Abstract
Antiplatelet therapies improve endothelial function in atherosclerosis, suggesting that platelets regulate vascular nitric oxide (NO) bioactivity in vivo. Herein, washed platelets consumed NO on activation in an aspirin-sensitive manner, and aspirin enhanced platelet NO responses in vitro. To examine whether in vivo aspirin can inhibit platelet NO consumption, a double-blind placebo-controlled study was conducted. After a 2-week nonsteroidal anti-inflammatory drug (NSAID)–free period, healthy men were randomly assigned and administered aspirin (75 mg/d orally) or identical placebo for 14 days, then crossed over to the opposite arm. Following in vivo aspirin, NO consumption by platelets was inhibited 91%. Rate of onset and recovery following aspirin withdrawal was consistent with cyclooxygenase 1 (COX-1) inhibition. In a small substudy, NO consumption by platelets from postmenopausal women was faster in hypercholesterolemics and less sensitive to aspirin (ie, 39% versus 76% inhibition for hypercholesterolemics or normocholesterolemics, respectively). However, 150 mg aspirin/day increased inhibition of NO consumption by platelets of hypercholesterolemics to 80%. Comparisons of platelet COX-1 or -2 expression and urinary 11-dehydro-thromboxane B2 excretion suggested that aspirin was less able to block platelet activation in vivo in hypercholesterolemia. In conclusion, aspirin inhibits NO consumption by platelets from healthy subjects, but its beneficial effects on NO bioactivity may be compromised in some hypercholesterolemic patients.
Introduction
The free radical gas nitric oxide (NO) is a bioactive mediator formed in the vasculature that has important roles in regulating vascular tone in vivo.1-4 In vascular disease, impaired NO signaling through its accelerated removal is often observed. NO removal may occur through its reaction with free radicals formed by enzymes that are up-regulated as part of the disease. Free radical pathways that are induced and can consume NO include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, lipoxygenases (LOXs), heme peroxidases, and cyclooxygenases (COXs).5-8 In particular, an involvement of platelet COX in regulating NO bioactivity is suggested by numerous clinical studies. For example, in hypercholesterolemia and hypertension, impaired acetylcholine-induced vasodilatation is restored by acute intravenous or oral low-dose aspirin.9-12 Also, antiplatelet glycoprotein IIbIIIa (GPIIbIIIa) antagonists improve flow-mediated dilatation of patients with coronary artery disease, suggesting a direct role for platelets in modulating NO bioactivity in vivo.12 Finally, we previously found that platelet COX-1 can consume NO in vitro.6
Aspirin is routinely prescribed to patients with vascular disease as an inhibitor of COX-1. Therefore, this study examined whether aspirin could block platelet COX-1–dependent NO consumption and enhance platelet NO responses in vitro. Additionally, the ability of clinically used doses of oral aspirin to inhibit platelet NO consumption were examined in a double-blind crossover study. Results show that in vitro or in vivo aspirin effectively inhibits NO consumption by platelets from healthy male subjects and can partially restore cyclic guanosine monophosphate (cGMP) levels following platelet activation. In a separate substudy, NO consumption by platelets from hypercholesterolemic patients was faster and relatively less sensitive to in vivo aspirin than NO consumption by platelets from corresponding control subjects. The data suggest that aspirin may improve NO bioactivity by inhibiting platelet COX-1–dependent NO consumption, although this beneficial effect may be compromised in some patients with hypercholesterolemia.
Patients, materials, and methods
Isolation of washed platelets
Whole blood was collected from healthy volunteers free from nonsteroidal anti-inflammatory drugs (NSAIDs) for at least 14 days, into acid-citrate-dextrose (ACD; trisodium citrate, 2.5 g/100 mL, citric acid, 1.37 g/100 mL, glucose, 2 g/100 mL) (blood-ACD ratio, 8.1:1.9 vol/vol) and centrifuged at 250g for 10 minutes at room temperature. The platelet-rich plasma was recentrifuged at 900g for 10 minutes, and the pellet was resuspended in Tyrode buffer (134 mM NaCl, 12 mM NaHCO3, 2.9 mM KCl, 0.34 mM Na2HPO4, 1 mM MgCl2, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 5 mM glucose, pH 7.4) containing ACD (1:9 vol/vol). The platelets were washed by centrifuging at 800g for 10 minutes then resuspended in Tyrode buffer.
Measurement of NO consumption by washed platelets
Anaerobic solutions of NO were prepared as described and measured electrochemically using an NO sensor (AmiNO100; Harvard Apparatus, Kent, United Kingdom).6,7 When NO loss was not linear, rates are given as first-order rate constants (kobs) calculated by determining the slope of ln[NO] versus time according to the following equation: d[NO]/dt = kobs[NO], where NO loss on addition of arachidonate was linear, the slope of the initial rate of NO disappearance was determined. For all NO uptake rates and first-order rate constants, data are the means ± SEMs (n ≥ 3 determinations per donor, with n ≥ 3 donors per group). For platelet NO consumption, NO (3.8 μM) was added to 0.5 mL Tyrode buffer containing 1 mM CaCl2, and 200×109 platelets/L with stirring. Once the response had stabilized, 50 μM arachidonate or ethanol vehicle was added, and rates of NO consumption were monitored.
In vitro treatment of washed platelets with aspirin
Washed platelets from healthy volunteers or women with hypercholesterolemia were incubated with 1 mM aspirin for 30 minutes at room temperature, before being assayed for NO consumption. For determination of cGMP, 0.5 × 109 platelets with or without aspirin pretreatment (1 mM, 30 minutes) were added to Tyrode buffer, 5 mM CaCl2, 1 mM isobutyl methyl xanthine (IBMX), 1 mM l-nitroarginine-methyl-ester and incubated with or without 1.9 μM NO, 40 μM arachidonate for 37°C for 5 minute. Following this, samples were taken in triplicate for determination of cGMP using radioimmunoassay.6 Platelet aggregation in response to thrombin (0.4 U/mL) was determined using a PAP4 optical aggregometer (BioData Corp, Horsham, PA). Aspirin-treated or control platelets were activated with thrombin in Tyrode buffer, 5 mM CaCl2, 10 seconds before addition of 1.9 μM NO.
Recruitment of subjects for in vivo studies
All human studies were approved by Bro Taf Local Research Ethics Committee (Study No. 01/4302). Normocholesterolemic male volunteers (NM; age, 22-33 years; cholesterol < 5.9 mM) were recruited from workplace colleagues. Normocholesterolemic postmenopausal women (NF; age, 50-60 years; cholesterol < 5.9 mM) or hypercholesterolemic postmenopausal women (HF; age, 56-66 years; cholesterol > 6.4 mM) were recruited by advertisement. Exclusion criteria were smoking (< 6 months prior to study), previous treatment with lipid-lowering medication, recent myocardial infarction (< 3 months), hypertension (blood pressure, > 140/80 mmHg), diabetes, gastric bleeding, peptic ulcer disease, and a known sensitivity to aspirin. All subjects gave written informed consent, according to the Declaration of Helsinki.
Effect of in vivo aspirin supplementation on NO consumption
Subjects abstained from NSAID-containing medication throughout the study. Following a 14-day NSAID-free washout period, blood and urine samples were obtained for baseline determinations of washed platelet NO consumption, cholesterol levels, and in vivo thromboxane generation. Subjects were randomly assigned to either 75 mg oral aspirin/day or identical placebo for 14 days (arm 1), in a double-blinded fashion, then blood and urine samples were again obtained. Following a second 14-day NSAID-free washout, subjects crossed over (arm 2) and received either 75 mg aspirin/day or identical placebo, as appropriate for 14 days. At the end, subjects returned to the clinic to provide final blood and urine samples. Urine samples were stored at –80°C until analysis by gas chromatography–mass spectrometry (GC-MS). To examine onset and recovery of platelet NO consumption, healthy men were administered 75 mg aspirin/day for 3 days. Blood samples were taken daily prior to aspirin, during treatment, and for a further 3 days following the last aspirin dose. In another study, hypercholesterolemic females were recalled and administered aspirin 150 mg/day for 14 days followed by returning to the clinic to provide a blood sample.
GC-MS determination of thromboxane in urine
11-Dehydro-TXB2 was quantified in urine using a highly precise and accurate gas chromatographic–mass spectrometric method using stable isotope dilution techniques.13
Immunodetection of COX isoforms and myeloperoxidase (MPO) in platelets
Western blotting (3 μg protein) was performed using goat anti–human COX-2 polyclonal antibody (1:200; Santa Cruz Biotech, Santa Cruz, CA), rabbit antiactin (Sigma, Poole, United Kingdom) or goat anti–human COX-1 (1:200; Santa Cruz Biotech) and visualized using enhanced chemiluminescence (ECL; Amersham, Buckinghamshire, United Kingdom), following incubation with anti–goat or anti–rabbit immunoglobulin G–horseradish peroxidase (IgG-HRP). Densitometry of bands was analyzed using Gene-Tools (Syngene, Cambridge, United Kingdom). MPO was detected using rabbit anti–human MPO (1:1000; Calbiochem, Nottingham, United Kingdom) and visualized using ECL with anti–rabbit IgG-HRP. Human neutrophils isolated by dextran sedimentation were used as positive control (1.5 μg protein).
Statistical analysis
All measurements of NO consumption were run in triplicate for each donor at each time point. Data are shown as mean ± SEM, with paired t test P less than .05 being considered statistically significant.
Results
Characterization of NO loss in reaction systems
Nitric oxide decay in Tyrode buffer followed first-order kinetics with rate constants (kobs) of typically 3×10–3 to 8 × 10–3/second. Although aerobic oxidation of NO is second order, at the low NO concentrations used, first-order reactions predominate that include gas-phase diffusion and electrode NO consumption. By using the kobs that was determined each day, rates of background NO loss at any concentration were determined and subtracted from platelet-dependent rates.
Aspirin inhibits platelet NO consumption by washed platelets from NM volunteers in vitro
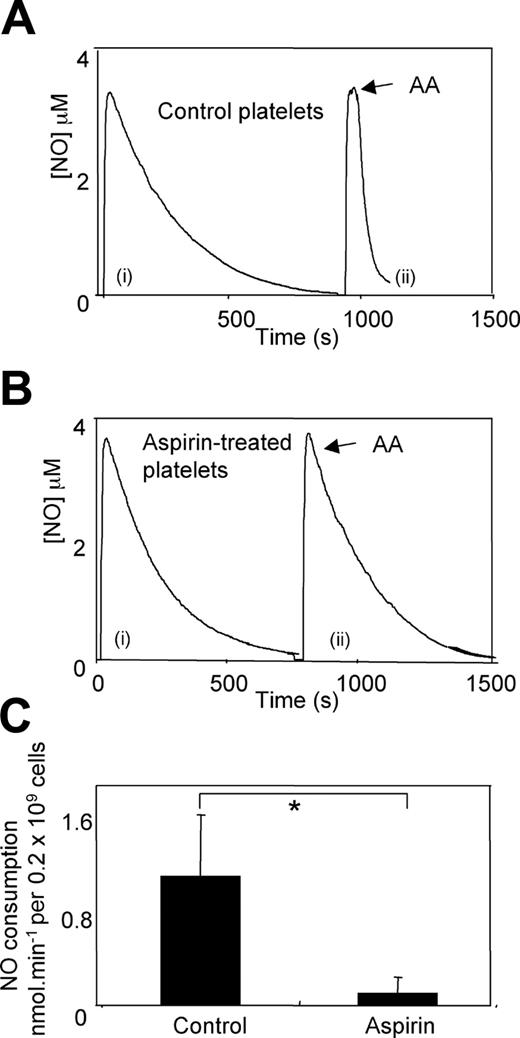
Resting washed platelets from NM donors (mean total cholesterol, 5.5 mM) did not consume NO, with first-order rates of decay similar to NO in buffer alone (kobs, 3.6 ± 0.27/second versus 3.9 ± 0.25/second, for buffer alone versus platelets, respectively). However, on addition of 50 μM arachidonate, rates of NO decay accelerated significantly (1.05 ± 0.5 nmol/minute per 0.2 × 109 platelets) (Figure 1A). Preincubation with 1 mM aspirin for 30 minutes inhibited arachidonate-stimulated NO consumption by 91% (0.09 ± 0.13 nmol/minute per 0.2 × 109 platelets) (Figure 1B). There was a range in control rates of arachidonate-stimulated NO consumption (ranging from 0.5 to 1.8 nmol/minute per 0.2 × 109 platelets), reflecting donor variability (Figure 1C). These data demonstrate that inhibition of COX-1 by aspirin in vitro prevents NO consumption by washed platelets.
Time course and dose response of aspirin inhibition of NO consumption in washed platelets
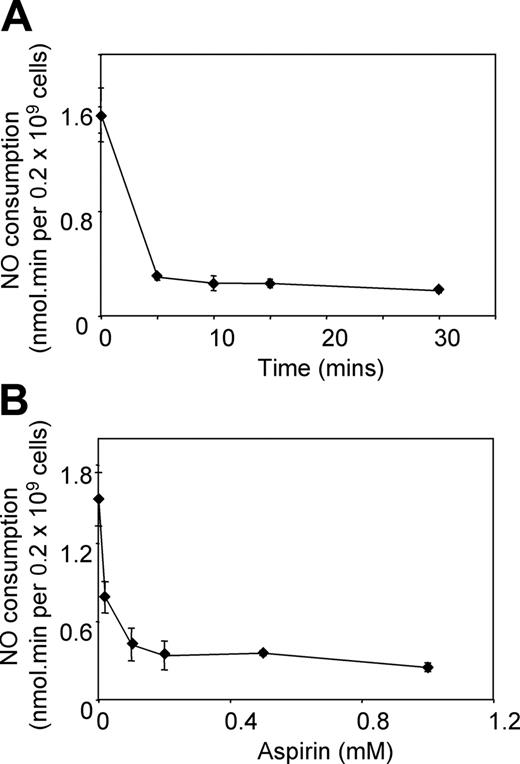
To characterize the sensitivity of platelets to aspirin inhibition of NO, time course and dose-response curves were constructed. Time course experiments showed that even 5 minutes is sufficient for almost complete inhibition of NO consumption by 1 mM aspirin (Figure 2A), whereas varying doses showed that aspirin concentrations as low as 0.1 mM inhibit effectively within 15 minutes at room temperature (Figure 2B).
Aspirin inhibits platelet NO consumption. (A) Aspirin-treated or -untreated platelets (1 mM, 30 minutes, room temperature) were placed in the chamber of the NO electrode at 0.2 × 109/mL in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C. NO (3.8 μM) was added, and decay was monitored, with and without addition of 50 μM arachidonate. Representative trace showing NO consumption by control platelets from one subject, (i) control, (ii) with arachidonate added at arrow. (B) Representative trace showing NO consumption by aspirin-treated platelets from the same subject as in A, (i) control, (ii) with arachidonate added at arrow. (C) Cumulative data on the effect of aspirin treatment on arachidonate-stimulated platelet NO consumption rates (mean ± SEM; n = 3 separate donors; *P < .05, Student unpaired t test).
Aspirin inhibits platelet NO consumption. (A) Aspirin-treated or -untreated platelets (1 mM, 30 minutes, room temperature) were placed in the chamber of the NO electrode at 0.2 × 109/mL in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C. NO (3.8 μM) was added, and decay was monitored, with and without addition of 50 μM arachidonate. Representative trace showing NO consumption by control platelets from one subject, (i) control, (ii) with arachidonate added at arrow. (B) Representative trace showing NO consumption by aspirin-treated platelets from the same subject as in A, (i) control, (ii) with arachidonate added at arrow. (C) Cumulative data on the effect of aspirin treatment on arachidonate-stimulated platelet NO consumption rates (mean ± SEM; n = 3 separate donors; *P < .05, Student unpaired t test).
Time course and dose response of aspirin inhibition of NO consumption by washed platelets in vitro. Aspirin-treated or -untreated platelets (room temperature, varying time and/or dose) were placed in the chamber of the NO electrode at 2 × 108/mL in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C. NO (3.8 μM) was added, and decay was monitored, with and without addition of 50 μM arachidonate. (A) NO consumption rates were monitored following incubation with 1 mM aspirin for varying times. (B) NO consumption rates were monitored following incubation for 15 minutes with varying aspirin concentrations (mean ± SEM, n = 3).
Time course and dose response of aspirin inhibition of NO consumption by washed platelets in vitro. Aspirin-treated or -untreated platelets (room temperature, varying time and/or dose) were placed in the chamber of the NO electrode at 2 × 108/mL in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C. NO (3.8 μM) was added, and decay was monitored, with and without addition of 50 μM arachidonate. (A) NO consumption rates were monitored following incubation with 1 mM aspirin for varying times. (B) NO consumption rates were monitored following incubation for 15 minutes with varying aspirin concentrations (mean ± SEM, n = 3).
Aspirin partially restores NO-dependent elevation of cGMP and enhances the inhibitory effects of NO on aggregation, following platelet activation
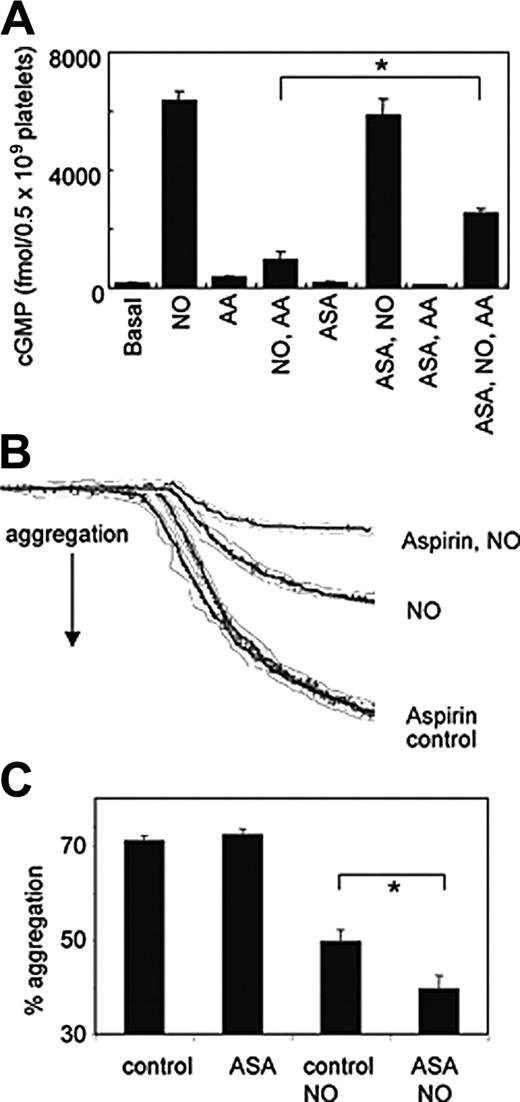
To determine whether aspirin could enhance platelet NO bioactivity, aggregation and cGMP responses were determined following addition of NO to activated platelets. Incubation with 1.9 μM NO caused a large increase in washed platelet cGMP, that was significantly attenuated by arachidonate (Figure 3A). If platelets were pretreated with aspirin, the ability of NO to elevate cGMP in the presence of arachidonate was significantly restored (Figure 3A). Aggregation of platelets to high-dose thrombin (0.4 U/mL) was unaffected by aspirin (Figure 3B-C). However, the inhibitory effect of NO on aspirinized platelets was significantly greater than for controls (Figure 3B-C). Because NO or cGMP analogs can effectively block thrombin-stimulated arachidonate release by inhibiting phospholipase A2, the order of addition in these experiments was reversed, with NO added 10 seconds after thrombin.14-16 These data indicate that the ability of NO to block platelet responses is enhanced by aspirin.
In vivo aspirin supplementation inhibits platelet NO consumption by washed platelets from healthy volunteers
At baseline, washed platelets from NM volunteers (n = 5; mean total cholesterol, 4.74 mM) did not consume NO (Figure 4A). On addition of 50 μM arachidonate, platelets consumed NO at rates of 1.31 ± 0.37 nmol/minute per 0.2 × 109 platelets. Following placebo, platelet NO consumption was unchanged from baseline; however, aspirin (75 mg/day) supplementation for 14 days reduced arachidonate-stimulated NO consumption by 95% (1.35 ± 0.47 versus 0.07 ± 0.02 nmol/minute per 0.2 × 109 platelets, for placebo or aspirin, respectively), indicating that in vivo inhibition of COX-1 by aspirin effectively prevents platelet NO consumption (Figure 4A-B). To characterize the onset of inhibition of NO consumption, and its recovery following aspirin withdrawal, healthy male volunteers (n = 3) were administered aspirin (75 mg/day) for 3 days, and NO consumption was measured daily, before, during, and 3 days after aspirin withdrawal. Onset of inhibition was gradual and essentially complete by day 3. Following aspirin withdrawal, NO consumption recovered over the following 3 days and was back to baseline by day 6 (Figure 4C). These data indicate that inhibition of NO consumption by aspirin is transient and parallels inhibition of COX-1 in vivo.17
Aspirin partially restores cGMP generation following arachidonate activation of platelets and enhances the inhibitory effects of NO on thrombin-stimulated platelet activation. (A) Platelet cGMP levels were determined using radioimmunoassay following incubation of washed platelets (± 1 mM aspirin preincubation) with 1.9 μM NO ± 40 μM arachidonate (AA) for 5 minutes, 37°C (n = 3, mean ± SEM; *P < .05, Student unpaired t test).ASAindicates aspirin. (B) Representative trace showing aggregation of washed platelets (control or aspirin treated) at 200 × 109/L in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C following addition of 0.4 U/mL thrombin, with and without 1.9 μM NO (added 10 seconds after thrombin). (C) Cumulative data showing the effect of aspirin treatment on the sensitivity of platelets to NO during thrombin-stimulated aggregation (mean ± SEM, n = 3; *P < .05, Student unpaired t test).
Aspirin partially restores cGMP generation following arachidonate activation of platelets and enhances the inhibitory effects of NO on thrombin-stimulated platelet activation. (A) Platelet cGMP levels were determined using radioimmunoassay following incubation of washed platelets (± 1 mM aspirin preincubation) with 1.9 μM NO ± 40 μM arachidonate (AA) for 5 minutes, 37°C (n = 3, mean ± SEM; *P < .05, Student unpaired t test).ASAindicates aspirin. (B) Representative trace showing aggregation of washed platelets (control or aspirin treated) at 200 × 109/L in 500 μL Tyrode buffer containing 1 mM CaCl2 at 37°C following addition of 0.4 U/mL thrombin, with and without 1.9 μM NO (added 10 seconds after thrombin). (C) Cumulative data showing the effect of aspirin treatment on the sensitivity of platelets to NO during thrombin-stimulated aggregation (mean ± SEM, n = 3; *P < .05, Student unpaired t test).
Aspirin supplementation inhibits NO consumption by platelets from NM volunteers. Baseline NO consumption following a 14-day NSAID-free washout period of washed platelets was determined as described in “Patients, materials, and methods,” then subjects were randomly assigned to receive either aspirin or placebo as described in “Patients, materials, and methods,” before repeat determinations of platelet NO consumption. (A) Representative trace showing NO consumption by platelets from one donor (i) at baseline, (ii) following aspirin supplementation, (iii) after placebo, with 50 μM arachidonate added at arrow. (B) Cumulative data showing the effect of aspirin supplementation on platelet NO consumption rates (mean ± SEM, n = 5 separate donors; *P < .01, Student unpaired t test). (C) Onset and recovery of inhibition of NO consumption by aspirin. Following a baseline determination of arachidonate-stimulated platelet NO consumption, subjects were administered 75 mg aspirin/day for 3 days. NO consumption was measured daily during and for 3 days after aspirin (mean ± SEM, n = 3 separate donors).
Aspirin supplementation inhibits NO consumption by platelets from NM volunteers. Baseline NO consumption following a 14-day NSAID-free washout period of washed platelets was determined as described in “Patients, materials, and methods,” then subjects were randomly assigned to receive either aspirin or placebo as described in “Patients, materials, and methods,” before repeat determinations of platelet NO consumption. (A) Representative trace showing NO consumption by platelets from one donor (i) at baseline, (ii) following aspirin supplementation, (iii) after placebo, with 50 μM arachidonate added at arrow. (B) Cumulative data showing the effect of aspirin supplementation on platelet NO consumption rates (mean ± SEM, n = 5 separate donors; *P < .01, Student unpaired t test). (C) Onset and recovery of inhibition of NO consumption by aspirin. Following a baseline determination of arachidonate-stimulated platelet NO consumption, subjects were administered 75 mg aspirin/day for 3 days. NO consumption was measured daily during and for 3 days after aspirin (mean ± SEM, n = 3 separate donors).
In vivo aspirin supplementation only partially inhibits platelet NO consumption by washed platelets from HF subjects
Resting washed platelets from a group of HF subjects (n = 6, mean total cholesterol, 7.01 mM) did not consume NO (Figure 5A-B). On addition of 50 μM arachidonate, platelets consumed NO at rates of 2.1 ± 0.69 nmol/minute per 0.2 × 109 platelets. Rates were unchanged following placebo; however, aspirin supplementation (75 mg/day, 14 days) reduced NO consumption by only 39% (2.04 ± 0.37 versus 1.28 ± 0.5 nmol/minute per 0.2 × 109 platelets for placebo or aspirin, respectively), and this was not statistically significant. This indicates that in vivo aspirin only partially inhibits platelet NO consumption in HF subjects (Figure 5A-B). Indeed, in 3 of the 6 subjects, rates of NO consumption following aspirin treatment were either unchanged or slightly increased over baseline (not shown). NO consumption was then determined in a group of NF subjects (n = 4, mean total cholesterol, 5.3 mM), as controls. Resting platelets consumed NO at rates that were unchanged by placebo, but significantly inhibited (76%) by aspirin supplementation (1.47 ± 0.25 versus 0.3 ± 0.18 nmol/minute per 0.2 × 109 platelets for placebo or aspirin, respectively) (Figure 5C-D). Finally, rates of NO consumption at baseline for HF subjects were almost double that for NFs or NMs (1.23 ± 0.2 versus 2.13 ± 0.54 nmol/minute per 0.2 × 109 platelets for NF or HF subjects, respectively). These data indicate that aspirin is far less effective at blocking platelet NO consumption in hypercholesterolemic patients than in normocholesterolemic control subjects.
In vitro inhibition of platelet NO consumption in hypercholesterolemic subjects
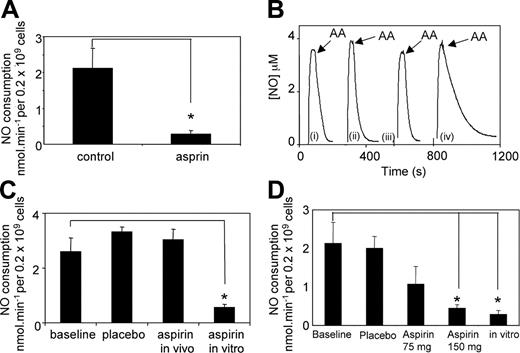
Following our observation of less inhibition of NO consumption by in vivo aspirin supplementation in HF volunteers, in vitro inhibition of platelet NO consumption by aspirin was examined in 4 of the original subjects, following a 14-day NSAID-free period. The remaining 2 had commenced lipid-lowering therapy by this time and were therefore excluded. The data show that aspirin incubation in vitro inhibits NO consumption by platelets from the HF group by 87% (Figure 6A; baseline, 2.13 ± 0.54 nmol/minute per 0.2 × 109 platelets; aspirin, 0.28 ± 0.1 nmol/minute per 0.2 × 109 platelets). This is similar to the extent of in vitro inhibition of NO consumption by platelets from NM subjects (Figure 1) and shows that the ability of aspirin to inhibit NO consumption by HF platelets in vitro is retained. To further illustrate the difference between the ability of low-dose in vivo and in vitro aspirin to block NO consumption in this group, data from one subject showed that in vivo supplementation had been totally without effect on platelet NO consumption (baseline, 2.6 ± 0.49 nmol/minute per 0.2 × 109 platelets; placebo, 3.3 ± 0.17 nmol/minute per 0.2 × 109 platelets; in vivo aspirin, 3.04 ± 0.07 nmol/minute per 0.2 × 109 platelets); however, in vitro aspirin inhibited platelet NO consumption from this volunteer by 80% (in vitro aspirin, 0.55 ± 0.12 nmol/minute per 0.2 × 109 platelets) (Figure 5B-C). These data indicate that the aspirin-insensitive NO consumption in these HF subjects is still primarily COX-dependent and not caused by an additional NO consumption pathway present in hypercholesterolemia. To examine whether a higher oral dose of aspirin could facilitate inhibition of NO consumption in this group, patients were recalled a final time and administered 150 mg aspirin/day for 14 days. Following this treatment, arachidonate-stimulated NO consumption was significantly inhibited (80%) as compared with baseline (2.1 ± 0.69 nmol/minute per 0.2 × 109 platelets versus 0.45 ± 0.09 nmol/minute per 0.2 × 109 platelets for baseline or aspirin-treated, respectively), and approached the extent of inhibition found in vitro for this group (87%) (Figure 6D). These data indicate that a higher dose of aspirin may be more effective at improving NO bioactivity in hypercholesterolemic women.
Aspirin supplementation is less effective at inhibiting NO consumption in platelets from HF than from NF volunteers. Baseline NO consumption following a 14-day NSAID-free washout period of washed platelets was determined as described in “Patients, materials, and methods,” then subjects were randomly assigned to receive either aspirin or placebo in a crossover manner as described in “Patients, materials, and methods,” before repeat determinations of platelet NO consumption. (A-B) HF donors, (C-D) NF donors. (A) Representative trace showing NO consumption by platelets from one HF donor (i) at baseline, (ii) following aspirin supplementation, and (iii) after placebo, with 50 μM arachidonate added at arrow. (B) Cumulative data showing the effect of aspirin supplementation on HF platelet NO consumption rates (mean ± SEM, n = 6 separate donors; NS, not significant, Student unpaired t test). (C) Representative trace showing NO consumption by platelets from one NF donor (i) at baseline, (ii) following aspirin supplementation, and (iii) after placebo, with 50 μM arachidonate added at arrow. (D) Cumulative data showing the effect of aspirin supplementation on NF platelet NO consumption rates (mean ± SEM, n = 4 separate donors; *P < .02, Student unpaired t test).
Aspirin supplementation is less effective at inhibiting NO consumption in platelets from HF than from NF volunteers. Baseline NO consumption following a 14-day NSAID-free washout period of washed platelets was determined as described in “Patients, materials, and methods,” then subjects were randomly assigned to receive either aspirin or placebo in a crossover manner as described in “Patients, materials, and methods,” before repeat determinations of platelet NO consumption. (A-B) HF donors, (C-D) NF donors. (A) Representative trace showing NO consumption by platelets from one HF donor (i) at baseline, (ii) following aspirin supplementation, and (iii) after placebo, with 50 μM arachidonate added at arrow. (B) Cumulative data showing the effect of aspirin supplementation on HF platelet NO consumption rates (mean ± SEM, n = 6 separate donors; NS, not significant, Student unpaired t test). (C) Representative trace showing NO consumption by platelets from one NF donor (i) at baseline, (ii) following aspirin supplementation, and (iii) after placebo, with 50 μM arachidonate added at arrow. (D) Cumulative data showing the effect of aspirin supplementation on NF platelet NO consumption rates (mean ± SEM, n = 4 separate donors; *P < .02, Student unpaired t test).
In vitro inhibition of NO consumption by aspirin and in vivo inhibition following 150 mg oral aspirin/day in platelets from HF subjects. Platelets from HF subjects were examined for inhibition by aspirin in vitro (1 mM aspirin, 30 minutes, room temperature). NO consumption was determined as described in “Patients, materials, and methods.” (A) Cumulative data showing rates of NO consumption following in vitro aspirin (n = 4, mean ± SEM; *P < .005, Student unpaired t test). (B) Representative traces from one HF subject showing NO consumption by platelets (i) at baseline, (ii) following aspirin supplementation, (iii) after placebo, and (iv) after in vitro aspirin incubation, with 50 μM arachidonate added at arrow. (C) Cumulative data from the HF subject in B, showing the effect of aspirin in vivo or in vitro on platelet NO consumption rates (mean ± SEM, n = 3 separate determinations; *P < .002, Student unpaired t test). (D) Cumulative data showing the effect of supplementation with 150 mg aspirin/day on HF platelet NO consumption rates, compared with baseline, placebo, low-dose aspirin, and in vitro aspirin (mean ± SEM, n = 4 separate donors; *P < .05, Student unpaired t test). Note: baseline, placebo, 75 mg/day aspirin and in vitro rates are provided for comparison with 150 mg/day dose and are already shown in Figures 5B and 6A.
In vitro inhibition of NO consumption by aspirin and in vivo inhibition following 150 mg oral aspirin/day in platelets from HF subjects. Platelets from HF subjects were examined for inhibition by aspirin in vitro (1 mM aspirin, 30 minutes, room temperature). NO consumption was determined as described in “Patients, materials, and methods.” (A) Cumulative data showing rates of NO consumption following in vitro aspirin (n = 4, mean ± SEM; *P < .005, Student unpaired t test). (B) Representative traces from one HF subject showing NO consumption by platelets (i) at baseline, (ii) following aspirin supplementation, (iii) after placebo, and (iv) after in vitro aspirin incubation, with 50 μM arachidonate added at arrow. (C) Cumulative data from the HF subject in B, showing the effect of aspirin in vivo or in vitro on platelet NO consumption rates (mean ± SEM, n = 3 separate determinations; *P < .002, Student unpaired t test). (D) Cumulative data showing the effect of supplementation with 150 mg aspirin/day on HF platelet NO consumption rates, compared with baseline, placebo, low-dose aspirin, and in vitro aspirin (mean ± SEM, n = 4 separate donors; *P < .05, Student unpaired t test). Note: baseline, placebo, 75 mg/day aspirin and in vitro rates are provided for comparison with 150 mg/day dose and are already shown in Figures 5B and 6A.
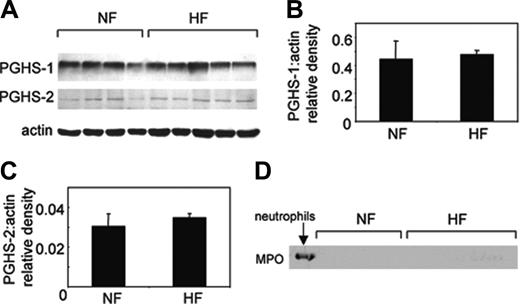
Expression of COX isoforms and MPO in platelets from NF or HF volunteers
To examine whether the elevated NO consumption rates could be related to expression of COX isoforms in hypercholesterolemics, COX-1 and -2 expressions were compared in HF or NF platelets. As expected, both groups expressed COX-1, and following actin-normalization expression levels were found to be similar (Figure 7A-B). Analysis for COX-2 revealed some weak staining for this isoform that was similar in both HF and NF groups (Figure 7A,C). Expression of COX-2 in platelets is controversial and could indicate leukocyte contamination or crossreactivity of the anti–COX-2 antibody with COX-1.18-20 To exclude leukocyte contamination, Western blots were examined for expression of the leukocyte protein myeloperoxidase. As shown, we did not detect MPO in our patient samples and additionally did not find leukocytes in our platelets when viewed by hemocytometer following isolation (Figure 7D; data not shown).
Expression of COX-1, -2, and MPO in HF and NF platelets. (A) Protein from washed platelets (3 μg protein) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described in “Patients, materials, and methods.” Membranes were probed using anti–COX-1, anti–COX-2, or antiactin, and immunoreactivity was revealed using anti–IgG-HRP secondary conjugates and ECL. Arrow shows bands corresponding to HF subject studied in Figure 5. (B) Relative density of COX-1 and actin in HF and NF samples shows no difference in COX-1 expression between subject groups. (C) Relative density of COX-2 and actin in HF and NF samples shows no difference in COX-2 expression between subject groups. Mean ± SEM, n = 4–5 separate patients. (D) Protein from washed platelets (3 μg protein) or human neutrophils (1.5 μg) was separated by SDS-PAGE as described in “Patients, materials, and methods” and probed using anti–human MPO and revealed using anti–IgG-HRP secondary antibody and ECL.
Expression of COX-1, -2, and MPO in HF and NF platelets. (A) Protein from washed platelets (3 μg protein) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described in “Patients, materials, and methods.” Membranes were probed using anti–COX-1, anti–COX-2, or antiactin, and immunoreactivity was revealed using anti–IgG-HRP secondary conjugates and ECL. Arrow shows bands corresponding to HF subject studied in Figure 5. (B) Relative density of COX-1 and actin in HF and NF samples shows no difference in COX-1 expression between subject groups. (C) Relative density of COX-2 and actin in HF and NF samples shows no difference in COX-2 expression between subject groups. Mean ± SEM, n = 4–5 separate patients. (D) Protein from washed platelets (3 μg protein) or human neutrophils (1.5 μg) was separated by SDS-PAGE as described in “Patients, materials, and methods” and probed using anti–human MPO and revealed using anti–IgG-HRP secondary antibody and ECL.
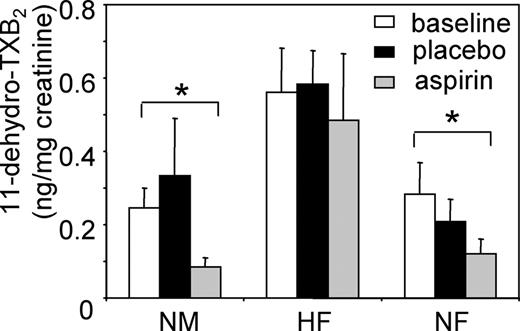
Urinary thromboxane excretion after aspirin supplementation in the 3 groups
To examine the effect of in vivo aspirin on COX-1 activity in the study groups, urinary levels of 11-dehydro-TXB2 were determined by GC-MS. In NM or NF subjects, placebo had no effect while aspirin supplementation reduced 11-dehydro-TXB2 levels by 66% or 57%, respectively, compared with baseline, whereas placebo had no effect (Figure 8). In contrast, 11-dehydro-TXB2 excretion was approximately doubled in the HF group and was not reduced by aspirin, compared with baseline. These data indicate that aspirin supplementation suppresses thromboxane generation in HM or HF subjects but not in this group of hypercholesterolemic postmenopausal women.
Discussion
In vitro, activated platelets consume NO via COX-1 peroxidase turnover, with 2 molecules of NO consumed per catalytic cycle.6 Platelet NO consumption could regulate both platelet and vascular wall responses to endothelial NO. However, we suggest that regulation of platelet NO bioactivity in vivo may be a less likely consequence, because NO consumption occurs after agonist stimulation. In contrast, platelets are stimulated to aggregate and secrete at sites of vascular injury, a setting in which vasodilation would not be beneficial. Therefore, a pathophysiologic role for platelet NO consumption in promoting hemostasis at sites of platelet plug formation is proposed.
Urinary 11-dehydro-TXB2excretion is not inhibited by aspirin in HF subjects. At baseline, and following aspirin or placebo supplementation, urine samples were obtained and analyzed for 11-dehydro-TXB2 using GC-MS in NM (n = 5), HF (n = 6), and NF (n = 4) samples (mean ± SEM; *P < .03, Student unpaired t test).
Urinary 11-dehydro-TXB2excretion is not inhibited by aspirin in HF subjects. At baseline, and following aspirin or placebo supplementation, urine samples were obtained and analyzed for 11-dehydro-TXB2 using GC-MS in NM (n = 5), HF (n = 6), and NF (n = 4) samples (mean ± SEM; *P < .03, Student unpaired t test).
In vivo, antiplatelet GPIIbIIIa antagonists or intravenous or oral low-dose aspirin improve endothelial dysfunction in patients with cardiovascular disease.9-12 Therefore, it is likely that the behavior of circulating platelets will be a critical determinant of the level of bioactive NO in cardiovascular disease. Our current study extended to inhibition of COX-1 in vivo and asked whether supplementation with aspirin, the universally prescribed inhibitor of this pathway, could alter platelet NO consumption. Herein, in vivo aspirin administration inhibited platelet NO consumption ex vivo in NM subjects by 95% (Figure 4A-B). Inhibition showed a gradual onset over 3 days and recovered during the 3 days following aspirin withdrawal (Figure 4C). This pattern is consistent with inhibition of COX-1 and its recovery in vivo as previously shown by measurement of urinary thromboxane metabolite excretion.17
We note that there are technical issues that preclude the use of thrombin in studies in which NO consumption rates are to be determined. In particular, NO suppresses thrombin-stimulated aggregation and arachidonate release,14-16 so thrombin is added before NO to ensure platelet activation occurs. This does not allow accurate determination of NO consumption, because a linear rate of NO decay is not detected by the electrode6 (Figure 4B). For this reason, we used arachidonate for quantitative assays because activation of COX-1 is achievable following addition of NO and its peak detection by the electrode (Figure 1Aii). Inhibition of thrombin-stimulated NO consumption by NO does not preclude its occurrence in vivo. Indeed, patients with vascular disease consistently show elevated thromboxane in vivo in tandem with impaired NO bioactivity that can be improved by aspirin, indicating that this phenomenon does occur in the vasculature.9-12,21
Resting washed platelets did not consume NO in vitro; however, circulating platelets are not resting in vivo. Detection of urinary thromboxane metabolites and their elevated levels in patients with vascular disease indicates platelet COX-1 turnover.21 Also, aspirin (including low-dose oral) improves NO bioactivity in patients with hypertension and vascular disease.9-12 Activation of platelets in vivo may result from laminar flow, plasma components, or other conditions that washed platelets are not exposed to following isolation. In this context, changes in cGMP mediated by aspirin/NO interactions may influence platelet activation state in vivo and could play an important role in preventing thrombosis. In vitro aspirin (1 mM, 30 minutes) inhibited arachidonate-stimulated platelet NO consumption by 91%, although lower doses down to 0.1 mM and shorter times (5 minutes) were also effective (Figures 1, 2A-B). Also, aspirin pretreatment partially restored NO-dependent cGMP synthesis that was inhibited by COX-1 turnover (Figure 3A). It is not clear why cGMP synthesis was only partially restored, because NO consumption was fully blocked. One potential explanation is activation of phosphodiesterases (PDEs) by NO, and, indeed, NO/cGMP is known to activate PDE5, the main enzyme responsible for cGMP hydrolysis in intact cells.22 Although we included IBMX (inhibitor of PDEs) in our experiments, we cannot exclude the possibility that incomplete inhibition of this pathway could have occurred.
Previously, we showed that inhibition of platelet aggregation by NO can be prevented by activation of COX-1. Specifically, aggregation of platelets triggered via the thromboxane receptor (TP) using U46619 is more sensitive to the inhibitory effects of NO than aggregation triggered by arachidonate. For both agonists, aggregation results from TP activation; however, U46619 is not a substrate for COX-1 and does not stimulate NO consumption.6 Although exogenous arachidonate may not be a physiologic substrate for platelet aggregation, thrombin also stimulated NO consumption and suppressed NO stimulation of cGMP generation in platelets via COX-1 turnover.6 Furthermore, herein, the ability of NO to block aggregation of platelets in response to high-dose thrombin was significantly greater in aspirinized than in control platelets (Figure 3B-C). Taken together, these data indicate that COX-1–dependent NO consumption provides a mechanism by which platelets could regulate NO bioactivity in vivo and by which endothelial dysfunction could be improved by aspirin supplementation.
In a small substudy, rates of NO consumption by platelets isolated from HF volunteers were almost twice those of healthy subjects, indicating that the ability of platelets to remove NO in hypercholesterolemia may be increased (Figure 5). Elevated NO consumption in HF subjects could result from elevated expression and/or activity of COX isoforms in platelets. Indeed, patients with hypercholesterolemia have elevated urinary thromboxane metabolites, as shown herein and by others21 (Figure 8). However, platelet levels of both COX isoform proteins were unaltered in these subjects (Figure 7), suggesting that COX-1 turnover is elevated in this group, rather than its expression. Detection of COX-2 in our samples was perhaps unexpected, because its expression in platelets is controversial; however, others have found this isoform in less than 10% of circulating platelets from healthy subjects.18-20
NO consumption by platelets from HF subjects was inhibited by in vivo aspirin only 39%, versus 76% for matched controls (Figure 5). In contrast, HF platelet NO consumption was inhibited 87% by aspirin in vitro (Figure 6A-C). This suggests a failure of low-dose aspirin to inhibit COX-1 in vivo in this group and is supported by findings that elevated urinary 11-dehydro TXB2 was also not suppressed at this dose (Figure 8). However, on administration of a 2-fold higher dose of aspirin for 14 days, NO consumption was more effectively blocked and approached in vitro levels of inhibition (Figure 6D). This indicates that COX-1 is still sensitive to aspirin in these patients, but that its bioavailability may be decreased.
Aspirin resistance has been suggested as a new independent predictor of vascular effects, although its exact definition is not standardized.23-31 It may transiently increase following vascular surgery, and estimations of its prevalence vary from 75% to 5.5%; however, studies vary widely in their platelet functional assays and agonist concentrations. Pharmacologically, aspirin resistance is defined as persistent production of thromboxane despite therapy.23-25 Although our sample size is small, it suggests a higher prevalence of aspirin resistance in hypercholesterolemia. Several mechanisms of aspirin resistance have been proposed, including (1) aspirin-insensitive COX-1, for example, with a mutation at or near serine 529; (2) thromboxane generation mediated by platelet or vascular COX-2; and (3) COX-1 expression in nucleated vascular cells. Our data showing that platelets resistant to low-dose aspirin in vivo are fully sensitive in vitro rule out a COX-1 mutation, at least in this small group of subjects (Figure 6). It is also unlikely that platelet COX-2 contributes to inability of aspirin to block NO consumption in our study, because (1) HF and NF subjects showedidentical levels of platelet COX-2 protein herein and (2) selective COX-2 inhibitors do not decrease systemic biosynthesis of thromboxane in vivo32 (Figure 7). Our data showing that higher doses of aspirin are required to inhibit NO consumption suggest altered in vivo pharmacokinetics of the drug, for example, increased binding to plasma proteins, or rate of hydrolysis in the gut content, gut wall, and liver. This could be investigated by determining bioavailability of systemic aspirin as has been previously done in healthy volunteers.33 It should be noted that our sample size was small, because of difficulties in recruiting newly diagnosed hypercholesterolemic subjects who had not been treated with medication for vascular disease. Notwithstanding this issue, significant effects of aspirin on NO consumption were observed in NM and NF groups. Additionally, several of our findings were consistent with previous observation of others: (1) we observed a doubling of thromboxane metabolites in hypercholesterolemics versus controls, which was significant; and (2) aspirin suppressed thromboxane excretion in healthy men to a degree that was identical to previously reported studies.21
The study focused on women because it was considerably more difficult to recruit newly diagnosed male patients who had never been prescribed aspirin or lipid-lowering therapies; however, there are reasons to suspect that our findings are more applicable to hypercholesterolemic women. For example, it has been previously noted that aspirin nonresponders tend to be women and/or older, and the Women's Health Study recently reported that aspirin is not cardioprotective against myocardial infarction in women, unlike men, but it does protect against ischemic stroke.31,34
In summary, this study shows that aspirin blocks platelet NO consumption in vitro and enhances (1) the inhibitory effects of NO on aggregation and (2) cGMP generation. Also in vivo aspirin effectively inhibits NO consumption by platelets from healthy male volunteers. In contrast, NO consumption by platelets from a small group of HF subjects was higher and less sensitive to aspirin. The findings suggest a role for platelet COX-1 in modulating NO bioactivity in vivo. However, the beneficial effects of antiplatelet therapy on NO bioactivity may be compromised in patients with hypercholesterolemia.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-02-0664.
Supported by the British Heart Foundation (M.J.C., P.C.W., V.B.O'D., M.J.L.), Wellcome Trust (V.B.O'D.), and the National Institutes of Health (grants GM15431, CA77839, DK48831) (J.D.M.). P.C.W. was a British Heart Foundation Junior Fellow;
M.J.C. was a British Heart Foundation Intermediate Fellow.
P.C.W., B.C., S.S., J.D.M., M.J.C., and V.B.O'D. performed the research; J.R.C. contributed to patient recruitment; M.J.L. and V.B.O'D. designed the research; and V.B.O'D. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal