ required for fibrinogen binding to αIIbβ3. Because agglutination is direct platelet-platelet interaction resulting from crosslinking of platelets by Ca2+-independent binding of VWF to GPIb-IX-V complexes on adjacent platelets, agglutination is not inhibited by EDTA, but is VWF dependent.9 Agglutination elicits a characteristic level of TxA2 production, about 2.5 ng/mL. The combination of agglutination and aggregation causes about 4 to 5 times more TxA2 production (about 12 ng/mL) than is caused by agglutination in the absence of aggregation (2.5 ng/mL) (Figure 1B).9
Lyn, but not Src, is required for aggregation, TxB2 production, and ATP secretion. (A) Aggregation and agglutination traces of washed wild-type (WT), Lyn–/–, and Src–/– mouse platelets treated with 0.5 μg/mL botrocetin (bt) and 10 μg/mL von Willebrand factor (VWF). WT platelets aggregated in the absence of EDTA and agglutinated in the presence of EDTA. EDTA chelates Ca2+, thereby preventing fibrinogen binding and platelet aggregation without affecting agglutination.9 Lyn-deficient platelets agglutinated, but failed to aggregate. Src–/– platelets underwent delayed aggregation. (B) The level of TxA2 produced by bt/VWF-stimulated WT platelets in the presence of EDTA is characteristic of agglutination elicited TxA2 production. Lyn–/– platelets did not produce TxA2 or secrete ATP, but Src–/– platelets displayed about a 50% decrease of those functions. The numbers above the bars denote TxA2 produced (ng/mL) or ATP secreted (nM) in response to the indicated treatments. The error bars represent standard deviation, n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
Lyn, but not Src, is required for aggregation, TxB2 production, and ATP secretion. (A) Aggregation and agglutination traces of washed wild-type (WT), Lyn–/–, and Src–/– mouse platelets treated with 0.5 μg/mL botrocetin (bt) and 10 μg/mL von Willebrand factor (VWF). WT platelets aggregated in the absence of EDTA and agglutinated in the presence of EDTA. EDTA chelates Ca2+, thereby preventing fibrinogen binding and platelet aggregation without affecting agglutination.9 Lyn-deficient platelets agglutinated, but failed to aggregate. Src–/– platelets underwent delayed aggregation. (B) The level of TxA2 produced by bt/VWF-stimulated WT platelets in the presence of EDTA is characteristic of agglutination elicited TxA2 production. Lyn–/– platelets did not produce TxA2 or secrete ATP, but Src–/– platelets displayed about a 50% decrease of those functions. The numbers above the bars denote TxA2 produced (ng/mL) or ATP secreted (nM) in response to the indicated treatments. The error bars represent standard deviation, n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
These results in Figure 1 demonstrate that Lyn is required for the agglutination-driven signaling that causes TxA2 production and therefore αIIbβ3 activation, granule secretion, and platelet aggregation.9 Although our results demonstrate that both Lyn and Src play a role in bt/VWF-induced GPIb signaling in mouse platelets, Lyn is essential, while Src only enhances the response.
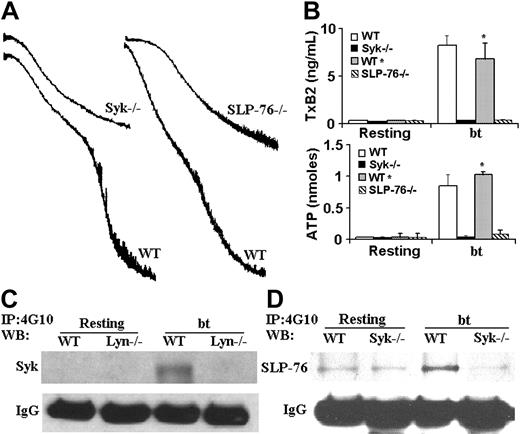
The role(s) of Syk and SLP-76 in bt/VWF signaling that induces TxA2 production
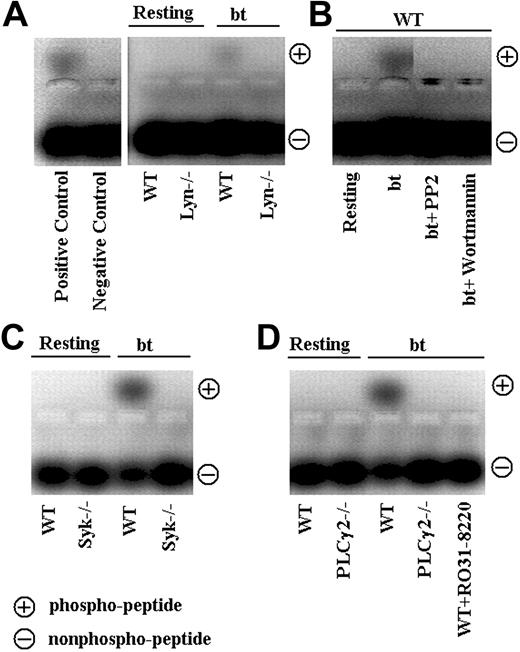
The nonreceptor tyrosine kinase Syk has also been implicated in VWF/GPIb signaling induced by bt/VWF and by shear stress.19,20 In the latter study, immunoprecipitation revealed an association between Syk and PI3K. We therefore used Syk–/– platelets to ascertain whether Syk is required for agglutination-elicited TxA2 production in mouse platelets stimulated with bt/VWF. The Syk–/– platelets agglutinated normally, but did not aggregate (Figure 2A), produce TxA2, or secrete ATP (Figure 2B). Thus, Syk is required for the bt/VWF-induced GPIb signaling that causes TxA2 production and the resulting platelet aggregation. Immunoprecipitation studies with the antiphosphotyrosine antibody 4G10, demonstrated that Lyn is upstream of Syk, since Syk was tyrosine phosphorylated in wild-type but not Lyn–/– platelets in response to bt/VWF (Figure 2C).
Because of the common requirement of Syk and SLP-76 in the regulation of blood and lymphatic vascular separation21 and in collagen-induced signaling,22 coupled with the requirement for Syk in bt/VWF-stimulated signaling, SLP-76–/– platelets were also analyzed. As with Syk–/– platelets, the SLP-76–/– platelets agglutinated, but did not aggregate (Figure 2A), produce TxA2, or secrete ATP (Figure 2B) in response to bt/VWF. In contrast to wild-type platelets, SLP-76 was not phosphorylated in bt/VWF-stimulated Syk–/– platelets, demonstrating that Syk is upstream of SLP-76 (Figure 2D).
Syk and SLP-76 are required for aggregation, TxA2 production, and ATP secretion; Lyn is required for the phosphorylation of Syk and SLP-76; and Syk is required for the phosphorylation of SLP-76 in response to bt/VWF. (A) Syk-deficient and SLP-76–deficient platelets each agglutinated, but did not aggregate in response to bt/VWF stimulation, and (B) did not produce TxA2 or secrete ATP. (C) Immunoprecipitation (IP) was run on platelet lysate as described in “Immunoprecipitation and Western blotting” using monoclonal antibody 4G10. Syk was not phosphorylated in Lyn-deficient platelets in response to bt/VWF. (D) SLP-76 was not phosphorylated in Syk-deficient platelets in response to bt/VWF. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
Syk and SLP-76 are required for aggregation, TxA2 production, and ATP secretion; Lyn is required for the phosphorylation of Syk and SLP-76; and Syk is required for the phosphorylation of SLP-76 in response to bt/VWF. (A) Syk-deficient and SLP-76–deficient platelets each agglutinated, but did not aggregate in response to bt/VWF stimulation, and (B) did not produce TxA2 or secrete ATP. (C) Immunoprecipitation (IP) was run on platelet lysate as described in “Immunoprecipitation and Western blotting” using monoclonal antibody 4G10. Syk was not phosphorylated in Lyn-deficient platelets in response to bt/VWF. (D) SLP-76 was not phosphorylated in Syk-deficient platelets in response to bt/VWF. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
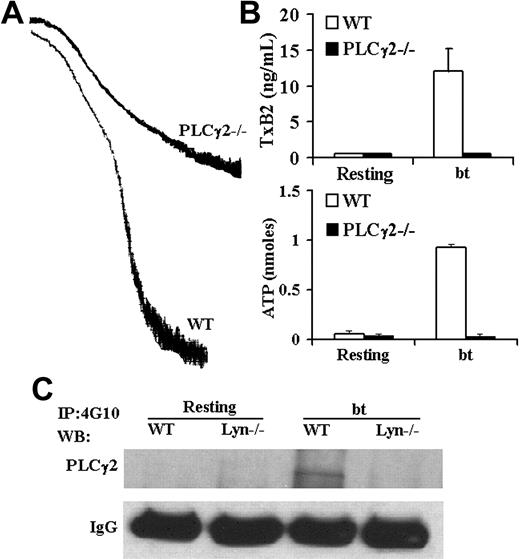
The role(s) of PLCγ2 in bt/VWF-induced TxA2 production
Although PLCγ2 has been shown to play a role in GPIb signaling,23-25 the role(s) of PLCγ2 in signaling induced by bt/VWF-mediated platelet agglutination has not been previously examined. Accordingly, PLCγ2-deficient platelets were stimulated with bt/VWF. The bt/VWF-stimulated PLCγ2–/– platelets agglutinated normally, but failed to aggregate (Figure 3A), produce TxA2, or secrete ATP (Figure 3B). These results demonstrate that PLCγ2 functions in the signaling elicited by GPIb-mediated agglutination that causes TxA2 production, an early step required for bt/VWF-induced αIIbβ3 activation.9 Immunoprecipitation and Western blot analysis of tyrosine-phosphorylated PLCγ2 revealed that Lyn is upstream of PLCγ2 (Figure 3C).
PLCγ2 function is required for bt/VWF-induced platelet aggegation, TxA2 production, and ATP secretion; Lyn is required for tyrosine phosphorylation of PLCγ2. (A) PLCγ2–/– platelets agglutinated, but did not aggregate, (B) produce TxA2, or secrete ATP in response to bt/VWF. (C) Lyn was required for tyrosine phosphorylation of PLCγ2 in response to bt/VWF. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
PLCγ2 function is required for bt/VWF-induced platelet aggegation, TxA2 production, and ATP secretion; Lyn is required for tyrosine phosphorylation of PLCγ2. (A) PLCγ2–/– platelets agglutinated, but did not aggregate, (B) produce TxA2, or secrete ATP in response to bt/VWF. (C) Lyn was required for tyrosine phosphorylation of PLCγ2 in response to bt/VWF. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
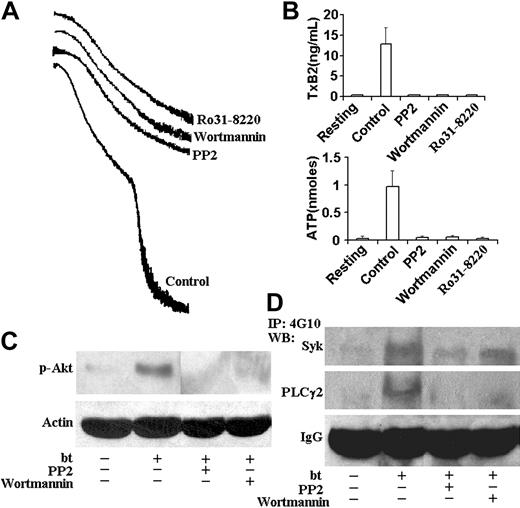
PP2, wortmannin, and Ro31-8220 inhibited aggregation, TxA2 production, and ATP secretion; PP2 inhibited Akt, Syk, and PLCγ2 phosphorylation; and wortmannin inhibited Akt and PLCγ2, but not Syk phosphorylation. (A) Aggregation and agglutination traces of wild-type platelets treated with bt/VWF in the presence of 10 μM PP2, 100 nM wortmannin, and 10 μM Ro31-8220. All of these inhibitors eliminated the aggregation, but did not affect agglutination. (B) PP2, wortmannin, and Ro31-8220 prevented TxA2 production and ATP secretion. (C) PP2 and wortmannin inhibited the phosphorylation of Akt. (D) PP2 inhibited the Syk and PLCγ2 phosphorylation. Wortmannin inhibited phosphorylation of PLCγ2, but not Syk. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
PP2, wortmannin, and Ro31-8220 inhibited aggregation, TxA2 production, and ATP secretion; PP2 inhibited Akt, Syk, and PLCγ2 phosphorylation; and wortmannin inhibited Akt and PLCγ2, but not Syk phosphorylation. (A) Aggregation and agglutination traces of wild-type platelets treated with bt/VWF in the presence of 10 μM PP2, 100 nM wortmannin, and 10 μM Ro31-8220. All of these inhibitors eliminated the aggregation, but did not affect agglutination. (B) PP2, wortmannin, and Ro31-8220 prevented TxA2 production and ATP secretion. (C) PP2 and wortmannin inhibited the phosphorylation of Akt. (D) PP2 inhibited the Syk and PLCγ2 phosphorylation. Wortmannin inhibited phosphorylation of PLCγ2, but not Syk. The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
The effects of inhibitors of Src family kinases, PI3K, and PKC on TxA2 production, ATP secretion, and phosphorylation of Akt, Syk, and PLCγ2 elicited by bt/VWF-mediated agglutination
Phosphoinositide 3-kinase (PI3K) associates constitutively with the cytoplasmic domain of GPIb,26 and this binding mediates the association of the Src family kinases, Lyn and Src, with the cytoplasmic domain of GPIb as a function of GPIb signaling.17,26 In addition, PI3K has been shown to be required for GPIb-induced αIIbβ3 activation depending on the conditions used to elicit that activation.8,27-29 Bt/VWF-induced αIIbβ3 activation requires TxA2 production,9 so we also evaluated the role of PI3K in bt/VWF-induced TxA2 production. This was done by treating bt/VWF-stimulated wild-type platelets with the PI3K-selective inhibitor, wortmannin. Wortmannin treatment did not affect agglutination, but it inhibited bt/VWF-induced platelet aggregation (Figure 4A), TxA2 production, and ATP secretion (Figure 4B). PI3K activation in response to bt/VWF was confirmed by assaying Akt activation (measured as activation-specific Akt phosphorylation) since Akt activation is PI3K dependent in human and mouse platelets. Unlike resting platelets, bt/VWF-stimulated platelets activated Akt, indicating activation of PI3K since Akt phosphorylation was inhibited by wortmannin (Figure 4C).
The Src family kinase inhibitor PP2 prevented bt/VWF-induced Akt phosphorylation, demonstrating that PI3K activation is dependent on Src family kinase activity (Figure 4C). Likewise, PP2 inhibted Syk and PLCγ2 phosphorylation (Figure 4D). Wortmannin modestly decreased (approximately 25% decrease in Syk phosphorylation as revealed by densitometric analysis), but did not prevent Syk phosphorylation, and it nearly eliminated PLCγ2 phosphorylation (Figure 4D). Densitometric analyses revealed that the decrease of Syk phosphorylation associated with wortmannin treatment was roughly equivalent to the decrease caused by EDTA treatment (data not shown), so the decrease may be a consequence of the absence of aggregation. Despite the apparent residual phosphorylation of PLCγ2 in platelets treated with wortmannin, there were no measurable differences between the phosphorylation densities of PLCγ2 in resting platelets and platelets treated with PP2 or wortmannin (not shown). These results indicate that Src family kinase activity is upstream of Syk and Syk is upstream of PI3K, and that most, if not all PLCγ2 phosphorylation requires PI3K.
The PKC selective inhibitor Ro31-8220 prevented TxA2 production, demonstrating that PKC is required for TxA2 production
PI3K is upstream of PLCγ2 and is activated by signaling that originates from Lyn and is propagated through Syk and SLP-76
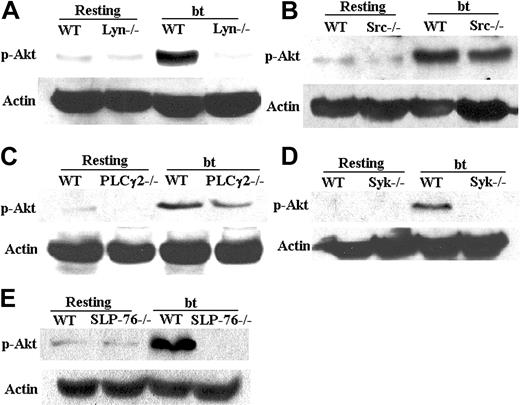
Platelets from mice deficient in Lyn, Syk, SLP-76, or PLCγ2 were stimulated with bt/VWF and assayed for Akt activation as a means of elucidating the pathway of PI3K activation. Those platelets were used for this test because all of them fail to produce TxA2 in response to bt/VWF stimulation. Lyn–/– platelets did not activate Akt (Figure 5A), whereas Src deficiency did not affect Akt activation (Figure 5B). In contrast to PLCγ2–/– platelets (Figure 5C), which diminished Akt phosphorylation by about 20%, both Syk-(Figure 5D) and SLP-76–deficient platelets (Figure 5E) failed to activate Akt in response to bt/VWF. These results confirm that PI3K is activated by signaling that probably originates from Lyn and is propagated via Syk to SLP-76, and that PI3K is upstream of PLCγ2. Further work is required to elucidate the basis of the diminished Akt phosphorylation by the bt/VWF-stimulated PLCγ2–/– platelets.
The roles of FcRγ-chain and LAT in bt/VWF-induced TxA2 production and platelet aggregation
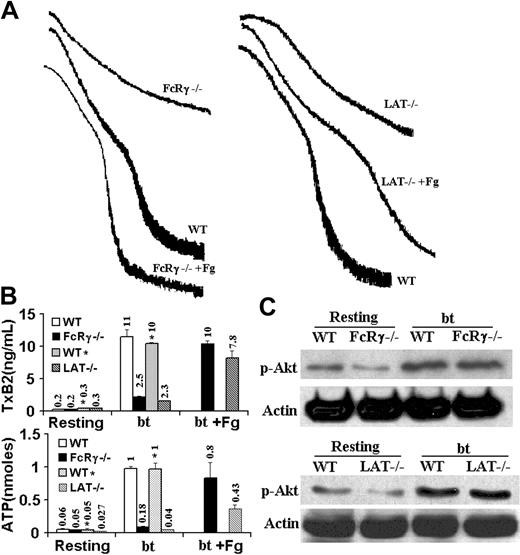
FcRγ-chain function has been shown to be not required for VWF-induced activation of αIIbβ3, shape change, and Ca2+ mobilization,8 but the FcRγ-chain has been shown to be phosphorylated in response to GPIb signaling18,25 and to be required for bt/VWF-induced aggregation of washed platelets.25 Because of this involvement of the FcRγ-chain in GPIb-elicited signaling, we examined bt/VWF-mediated signaling in FcRγ-chain–deficient platelets. The bt/VWF-stimulated FcRγ-chain–/– platelets agglutinated, and produced the normal agglutination-induced level of TxA2 (2.5 ng/mL) (Figure 6A-B), and secreted a low (0.18 nM) but greater than resting level (0.05 nM) of ATP (P < .05, n = 5), and did not aggregate (Figure 6A). Consistent with these results, the bt/VWF-stimulated FcRγ-chain–/– platelets activated Akt (Figure 6C). Exogenous fibrinogen enabled the FcRγ-chain–/– platelets to aggregate, confirming the observation that FcRγ-chain function is not required for activation of αIIbβ3 in response to GPIb-mediated signaling.8 Thus, the data presented here demonstrate that FcRγ-chain function is not required for agglutination-elicited TxA2 production, but is required for α-granule secretion, and therefore the subsequent aggregation-dependent enhancement of TxA2 production and ATP secretion.9
Phosphorylation of Akt (Ser473) requires Lyn, Syk, and SLP-76, but not Src and PLCγ2. (A) Akt was not phosphorylated in Lyn–/– platelets stimulated by bt/VWF. (B) Akt was phosphorylated in Src–/– platelets. (C) Akt was phosphorylated in PLCγ2–/– platelets in response to bt/VWF. (D) Akt was not phosphorylated in Syk–/– platelets stimulated by bt/VWF. (E) Akt was not phosphorylated in SLP-76–/– platelets in response to bt/VWF. The results shown here are representative of 4 experiments.
Phosphorylation of Akt (Ser473) requires Lyn, Syk, and SLP-76, but not Src and PLCγ2. (A) Akt was not phosphorylated in Lyn–/– platelets stimulated by bt/VWF. (B) Akt was phosphorylated in Src–/– platelets. (C) Akt was phosphorylated in PLCγ2–/– platelets in response to bt/VWF. (D) Akt was not phosphorylated in Syk–/– platelets stimulated by bt/VWF. (E) Akt was not phosphorylated in SLP-76–/– platelets in response to bt/VWF. The results shown here are representative of 4 experiments.
Because of the relationship between LAT and PLCγ2 in GPVI-mediated signaling30 and the demonstration that bt/VWF stimulation of washed platelets caused LAT phosphorylation,25 LAT–/– platelets were also analyzed. Unlike the littermate wild-type control platelets, LAT–/– platelets stimulated with bt/VWF agglutinated and did not aggregate (Figure 6A), and produced an 80% diminished level of TxA2 (Figure 6B), but did not secrete ATP (Figure 6B). Exogenous fibrinogen enabled platelet aggregation, demonstrating that the bt/VWF-stimulated LAT–/– platelets had activated αIIbβ3 (Figure 6A). This aggregation enabled by exogenous fibrinogen establishes the fact that LAT is not required for TxA2 production because αIIbβ3 activation requires TxA2 production.9 The normal phosphorylation of Akt in bt/VWF-treated LAT–/– platelets indicates that LAT is not required for PI3K activation (Figure 6C). So, although LAT function is not absolutely required for GPIb-induced TxA2 production, it does enhance TxA2 production and appears to be required for ATP secretion, and therefore α-granule secretion and aggregation of washed platelets.9
FcRγ-chain and LAT are not required for TxA2 production, Akt phosphorylation, or αIIbβ3 activation by washed platelets stimulated with bt/VWF. (A) FcRγ-chain–deficient and LAT-deficient platelets agglutinated, but did not aggregate. Fibrinogen (Fg) restored normal aggregation to both the FcRγ-chain–/– platelets and the LAT–/– platelets. (B) FcRγ-chain–deficient platelets produced the agglutination-elicited level of TxA2 and secreted the level of ATP elicited by agglutination. LAT-deficient platelets produced the agglutination-elicited level of TxA2, but did not secrete ATP. Fibrinogen (Fg) enhanced TxB2 production and ATP secretion by both the FcRγ-chain–/– and the LAT–/– platelets. (C) FcRγ-chain–deficient and LAT-deficient platelets phosphorylated Akt in response to stimulation by bt/VWF. The numbers over the bars represent the amount of TxA2 production (ng/mL) or ATP secretion (nM). The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
FcRγ-chain and LAT are not required for TxA2 production, Akt phosphorylation, or αIIbβ3 activation by washed platelets stimulated with bt/VWF. (A) FcRγ-chain–deficient and LAT-deficient platelets agglutinated, but did not aggregate. Fibrinogen (Fg) restored normal aggregation to both the FcRγ-chain–/– platelets and the LAT–/– platelets. (B) FcRγ-chain–deficient platelets produced the agglutination-elicited level of TxA2 and secreted the level of ATP elicited by agglutination. LAT-deficient platelets produced the agglutination-elicited level of TxA2, but did not secrete ATP. Fibrinogen (Fg) enhanced TxB2 production and ATP secretion by both the FcRγ-chain–/– and the LAT–/– platelets. (C) FcRγ-chain–deficient and LAT-deficient platelets phosphorylated Akt in response to stimulation by bt/VWF. The numbers over the bars represent the amount of TxA2 production (ng/mL) or ATP secretion (nM). The error bars represent standard deviation; n = 3. In some cases, the values of the SD were so small that the bars cannot be seen.
PKC activation elicited by bt/VWF is dependent on Lyn, Syk, PI3K, and PLCγ2. This assay demonstrates activation of PKC as exemplified by phosphorylation of PKC-specific peptide substrate. Phosphorylated peptide substrate moves toward the positive electrode in the assay. (A) In contrast to wild-type platelets, PKC was not activated in Lyn–/– platelets stimulated with bt/VWF. (B) PP2 and wortmannin each blocked the activation of PKC. (C) Syk and (D) PLCγ2 also are required for PKC activation. The results shown here are representative of 3 experiments.
PKC activation elicited by bt/VWF is dependent on Lyn, Syk, PI3K, and PLCγ2. This assay demonstrates activation of PKC as exemplified by phosphorylation of PKC-specific peptide substrate. Phosphorylated peptide substrate moves toward the positive electrode in the assay. (A) In contrast to wild-type platelets, PKC was not activated in Lyn–/– platelets stimulated with bt/VWF. (B) PP2 and wortmannin each blocked the activation of PKC. (C) Syk and (D) PLCγ2 also are required for PKC activation. The results shown here are representative of 3 experiments.
Botrocetin/VWF-induced PKC activation results from signaling initiated by Lyn, and propagated via Syk, PI3K, and PLCγ2
Previous work by us has demonstrated that PKC activity is required for bt/VWF/-induced TxA2 production.9 This result was confirmed by the data in Figure 4A. Establishing the position of PKC relative to Lyn, Syk, PI3K, and PLCγ2 in the signaling pathway used by bt/VWF-stimulated platelets to elicit TxA2 production was accomplished by combining the results of a PKC activation assay (Figure 7) with those obtained from immunoprecipitation and Western blotting studies. The results were unequivocal: PKC is downstream of signaling apparently initiated by Lyn and propagated via Syk, SLP-76, PI3K, and PLCγ2. This preliminary pathway is based in part on the observations that Syk, PI3K, PLCγ2, and PKC activation are Lyn dependent (Figures 2C, 3C, 5A, and 7A, respectively). Further resolution of the pathway was obtained by showing that PI3K is downstream of Syk, and SLP-76 (Figure 5D-E) and upstream of PLCγ2 (Figure 5C). This was accomplished by demonstrating that bt/VWF-stimulated Syk–/– (Figure 5D) and SLP-76–/– (Figure 5E) platelets did not activate PI3K, and that PI3K is upstream of PLCγ2 (Figures 4D, 5C). The latter point was established by showing that wortmannin prevented most, if not all phosphorylation of PLCγ2 (Figure 4D). Finally, bt/VWF-stimulated Syk–/– (Figure 7C) and PLCγ2–/– platelets (Figure 7D) failed to activate PKC, demonstrating that PKC is downstream of Syk and PLCγ. Thus, these data demonstrate that bt/VWF-induced signaling activates PKC via a signaling cascade that apparently originates with Lyn and activates PKC in response to propagation of that signaling through Syk, SLP-76, PI3K, and PLCγ2, and presumably other unidentified signaling and adaptor molecules.
Discussion
Under appropriate conditions, bt/VWF stimulation of washed murine platelets causes platelet activation and a biphasic agglutination/aggregation response. The GPIbα-mediated agglutination initiates a signaling cascade that elicits TxA2 production. In this system, activation of αIIbβ3 is dependent on the TxA2 production elicited by GIbα-mediated agglutination.9 Granule secretion requires TxA2-mediated signaling. The granule secretion provides the fibrinogen and ADP required to enable aggregation and αIIbβ3-mediated outside-in signaling. The αIIbβ3-dependent outside-in signaling is required for the majority of the TxA2 production and ATP secretion resulting from stimulation of platelets with bt/VWF.9 Contact-dependent signaling may also be required for the αIIbβ3-dependent TxA2 production and ATP secretion.31 The enhancement of TxA2 production and ATP secretion that is dependent on αIIbβ3 outside-in signaling also requires Ca2+ mobilization, and uses signaling mediated by TxA2 receptors, P2Y1, Gαq, P2Y12, and Gi.9 The results presented here extend our understanding of bt/VWF-elicited signaling by examining the signaling used by platelets to elicit TxA2 production in response to bt/VWF-mediated agglutination.
The results obtained here using Lyn–/– and Src–/– platelets advance our understanding of Src family kinases in GPIb-mediated signaling, but also raise new questions about their functions. It is known that the Src family kinase selective inhibitors PP1 and PP2 inhibit GPIb-induced Ca2+ oscillations,8,23 cytoskeleton reorganization,23 and αIIbβ3 activation.8 PP2 also inhibits bt/VWF-induced aggregation of washed mouse platelets (Figure 4A) as well as TxA2 production and ATP secretion (Figure 4B). The Src family kinases Lyn and Src have been shown to be dynamically associated with GPIb in response to bt/VWF stimulation.17 However, Src, but not Lyn, was activated by the A1 domain of VWF, a monomeric ligand, in the presence of botrocetin. This suggests that the binding of a monomeric ligand to GPIb (which presumably does not cause receptor clustering) elicits the physical association of both Lyn and Src with GPIb, but only the activation of Src.17 This observation lead to the suggestion that Src is more likely than Lyn to be responsible for GPIb-mediated tyrosine phosphorylation.17 The results presented here appear to contradict this conclusion since Lyn, not Src, was required for bt/VWF-induced platelet activation (Figure 1A). The explanation for this unexpected result may be that even though Src was activated by monomeric ligand, it did not elicit the phosphorylation of FcRγ-chain, Syk, or PLCγ2, proteins that were phosphorylated in response to multimeric VWF and botrocetin.17 That is, Lyn may have been activated in response to VWF, as a multimeric ligand, and thus been responsible for the phosphorylation of these proteins. Alternatively, this discrepancy might simply reflect differences in GPIb signaling between humans and mice. Regardless, the data presented document an absolute requirement for Lyn in bt/VWF-induced GPIb signaling in mouse platelets. Additionally, Lyn appears to be the first kinase used in the bt/VWF-induced signaling pathway.
The roles of Syk and SLP-76 in bt/VWF-initiated GPIb signaling were investigated using platelets deficient in Syk and platelets deficient in SLP-76. The bt/VWF-stimulated Syk–/– platelets agglutinated, but did not aggregate (Figure 2A), produce TxA2, or secrete ATP (Figure 2B). The data in Figure 2C and 2D, respectively, demonstrate that Lyn is upstream of Syk, and that Syk may be immediately upstream of SLP-76 because Syk is required for SLP-76 phosphorylation (Figure 2D). As with the Syk–/– platelets, the bt/VWF-stimulated SLP-76–/– platelets agglutinated, but did not aggregate (Figure 2A), produce TxA2, or secrete ATP (Figure 2B). Also, both Syk (Figure 5D) and SLP-76 (Figure 5E) were required for the activation of PI3K. Thus, Syk and SLP-76 are essential for an early step in TxA2 production induced by agglutination. These results are consistent with the ability of Syk from mouse platelets stimulated with collagen-related peptide to phosphorylate SLP-76 tyrosine residues 113, 128, and 145 in vitro.22 Further, SLP-76 was not phosphorylated in CRP-treated Syk–/– platelets,22 revealing further similarity between GPIb and GPVI signaling.
Previous work has demonstrated that PLCγ2 function affects spreading and cytoskeletal rearrangement in mouse platelets in response to bt/VWF stimulation,23 but its role in bt/VWF-mediated signaling has not been clarified. The behavior of the PLCγ2–/– platelets in response to bt/VWF in this study revealed that PLCγ2 is required for GPIb-induced TxA2 production, ATP secretion, and aggregation (Figure 3). PI3K activation was Syk and SLP-76 dependent (Figure 5D-E), and was required for most, if not all PLCγ2 phosphorylation (Figure 4D). In contrast to GPVI signaling, PLCγ2 phosphorylation was not dependent on FcRγ-chain (Figure 6). Again, this is clear because TxA2 production was PLCγ2 dependent, but not FcRγ-chain dependent. Thus, in a similar manner to αIIbβ3 activation induced by platelet adhesion to VWF,8 activation of PLCγ2 by bt/VWF apparently is not immunoreceptor tyrosine-based activation motif (ITAM) dependent. The data in Figure 6 also demonstrate that PLCγ2 activation is not LAT dependent since LAT, unlike PLCγ2, is not required for TxA2 production. Also, it is clear that PLCγ2 is required for PKC activation (Figure 7D) and that PKC is required for TxA2 production (Figure 4B).9
The effects of wortmannin on bt/VWF-induced signaling confirm and extend previous observations that PI3K function is required for αIIbβ3 activation induced by GPIb-mediated signaling in a variety of model systems.8,17,20 The results presented here demonstrate that PI3K is not required for agglutination, but is required for bt/VWF-induced aggregation (Figure 4A), TxA2 production, and dense granule secretion (Figure 4B). The results in Figures 1, 2, and 5 demonstrate that the GPIb-elicited signaling appears to travel from Lyn (Figure 1) to Syk (Figure 2), and then via the adaptor SLP-76 (Figure 2) to PI3K (Figure 5E). PI3K is required for most, if not all phosphorylation of PLCγ2 (Figure 4D) and is required for activation of PKC (Figure 7B), results consistent with the widely reported role of PI3K in PLCγ2 activation, and the role of PLCγ2 in PKC activation.32 Further work is required to establish if these signaling molecules function as a branched network rather than as a linear sequence to elicit TxA2 production. These observations are consistent with the suggestion that GPIb forms a signaling complex that includes an Src family kinase, Syk, SLP-76, and PI3K.8
Although PI3K activation is essential for platelet activation in response to both bt/VWF-mediated agglutination (Figure 4) and pathologic shear stress applied in a cone-plate viscometer,20 the mechanisms of activation of PI3K appear to be essentially different in these 2 systems. Unlike the situation in response to shear stress,20 PI3K activation elicited by GPIb-mediated agglutination is not dependent on ATP secretion or P2Y12-dependent signaling.9 This is evident because the P2Y12 antagonist, AR-C69931MX, inhibited activation of PI3K in response to shear stress,20 but was without effect on agglutination-elicited TxA2 production in the bt/VWF system.9 Despite this difference and the fact that platelet activation in response to shear stress is TxA2 independent, Syk was required for PI3K activation in both systems (Figure 5D).20 These results demonstrate that GPIb-dependent signaling activated by different environmental agents can use essentially different mechanisms to activate signaling molecules common to both systems, demonstrating the versatility of GPIb-dependent signaling.9
The literature contains data that demonstrate a role(s) for the FcRγ-chain in GPIb-mediated signaling. Despite this demonstration of a requirement for FcRγ-chain function in bt/VWF-induced platelet aggregation,25 recent work has shown that FcRγ-chain–/– platelets undergo normal cytoskeletal reorganization (spreading and filopodia formation) and Ca2+ mobilization following adhesion to a VWF matrix,23 and that FcRγ-chain function is not required for αIIbβ3 activation under these conditions.8 Nevertheless, another group has demonstrated that, in contrast to washed FcRγ-chain+/+ platelets, washed FcRγ-chain–/– platelets fail to aggregate in response to bt/VWF stimulation.25 The data presented in Figure 6B demonstrate that FcRγ-chain is not required for agglutination-elicited TxA2 production. Therefore, it is clear that FcRγ-chain is not upstream of PLCγ2 because PLCγ2 is required for agglutination-dependent TxA2 production, whereas FcRγ-chain is not. The fact that fibrinogen enables the bt/VWF-stimulated FcRγ-chain–/– platelets to aggregate demonstrates that these platelets had activated αIIbβ3, confirming a previous study,8 and that their functional deficiency was apparently due to insufficient α-granule secretion.
Finally, the data presented here demonstrate the versatility of GPIb signaling in using a common set of signaling molecules that can be activated by essentially different mechanisms in response to different environmental stimuli. For example, PI3K activation is required and dependent on Syk in response to both shear stress20 and bt/VWF stimulation,9 but in the former case, activation of PI3K requires ATP secretion and P2Y12 function,20 whereas, in the latter case, PI3K activation is independent and upstream of ATP secretion and P2Y12 function.9 Thus, not only is GPIb a central player in hemostasis, but it may also be able to signal using a common set of signaling molecules (an Src family kinase[s], Syk, PI3K, PLCγ2, and PKC) that are activated by essentially different mechanisms, ultimately leading to different mechanisms of activating αIIbβ3. Understanding the versatility of GPIb signaling may therefore prove useful in developing a rationale for the design of clinically useful antithrombotic agents targeted to GPIb function.
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2005-04-1667.
Supported in part by HL63216 from the National Heart, Lung, and Blood Institute; P30CA21765 from the Cancer Center Support Grant; and P01CA20180 from the National Cancer Institute; Public Health Services; the National Health and Medical Research Council of Australia; the American Lebanese Syrian Associated Charities; and the W. Harry Feinstone Center for Genomic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs V. Pivniouk and R. S. Geha for providing us with breeding stock used to produce the SLP-76–deficient mice used in this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal