Abstract
Iron regulatory protein 2 (IRP2)-deficient mice have been reported to suffer from late-onset neurodegeneration by an unknown mechanism. We report that young adult Irp2-/- mice display signs of iron mismanagement within the central iron recycling pathway in the mammalian body, the liver-bone marrow-spleen axis, with altered body iron distribution and compromised hematopoiesis. In comparison with wild-type littermates, Irp2-/- mice are mildly microcytic with reduced serum hemoglobin levels and hematocrit. Serum iron and transferrin saturation are unchanged, and hence microcytosis is not due to an overt decrease in systemic iron availability. The liver and duodenum are iron loaded, while the spleen is iron deficient, associated with a reduced expression of the iron exporter ferroportin. A reduction in transferrin receptor 1 (TfR1) mRNA levels in the bone marrow of Irp2-/- mice can plausibly explain the microcytosis by an intrinsic defect in erythropoiesis due to a failure to adequately protect TfR1 mRNA against degradation. This study links a classic regulator of cellular iron metabolism to systemic iron homeostasis and erythropoietic TfR1 expression. Furthermore, this work uncovers aspects of mammalian iron metabolism that can or cannot be compensated for by the expression of IRP1. (Blood. 2005;106: 2580-2589)
Introduction
As both lack and excess of iron are pathologic, vertebrates control iron balance at the cellular systemic levels by coordinating iron uptake, storage, export, and distribution (for a recent review, see Hentze et al1 ). For systemic iron uptake, dietary iron is transported from the intestinal lumen into the cytoplasm of duodenal enterocytes via divalent metal transporter 1 (DMT1)/solute carrier family 11 member 2 (Slc11A2).2 The ubiquitously expressed transferrin (Tf) receptor 1 (TfR1) is thought to supply body cells with iron by internalization of serum Tf. Some specialized macrophages acquire iron indirectly by breaking down heme following phagocytosis of senescent erythrocytes. Iron that enters cells and is not used can be sequestered within heteropolymers of ferritin H- and L-chain. The mechanisms by which cells export iron are less well understood. Ferroportin/solute carrier family 40 member 1 (Slc40A1), located at the basolateral membrane of duodenal enterocytes, exports iron into the bloodstream and loads it onto plasma apo-Tf,3 probably in conjunction with the feroxidase hephaestin. Ferroportin is also expressed by, for example, macrophages and hepatocytes3-5 where it acts in concert with ceruloplasmin.3,6
Systemic iron homeostasis requires communication between cells that need iron (mainly erythroid precursors) and cells that acquire (duodenal enterocytes), store (hepatocytes and tissue macrophages), or recycle (tissue macrophages) iron. As no pathway for regulated iron excretion is known, control of intestinal iron absorption is critical for maintenance of adequate body iron levels. Duodenal iron absorption responds to changes in dietary iron intake, the status of the iron stores, and erythropoietic activity.7 Hepcidin (Hamp), a soluble β-defensin-like polypeptide excreted mostly by the liver decreases duodenal iron absorption and iron release from macrophages by inhibition of ferroportin.8 Hamp mRNA levels decrease in response to iron deficiency and anemia, while dietary iron overload stimulates Hamp expression.9
Cellular iron metabolism is coordinately controlled by iron regulatory protein 1 (IRP1) and IRP2 that bind to iron-responsive elements (IREs), cis-regulatory RNA motifs present in untranslated regions (UTRs) of mRNAs encoding proteins of iron uptake (TfR1, DMT1), storage (ferritin H- and L-chain), export (ferroportin), or utilization (mitochondrial aconitase, 5-aminolevulinate synthase). Independently, both IRPs inhibit translation when bound to IREs present in the 5′ UTR (eg, ferritin, 5-aminolevulinate synthase, mitochondrial aconitase, ferroportin mRNAs), whereas their association with the IREs present in the 3′ UTR of the TfR1 mRNA prevents its degradation.1 The IRE-binding activity from both IRPs is high in iron-deficient cells and low under conditions of iron load. Failure to coordinate the expression of IRE-containing genes is associated with pathologic conditions, as illustrated by the autosomal dominant hyperferritinemia-cataract syndrome observed in patients carrying mutations in the ferritin L IRE,10,11 by the autosomal dominant iron overload in patients with a mutation in the ferritin H IRE,12 or by a progressive neurodegenerative disorder observed in aged mice lacking IRP2.13
The role of the IRP/IRE regulatory network in cellular homeostasis has been extensively investigated in cell culture.1,14-16 The respective roles of IRP1 and IRP2 in mammalian physiology are only beginning to be investigated.13,17-19 To address the role of the IRP/IRE regulatory network in systemic iron metabolism, we developed mouse lines with targeted disruptions of the Irp1 or Irp2 loci.18 A detailed analysis of the phenotype of mice lacking IRP2 uncovers a novel role of IRP2 in systemic iron metabolism. We address the mechanisms underlying the misregulation of systemic iron homeostasis in Irp2-/- mice.
Materials and methods
Mice
The generation of the IRP2-deficient mice has been described.18 A βGeo gene-trap construct was inserted into the second intron of the Irp2 locus to interrupt the open reading frame near the amino-terminus, thereby creating a functional null allele. The selection cassette was coinserted with LoxP sites flanking exon 3 for excision by the Cre recombinase. Heterozygotes on a mixed Sv129/Ola/C57BL6/J genetic background were intercrossed to obtain +/+, +/-, and -/- littermates. Animals were kept under a constant light/dark cycle. The iron content of the standard diet was 200 mg/kg. Mice were made iron deficient by feeding a low iron diet (< 10 mg/kg) versus a control diet (C1038 and C1000, respectively; Altromin, Lage, Germany) for 25 days starting from weaning age. Ten-week-old females were injected intraperitoneally with phenylhydrazine (60 mg/kg of body weight) or NaCl 0.9% on 2 consecutive days and killed 3 days after the last injection. Heparinized blood was collected by cardiac puncture. Longitudinal sections of the proximal duodenum were collected first. Bone marrow cells were flushed out of the femur with ice-cold phosphate-buffered saline (PBS) and pelleted by centrifugation for RNA and protein extraction. Animal handling was in accordance with institutional guidelines.
Hematology and iron determination
Serum iron and unsaturated iron binding capacity (UIBC) were determined using the Total iron assay and UIBC assay reagents, respectively (Diagnostic Chemicals, Charlottetown, PE), together with the calibrators and standards recommended by the manufacturer. Blood profiles and hemoglobin content were determined by Laboklin (Bad-Kissingen, Germany).
Determination of duodenal iron transport
Duodenal iron transfer was determined as described previously.22 Twelve-week-old mice were fasted overnight. A tied-off duodenal segment (2-3 cm) was flushed with saline (37°C), filled in situ with 50 to 100 μL physiologic medium (125 mM NaCl, 3.5 mM KCl, 10 mM d-glucose, 16 mM Na-HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4]), containing 100 μM 59Fe3+-nitrilotriacetate 1:2 (NEZ37; NEN, Dreieich, Germany). The ligated duodenal segment was removed after 15 minutes, ligating the mesenteric blood supply to avoid blood losses. The radioactivity associated with the carcass was measured in a whole-body counter (ARMAC 446; Packard, Palo Alto, CA).22
RNA analyses
Total RNA was extracted using the Trizol reagent (Invitrogen, Karlsruhe, Germany) and a polytron homogenizer (Kinematika, Lucerne, Switzerland). Northern blotting was done following standard procedures, using a β-actin probe to assess equal loading. Signals were quantified using a fluorimager (Fujifilm FLA-2000; Amersham Biosciences, Freiburg, Germany). Microarray experiments were carried out as described previously using the Mouse Version 3.023,24 of the “IronChip.”
Templates for RNase protection assays were generated by polymerase chain reaction (PCR) from murine cDNAs using the primers 5′-GATGAATGACTTCCTGAATGTC-3′ (sense) and 5′-TAGTCATCTGGACACCACTG-3′ (reverse) (DMT1 3′ variants); 5′-AACACTGTTGTCAGAGAAGTTG-3′ (sense) and 5′-ATTCACAGAATAACTTAGTTCTTC-3′ (reverse) (Tfr1); and 5′-AGATCTGTGAAGAATAGAGAGCCTAG-3′ (sense) and 5′-GCTGCAGGGGTGTAGAGAGGTC-3′ (reverse) (Hepcidin 1 and 2). The PCR products were cloned into the pCRII Topo vector (Invitrogen). The templates were linearized with BamHI (DMT1), XhoI (TfRI), or BglII (hepcidin) and antisense probes were generated by in vitro transcription using the T7 (DMT1, hepcidin) or the SP6 (TfRI) RNA polymerases (Stratagene, Amsterdam, the Netherlands). Total RNA (5 μg) was used for RNase protection assay using the RPA-III kit (Ambion, Huntingdon, Cambridgeshire, United Kingdom). RNA samples were cohybridized with a β-actin probe from the kit. Protected products were resolved on denaturing acrylamide gels and subjected to autoradiography using a fluorimager (Fujifilm FLA-2000; Amersham Biosciences).
Protein analyses
Protein extracts were prepared and subjected to Western blot analysis as described previously,18 except that for the detection of ferroportin the samples were not heated prior to loading. Ferritin H- and L-subunits were detected using the goat polyclonal antibodies sc-14416 and sc-14420, respectively (Santa Cruz Biotechnology, Heidelberg, Germany). β-Actin (clone AC-15) and TfR1 were detected using mouse monoclonal antibodies from Sigma and Zymed (Berlin, Germany), respectively. Ferroportin and IRP2 were detected using immunopurified rabbit polyclonal antibodies raised against a peptide corresponding to the C-terminus of ferroportin (GPDEKEVTDENQPNTS) or against a tandem of the 73 amino acid (a.a.) domain of IRP225 fused to glutathione-S-transferase (GST), respectively. The specificity of the ferroportin antibody was established using protein extracts from Hela cells transfected with a mouse ferroportin cDNA as described previously3 (not shown).
Tissues were washed in PBS and incubated in fresh 4% paraformaldehyde solution overnight at 4°C. After extensive washes in PBS, specimens were infused with a PBS/15% sucrose followed by PBS/30% sucrose. Finally, tissues were washed in PBS and embedded in tissue-Teck optimal cutting temperature (OCT) compound (Pelco, Redding, CA). Tissue sections (10 μm) were prepared on a cryostat (model CM3050; Leica, Wetzlar, Germany) and attached to SuperFrostPlus glass slides (Menzel-Glaser, Braunschweig, Germany). Immunostaining of ferritin H- and L-chain was performed using the goat polyclonal antibodies sc-14416 and sc-14420 (Santa Cruz Biotechnology) and a Vectastain kit (Vector Laboratories, Burlingame, CA). Images were acquired using Fire Cam 1.2.0 software (Leica) and a DC500 digital camera (Leica) connected to an Axiophot microscope equipped with a 20 ×/0.50 objective lens (Zeiss). Final pictures were prepared with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). For immunofluorescent staining, tissue slices were treated with trypsin and incubated in PBS/0.1% triton X100 for 20 minutes, washed in PBS, and placed in blocking solution (PBS, bovine serum albumin [BSA] 0.2%, normal goat serum 1/500; Santa Cruz Biotechnology). Samples were incubated with the antiferroportin antibody described in the previous paragraph and a rat monoclonal anti-F4/80 antibody (Serotec, Düsseldorf, Germany) to detect splenic macrophages. Immune complexes were detected with goat antirabbit (ferroportin) or antirat (F4/80) antibodies conjugated to Alexa 488 or Alexa 594 (Molecular Probes, Invitrogen). Nuclei were stained with 4,6 diamidino-2-phenylindole (Dapi; Molecular Probes, Invitrogen) and samples were mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL). Images were acquired using Axiovision 4.3 software and a black-and-white AxioCam HRm CCD camera (Zeiss) connected to a wide-field Axiovert 200M microscope equipped with a 25 ×/0.80 oil-immersion objective lens (Zeiss). Final pictures were prepared with Adobe Photoshop 6.0 software.
Results
Abnormal systemic iron distribution and microcytosis in IRP2-deficient mice
At 8 to 10 weeks of age, IRP2-deficient mice present without gross phenotypic abnormalities. Both males and females are fertile, apparently healthy, and display a normal overall posture and activity pattern. The mean weight is 28.6 ± 3.3 g (wild type) versus 27.8 ± 4.7 g (Irp2-/-) for males and 25.1 ± 3.4 g (wild type) versus 24.3 ± 5.2 g (Irp2-/-) for females.
To evaluate the consequences of IRP2 deficiency on systemic iron metabolism, tissue iron was measured in the liver, a site of iron storage; in the duodenum, the site of iron absorption; and in the spleen, a major site of iron recycling; as well as in the brain (Table 1). Nonheme iron content is increased in the liver (by ∼ 50%) and duodenum (by ∼ 80%), in agreement with earlier observations on older animals of a distinct IRP2 knock-out (KO) mouse line.13 By contrast, the spleen of Irp2-/- mice displays a relative iron deficiency (by ∼ 40%) that was not reported before. Nonheme iron levels in the brain are unchanged.
Abnormal iron distribution in Irp2−/−versus wild-type mice
. | +/+ (n) . | −/− (n) . |
|---|---|---|
| Liver | 195 ± 9 (27) | 300 ± 15 (24)* |
| Duodenum | 571 ± 41 (17) | 1058 ± 113 (13)* |
| Spleen | 2191 ± 191 (15) | 1331 ± 122 (11)* |
| Brain | 157 ± 11 (18) | 148 ± 13 (14) |
. | +/+ (n) . | −/− (n) . |
|---|---|---|
| Liver | 195 ± 9 (27) | 300 ± 15 (24)* |
| Duodenum | 571 ± 41 (17) | 1058 ± 113 (13)* |
| Spleen | 2191 ± 191 (15) | 1331 ± 122 (11)* |
| Brain | 157 ± 11 (18) | 148 ± 13 (14) |
Tissue nonheme iron content was determined using the bathophenantroline chromogen20 in 8- to 10-week-old mice and is given in milligram per gram of dried tissue. Results are presented as means ± standard error. The sample size (n) is indicated.
P = .001, Student t test.
To assess the impact of the abnormal iron distribution on the major systemic iron utilization pathway, we determined the hematologic parameters (Table 2). While leukocyte counts are normal, the hematocrit and serum hemoglobin values are significantly reduced in spite of normal erythrocyte counts, with lower MCV values (microcytosis) in both male and female Irp2-/- mice.
Hematologic parameters of Irp2−/−versus wild-type mice
. | Males (n) . | . | Females (n) . | . | ||
|---|---|---|---|---|---|---|
. | +/+ . | −/− . | +/+ . | −/− . | ||
| RBC count, 1012/L | 8.43 ± 0.25 (11) | 7.96 ± 0.20 (7) | 7.53 ± 0.23 (14) | 7.27 ± 0.15 (18) | ||
| MCV, fL | 53.04 ± 0.57 (10) | 46.78 ± 0.75 (7)† | 51.11 ± 0.58 (12) | 47.21 ± 0.52 (17)† | ||
| Hematocrit level, L/L | 0.45 ± 0.01 (11) | 0.37 ± 0.01 (7)† | 0.39 ± 0.01 (14) | 0.34 ± 0.01 (18)* | ||
| Hemoglobin level, g/L | 138 ± 3 (11) | 115 ± 3 (7)† | 128 ± 6 (14) | 108 ± 3 (18)* | ||
| Thrombocyte count, 109/L | 613 ± 28 (8) | 761 ± 56 (6) | 555 ± 44 (9) | 611 ± 65 (9) | ||
| Reticulocyte count, 109/L | 148.0 ± 12.8 (11) | 194.2 ± 35.6 (6) | 354.4 ± 100.4 (7) | 347.4 ± 121.5 (9) | ||
| WBC count, 109/L | 6.318 ± 0.643 (11) | 6.985 ± 0.883 (7) | 5.036 ± 0.414 (14) | 5.322 ± 0.396 (18) | ||
| Neutrophil count, 109/L | 0.725 ± 0.093 (7) | 0.937 ± 0.213 (6) | 0.716 ± 0.228 (9) | 0.454 ± 0.052 (9) | ||
| Lymphocyte count, 109/L | 5.601 ± 0.834 (7) | 5.157 ± 0.682 (6) | 4.115 ± 0.444 (9) | 5.038 ± 0.461 (9) | ||
| Monocyte count, 109/L | 0.121 ± 0.024 (7) | 0.128 ± 0.044 (6) | 0.266 ± 0.188 (9) | 0.252 ± 0.195 (9) | ||
. | Males (n) . | . | Females (n) . | . | ||
|---|---|---|---|---|---|---|
. | +/+ . | −/− . | +/+ . | −/− . | ||
| RBC count, 1012/L | 8.43 ± 0.25 (11) | 7.96 ± 0.20 (7) | 7.53 ± 0.23 (14) | 7.27 ± 0.15 (18) | ||
| MCV, fL | 53.04 ± 0.57 (10) | 46.78 ± 0.75 (7)† | 51.11 ± 0.58 (12) | 47.21 ± 0.52 (17)† | ||
| Hematocrit level, L/L | 0.45 ± 0.01 (11) | 0.37 ± 0.01 (7)† | 0.39 ± 0.01 (14) | 0.34 ± 0.01 (18)* | ||
| Hemoglobin level, g/L | 138 ± 3 (11) | 115 ± 3 (7)† | 128 ± 6 (14) | 108 ± 3 (18)* | ||
| Thrombocyte count, 109/L | 613 ± 28 (8) | 761 ± 56 (6) | 555 ± 44 (9) | 611 ± 65 (9) | ||
| Reticulocyte count, 109/L | 148.0 ± 12.8 (11) | 194.2 ± 35.6 (6) | 354.4 ± 100.4 (7) | 347.4 ± 121.5 (9) | ||
| WBC count, 109/L | 6.318 ± 0.643 (11) | 6.985 ± 0.883 (7) | 5.036 ± 0.414 (14) | 5.322 ± 0.396 (18) | ||
| Neutrophil count, 109/L | 0.725 ± 0.093 (7) | 0.937 ± 0.213 (6) | 0.716 ± 0.228 (9) | 0.454 ± 0.052 (9) | ||
| Lymphocyte count, 109/L | 5.601 ± 0.834 (7) | 5.157 ± 0.682 (6) | 4.115 ± 0.444 (9) | 5.038 ± 0.461 (9) | ||
| Monocyte count, 109/L | 0.121 ± 0.024 (7) | 0.128 ± 0.044 (6) | 0.266 ± 0.188 (9) | 0.252 ± 0.195 (9) | ||
Results were obtained from 8- to 10-week-old mice and are given ± standard errors. The sample size (n) is indicated. RBC indicates red blood cell; MCV, mean corpuscular volume; and WBC, white blood cell.
P = .01, Student t test.
P = .001, Student t test.
Considering the accumulation of iron in the duodenum and the liver, Irp2-/- mice could be microcytic because of decreased systemic iron availability. However, serum iron levels, the total iron binding capacity, and the serum transferrin saturation do not significantly differ between Irp2-/- mice and their wild-type littermates (Table 3). Thus, IRP2-deficient mice are mildly microcytic and display signs of iron mismanagement with abnormal body iron distribution. The normal serum iron and transferrin saturation values suggest that the microcytosis is not due to systemic iron deficiency.
Serum iron parameters in Irp2−/−versus wild-type mice
. | +/+, n = 14 . | −/−, n = 15 . |
|---|---|---|
| Serum Fe, μg/dL | 212 ± 18 | 212 ± 14 |
| TIBC, μg/dL | 416 ± 20 | 412 ± 20 |
| Tf saturation, % | 50.6 ± 3.0 | 51.3 ± 1.9 |
. | +/+, n = 14 . | −/−, n = 15 . |
|---|---|---|
| Serum Fe, μg/dL | 212 ± 18 | 212 ± 14 |
| TIBC, μg/dL | 416 ± 20 | 412 ± 20 |
| Tf saturation, % | 50.6 ± 3.0 | 51.3 ± 1.9 |
Results were obtained from 8- to 10-week-old mice and are presented as means ± standard error. TIBC (total iron binding capacity) = serum Fe + UIBC (unbound iron binding capacity). Tf saturation = (serum Fe/TIBC) × 100. The sample size (n) is indicated.
Duodenal iron metabolism in Irp2-/- mice
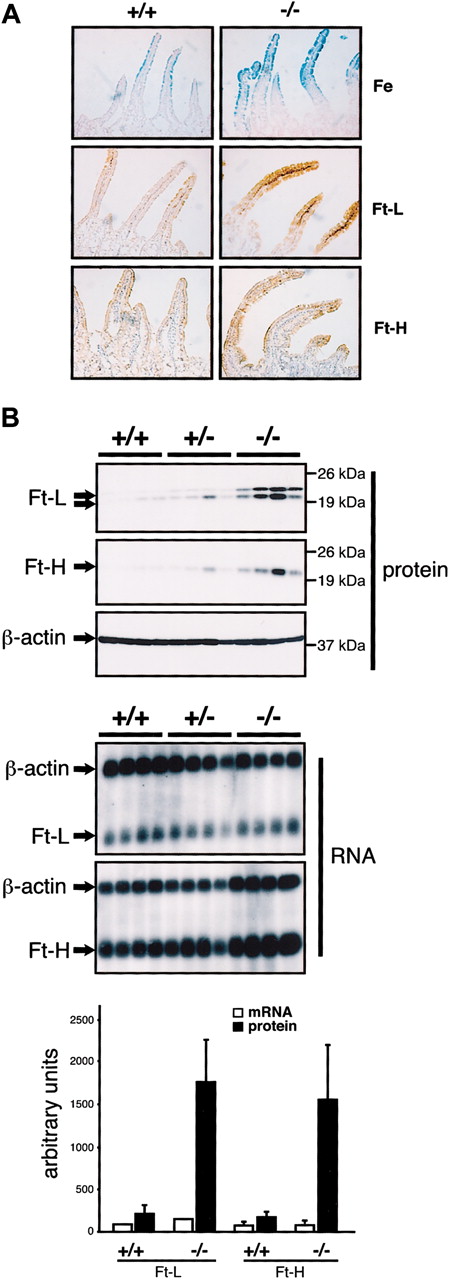
To better understand the iron accumulation in the duodenum of IRP2-deficient mice, we performed Prussian Blue staining (Figure 1A, upper panels). Confirming the increase in nonheme iron content detected with the bathophenantroline method (Table 1), iron deposits are evident in the duodenal villi but not crypts of Irp2-/- mice, suggesting that iron deposits do not result from increased uptake of plasma iron via TfR1, which is preferentially expressed in crypt cells.26 Immunostaining reveals ferritin H- and L-chain expression that mirrors the increase in iron (Figure 1A, lower panels). By Western blot analysis of protein extracts from 4 +/+,4 +/-, and 4 -/- mice (Figure 1B), ferritin H- and L-chain levels are increased 8-fold and 9-fold, respectively, in Irp2-/- mice. Some interindividual variability could be due to heterogeneity in the mixed genetic background. Ferritin H- and L-chain mRNAs are unchanged in the same animals (Northern blots in Figure 1B, lower panels), showing that accumulation of ferritin occurs posttranscriptionally.
A rise in iron and ferritin levels in Irp2-/- enterocytes could be explained by increased iron influx, decreased efflux, or retention of iron within ferritin that is translationally derepressed. Next, we examined the expression of DMT1 and ferroportin, the apical and the basolateral iron transporters, respectively. Four isoforms of DMT1 that differ in their N- and C-termini are generated by a combination of alternative splicing and the use of alternative promoters and polyadenylation sites.27 We analyzed DMT1 mRNA expression by RNase protection assay, focusing on the variants that differ by the absence (noIRE form) or the presence (IRE form) of an IRE motif in the 3′ UTR28 (Figure 2A). The increased DMT1 mRNA expression, especially of the IRE form, was ascertained in duodenal samples of iron-deficient mice (Figure 2A) as a positive control for regulation.29 Analysis of groups of 4 IRP2+/+, IRP2+/-, and Irp2-/- mice reveals no significant variation in the expression of the DMT1 mRNA isoforms. Confirming mRNA data, DMT1 protein expression was readily detected by Western blotting in iron-deficient mice, whereas basal DMT1 levels remained below the detection limit in both wild-type and Irp2-/- mice (data not shown).
In the same mice, ferroportin levels are subject to significant interindividual variability (Figure 3B), but combining the results obtained from 3 independent lots of mice shows that ferroportin expression is not significantly altered in Irp2-/- mice, either at the mRNA or the protein level. We analyzed ferroportin protein expression in total protein extracts, in which cell-surface-exposed protein and intracellular protein are not discriminated. However, internalized ferroportin is targeted for degradation,8 and should therefore contribute little to the total ferroportin signal.
Dcytb, an iron reductase thought to act in conjunction with DMT1, may act as a facilitator of iron overload in HFE KO mice.30 A comparison of duodenal mRNA expression patterns between Irp2-/- and IRP2+/+ mice using the IronChip24 revealed unchanged Dcytb mRNA expression (details of this experiment are presented in Supplemental Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article). Similarly, no alteration in the expression of hephaestin mRNA was observed.
These data show that the accumulation of iron and ferritin in the duodenal mucosa of IRP2-deficient mice occurs without detectable alteration in the expression of the known iron transporters. Among other possibilities, our data could be explained by partial derepression of ferritin translation as a direct consequence of IRP2 deficiency, and subsequent iron retention within the increased ferritin stores.
The lack of detectable changes in the levels of duodenal DMT1 and ferroportin in Irp2-/- mice with diminished hematocrit is intriguing. Duodenal expression of ferroportin and of DMT1 is modulated by nutritional iron deficiency15,31 and hemolytic anemia.32 Therefore, the unchanged ferroportin and DMT1 expression in IRP2-deficient mice could reflect impaired sensing and/or signaling of the reduction in hematocrit and serum hemoglobin values. To test whether Irp2-/- mice can respond normally to reduced hematocrit levels, mice were injected with phenylhydrazine (PHZ) to trigger hemolysis. This treatment similarly diminished the hematocrit in wild-type and mutant mice (data not shown). Wild-type mice respond to PHZ with the expected increase in ferroportin protein (Figure 2D) and DMT1 mRNA, especially the IRE-containing variant (Figure 2C) mirrored by the level of the corresponding DMT1 protein (data not shown). In addition, an IronChip analysis revealed the appropriate up-regulation of the Dcytb mRNA (data not shown). Of importance, the response of Irp2-/- mice to PHZ is identical to that of wild-type mice (Figure 2C-D). These data show that a mild reduction of the hematocrit and serum hemoglobin values in IRP2-deficient mice fails to elicit alterations in duodenal expression of DMT1 and ferroportin, although the regulatory network in Irp2-/- mice is able to respond to PHZ-induced anemia.
Iron accumulation and increased ferritin expression in the duodenum of IRP2-deficient mice. (A) The proximal part of the duodenum from 10-week +/+ and -/- males was analyzed by Prussian blue staining (top panels) or by immunostaining with anti-ferritin L-chain (middle panels) or anti-ferritin H-chain antibodies (bottom panels). Prussian blue coloration was counterstained with Nuclear Fast Red and ferritin immunostaining with hematoxylin. Pictures were acquired on a Axiophot microscope (Zeiss, Jena, Germany) with a × 20 objective. (B) Expression of ferritin H- and ferritin L-chains in groups of 4 +/+, +/-, and -/- 10-week-old males. Total protein (40 μg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blot analysis (top panels). Ferritin H- and L-chain mRNA levels were analyzed by Northern blotting using 10 μg total RNA from the same mice (bottom panels) and β-actin mRNA as a standard. Western blot and Northern blot signals were quantified and are presented as a histogram (bottom) after normalization for β-actin expression. Error bars indicate standard deviation. In Western blots, the ferritin L-subunit resolves into 2 bands, the bottom one corresponding to a cleavage product. Both bands were taken into account for quantification. IRP2 expression was below the detection limit.
Iron accumulation and increased ferritin expression in the duodenum of IRP2-deficient mice. (A) The proximal part of the duodenum from 10-week +/+ and -/- males was analyzed by Prussian blue staining (top panels) or by immunostaining with anti-ferritin L-chain (middle panels) or anti-ferritin H-chain antibodies (bottom panels). Prussian blue coloration was counterstained with Nuclear Fast Red and ferritin immunostaining with hematoxylin. Pictures were acquired on a Axiophot microscope (Zeiss, Jena, Germany) with a × 20 objective. (B) Expression of ferritin H- and ferritin L-chains in groups of 4 +/+, +/-, and -/- 10-week-old males. Total protein (40 μg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blot analysis (top panels). Ferritin H- and L-chain mRNA levels were analyzed by Northern blotting using 10 μg total RNA from the same mice (bottom panels) and β-actin mRNA as a standard. Western blot and Northern blot signals were quantified and are presented as a histogram (bottom) after normalization for β-actin expression. Error bars indicate standard deviation. In Western blots, the ferritin L-subunit resolves into 2 bands, the bottom one corresponding to a cleavage product. Both bands were taken into account for quantification. IRP2 expression was below the detection limit.
To investigate the functional consequences of increased ferritin levels in the duodenum, we studied duodenal iron transfer in situ by injecting 59Fe into the lumen of the gut and measuring the radioactivity recovered in the body after 15 minutes. The rate of iron transport across the duodenal mucosa is similar in Irp2-/- mice and wild-type littermate controls (Figure 2E). Thus, the increase in the ferritin content of enterocytes of Irp2-/- mice does not detectably affect the transport of 59Fe measured over a short period of time. It is possible that the increased ferritin expression favors duodenal iron retention over a more extended time course. Nonetheless, our data show that IRP2 deficiency has only a minor impact on duodenal iron metabolism and its ability to respond to hemolysis.
Expression of DMT1 and ferroportin, and 59Fe transport in the duodenum of IRP2-deficient mice. (A) DMT1 expression was analyzed by RNase protection assay. The antisense RNA probe matching a sequence common to the 2 3′ variants of the DMT1 mRNA isoforms plus a domain specific for the noIRE form is depicted (top). Total RNA (5 μg) was cohybridized with the DMT1 probe together with a β-actin probe as an input control. Arrows indicate the signals corresponding to full-length probes (DMT1: 500 nucleotide [nt], β-actin: 276 nt), to β-actin (250 nt), and to the IRE (320 nt)/no-IRE (400 nt) DMT1 isoforms. Total RNA from control and iron-deficient mice was used as a positive control for regulation of DMT1 expression. The histogram shows the levels of the DMT1 mRNA isoforms after normalization for β-actin. Error bars indicate standard deviation. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each). (B) Ferroportin expression was analyzed by Western blotting (top panels) and by Northern blotting (bottom panels). Equal loading was checked by detection of β-actin. The expression of ferroportin and of the DMT1 mRNA 3′ splice variants was determined by Western blotting (D) and RNase protection assay (C), respectively, in wild-type and Irp2-/- mice injected with phenylhydrazine (PHZ) versus a saline as a control (ctr). β-Actin mRNA was used as a standard. (E) Measurement of duodenal 59Fe transfer in vivo. Twelve-week-old males and females were fasted overnight and 59Fe transfer was analyzed as described in “Materials and methods.” The size (n) of the samples is indicated.
Expression of DMT1 and ferroportin, and 59Fe transport in the duodenum of IRP2-deficient mice. (A) DMT1 expression was analyzed by RNase protection assay. The antisense RNA probe matching a sequence common to the 2 3′ variants of the DMT1 mRNA isoforms plus a domain specific for the noIRE form is depicted (top). Total RNA (5 μg) was cohybridized with the DMT1 probe together with a β-actin probe as an input control. Arrows indicate the signals corresponding to full-length probes (DMT1: 500 nucleotide [nt], β-actin: 276 nt), to β-actin (250 nt), and to the IRE (320 nt)/no-IRE (400 nt) DMT1 isoforms. Total RNA from control and iron-deficient mice was used as a positive control for regulation of DMT1 expression. The histogram shows the levels of the DMT1 mRNA isoforms after normalization for β-actin. Error bars indicate standard deviation. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each). (B) Ferroportin expression was analyzed by Western blotting (top panels) and by Northern blotting (bottom panels). Equal loading was checked by detection of β-actin. The expression of ferroportin and of the DMT1 mRNA 3′ splice variants was determined by Western blotting (D) and RNase protection assay (C), respectively, in wild-type and Irp2-/- mice injected with phenylhydrazine (PHZ) versus a saline as a control (ctr). β-Actin mRNA was used as a standard. (E) Measurement of duodenal 59Fe transfer in vivo. Twelve-week-old males and females were fasted overnight and 59Fe transfer was analyzed as described in “Materials and methods.” The size (n) of the samples is indicated.
Hepatic iron metabolism in IRP2 deficiency
As the livers of Irp2-/- mice also display iron loading, ferritin H- and L-chain expression was analyzed by Western blotting (Figure 3, upper panels). L-chain expression is increased 6-fold in Irp2-/- mice. Ferritin H-chain expression is below the detection limit in the liver of wild-type mice but is detectably increased in Irp2-/- animals. Ferritin H- and L-chain mRNA levels are unchanged (Figure 3, lower panels), showing that ferritin expression is increased posttranscriptionally. By contrast, Irp2-/- mice display a faint reduction of TfR1 levels (data not shown), suggesting that the accumulation of iron and ferritin in the liver of IRP2-deficient mice occurs without constitutive increase in TfR1-dependent iron acquisition.
Posttranscriptional increase in ferritin expression in the liver of IRP2-deficient mice. Expression of ferritin H- and L-subunits was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting using β-actin as a standard. IRP2 expression is also shown; a nonspecific band is indicated with an asterisk. The Western blot and Northern blot signals were quantified and results are presented in a histogram (bottom) after normalization for β-actin. Error bars indicate standard deviation. Ferritin H-chain was below the detection limit in wild-type mice (nd) and was not quantified in Irp2-/- mice (nq) because of background interference. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each).
Posttranscriptional increase in ferritin expression in the liver of IRP2-deficient mice. Expression of ferritin H- and L-subunits was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting using β-actin as a standard. IRP2 expression is also shown; a nonspecific band is indicated with an asterisk. The Western blot and Northern blot signals were quantified and results are presented in a histogram (bottom) after normalization for β-actin. Error bars indicate standard deviation. Ferritin H-chain was below the detection limit in wild-type mice (nd) and was not quantified in Irp2-/- mice (nq) because of background interference. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each).
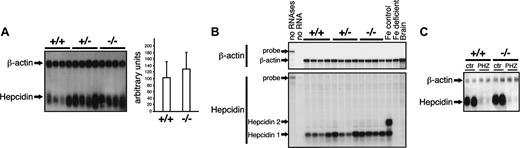
Considering both the liver iron accumulation (Table 1) and the reduced hematocrit (Table 2), we determined hepcidin expression. Hepcidin mRNA levels show interindividual variability in mutant mice as well as in wild-type littermates. Combining the results from 3 independent lots of mice, we observe a slight increase, although not statistically significant, in hepcidin mRNA expression in Irp2-/- versus IRP2+/+ mice (Figure 4A). Unlike humans, mice express 2 hepcidin genes. The 2 hepcidin proteins may play distinct roles in iron homeostasis.33 Given their 92% sequence identity,34 the hepcidin 1 and 2 mRNAs cannot be discriminated by Northern blotting. To check whether IRP2 mutant mice regulate hepcidin 1 and 2 mRNAs selectively, RNase protection experiments were performed with brain mRNA as a negative control (Figure 4B). As a control for regulation, we analyzed hepcidin mRNA expression in females that received an iron-poor versus a control diet. In control mice, both hepcidin mRNAs are detected (Figure 4B). Iron-deficient female mice display the expected decrease in both hepcidin 1 and hepcidin 2 mRNA levels, while hepcidin 2 mRNA is barely detectable in Irp2+/+ and Irp2-/- males, which express predominantly hepcidin 1. This difference is likely explained by the sex difference since sex has been shown to affect the hepcidin1-hepcidin2 ratio.35 In agreement with the Northern blot data, we do not observe significant changes in hepcidin 1 mRNA levels in IRP2-deficient compared with wild-type mice. This observation is surprising since a reduction in hematocrit and serum hemoglobin values is expected to elicit hepcidin down-regulation. To test whether IRP2-deficient mice can adequately adjust hepcidin expression in response to decreased hematocrit and serum hemoglobin values, mice were injected with PHZ as described in the previous section. Wild-type mice respond with the expected dramatic reduction in hepcidin mRNA expression (Figure 4C). Of importance, the response of Irp2-/- mice to PHZ is identical, showing that IRP2 deficiency does affect the control of hepcidin expression in response to hemolytic anemia.
Unchanged hepcidin mRNA expression in the liver of IRP2-deficient mice. (A) Expression of hepcidin was analyzed in groups of 4 +/+, +/-, and -/- mice by Northern blotting using β-actin as a standard. The histogram shows quantification of the hepcidin signals (normalized for β-actin) from the analysis of 3 independent lots of mice comprising 4 animals in each group. Error bars indicate standard deviation. (B) Hepcidin 1 and hepcidin 2 mRNA levels were assayed by RNase protection. Total RNA (10 μg) was cohybridized with the hepcidin probe together with a β-actin probe as an input control. The signals corresponding to full-length probes (hepcidin: 125 nt, β-actin: 334 nt), to β-actin (250 nt), and to the hepcidin 1 (40 nt) and hepcidin 2 (54 nt) mRNAs are indicated by arrows. Total RNA from control versus iron-deficient mice was used as a positive control for regulation of hepcidin expression. (C) Hepcidin mRNA levels were assayed by Northern blotting in mice injected with phenylhydrazine (PHZ) or a saline as a control (ctr), in wild-type versus IRP2-mutant mice. β-Actin was used as a standard.
Unchanged hepcidin mRNA expression in the liver of IRP2-deficient mice. (A) Expression of hepcidin was analyzed in groups of 4 +/+, +/-, and -/- mice by Northern blotting using β-actin as a standard. The histogram shows quantification of the hepcidin signals (normalized for β-actin) from the analysis of 3 independent lots of mice comprising 4 animals in each group. Error bars indicate standard deviation. (B) Hepcidin 1 and hepcidin 2 mRNA levels were assayed by RNase protection. Total RNA (10 μg) was cohybridized with the hepcidin probe together with a β-actin probe as an input control. The signals corresponding to full-length probes (hepcidin: 125 nt, β-actin: 334 nt), to β-actin (250 nt), and to the hepcidin 1 (40 nt) and hepcidin 2 (54 nt) mRNAs are indicated by arrows. Total RNA from control versus iron-deficient mice was used as a positive control for regulation of hepcidin expression. (C) Hepcidin mRNA levels were assayed by Northern blotting in mice injected with phenylhydrazine (PHZ) or a saline as a control (ctr), in wild-type versus IRP2-mutant mice. β-Actin was used as a standard.
Altered iron metabolism in the spleen of IRP2-deficient mice
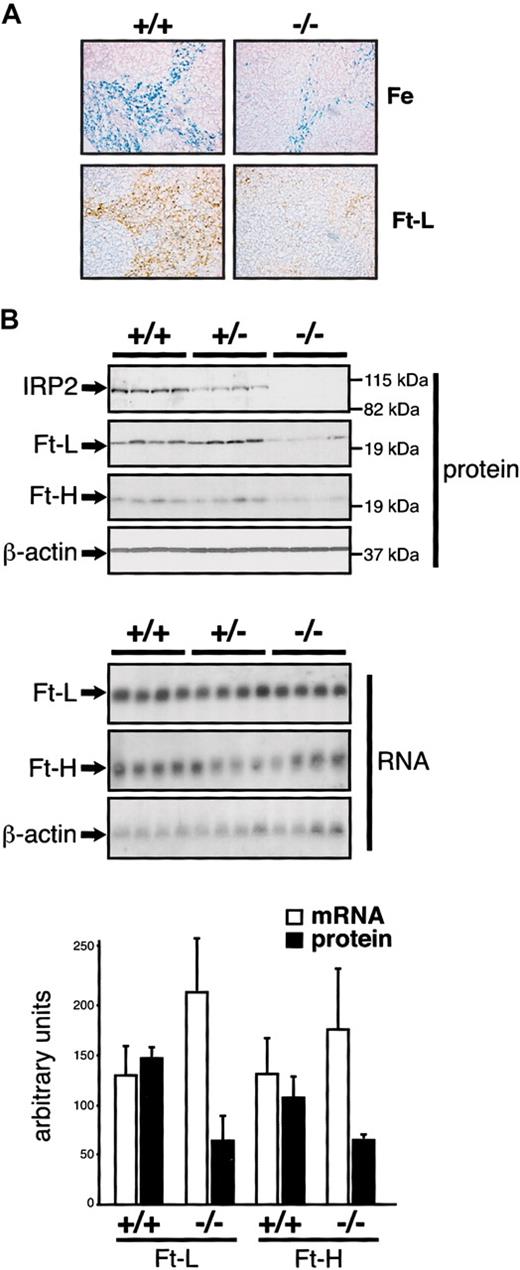
In agreement with a decrease in nonheme iron content (Table 1), sections from the spleens of Irp2-/- mice show weaker Prussian Blue staining in the red pulp (Figure 5A, upper panels). This staining reveals essentially macrophages and is associated with weaker immunostaining for the ferritin L-chain (Figure 5A, lower panels), and a reduction in ferritin H (∼ 1.5 fold) and L (∼ 2 fold) expression in Western blots of splenic extracts from Irp2-/- mice (Figure 5B, upper panels). The levels of the corresponding mRNAs are unchanged (Figure 5B, lower panels), suggesting posttranscriptional down-regulation. We detected no signs of erythroid hyperplasia that could explain the iron deficiency of splenic macrophages. Indeed, Irp2-/- mice display no splenomegaly, and the mRNA levels encoding the heme biosynthetic pathway enzymes ferrochelatase or erythroid 5-aminolevulinate synthase (eALAS) are normal as assessed with the IronChip (Supplemental Figure S1). By contrast, the levels of these mRNAs increase when the mice are made strongly anemic by PHZ injection (data not shown).
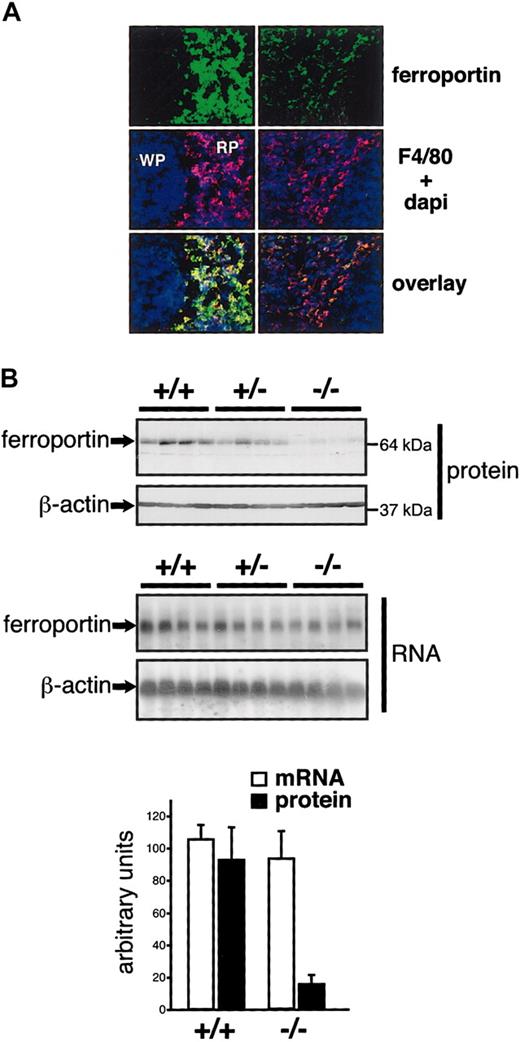
The iron deficiency of splenic macrophages could be due to increased iron export and/or decreased iron acquisition. Several observations indicate that ferroportin is a major mediator of iron efflux from macrophages.36,37 Overexpression (or activity) of ferroportin could hence explain an iron deficiency of splenic macrophages. Alternatively, if the splenic iron deficiency was caused by reduced iron acquisition, ferroportin mRNA translation might be repressed via IRP1. Thus, determination of ferroportin expression could help distinguish between these scenarios. In the spleen, ferroportin is detected mostly in macrophages expressing the F4/80 antigen.4 In F4/80-positive cells of Irp2-/- spleens, total ferroportin protein expression is reduced, without a concomitant change in ferroportin mRNA levels (Figure 6). This result directly supports the second hypothesis, according to which the iron deficiency is more readily explained by reduced iron acquisition than by increased export. A similar decrease in total ferroportin expression at the posttranscriptional level was observed in the bone marrow of Irp2-/- mice, another major site of iron recycling (data not shown).
Decreased erythroid TfR1 expression can account for the microcytosis
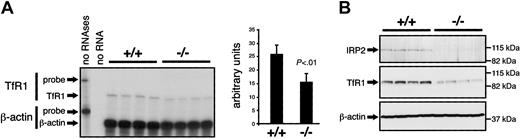
Microcytosis in Irp2-/- mice is associated with iron redistribution within the liver-bone marrow-spleen axis. Nonetheless, serum iron levels and transferrin saturation are not detectably altered, suggesting that the microcytosis could result from an intrinsic defect in hematopoiesis. To test this possibility, IRP target mRNAs were analyzed in the bone marrow. Translational derepression of ferritin due to lack of IRP2 may result in iron sequestration and impair hematopoiesis. However, Western blotting revealed no evidence for increased ferritin expression in the bone marrow of Irp2-/- versus wild-type mice (data not shown). Similarly, dysregulation of eALAS expression may affect heme synthesis, although we found eALAS protein accumulation to be unchanged in IRP2-deficient mice (data not shown). Of importance, TfR1 is essential for hematopoiesis.38 As IRP binding to IREs in the 3′ UTR of TfR1 mRNA sustains TfR1 expression, lack of IRP2 may negatively affect TfR1 expression in erythroid precursors and diminish their iron acquisition capacity. TfR1 mRNA levels in the bone marrow of Irp2+/+ and Irp2-/- mice were measured by RNase protection assay. In the bone marrow, most of the TfR1 is expressed in erythroid cells.39 Indeed, the level of TfR1 mRNA (Figure 7A) and protein (Figure 7B) is significantly reduced in Irp2-/- mice, a result that can plausibly explain the observed phenotype.
Diminished iron staining and ferritin expression in the spleen of IRP2-deficient mice. (A) The spleen from 10-week +/+ and -/- males was analyzed by Prussian Blue staining (top panels) or staining with an anti-ferritin L-chain antibody (bottom panels). Prussian blue coloration was counterstained with Nuclear Fast Red and ferritin immunostaining with hematoxylin. Pictures were acquired on a Axiophot microscope (Zeiss) with a × 20 objective. Ferritin H-chain expression was below the detection limit and is not shown. (B) IRP2 and ferritin H- and L-chain expression was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting from 40 μg total protein or 10 μg total RNA, respectively, using β-actin as a standard. The histogram represents the level of ferritin expression after normalization for β-actin. Error bars indicate standard deviation.
Diminished iron staining and ferritin expression in the spleen of IRP2-deficient mice. (A) The spleen from 10-week +/+ and -/- males was analyzed by Prussian Blue staining (top panels) or staining with an anti-ferritin L-chain antibody (bottom panels). Prussian blue coloration was counterstained with Nuclear Fast Red and ferritin immunostaining with hematoxylin. Pictures were acquired on a Axiophot microscope (Zeiss) with a × 20 objective. Ferritin H-chain expression was below the detection limit and is not shown. (B) IRP2 and ferritin H- and L-chain expression was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting from 40 μg total protein or 10 μg total RNA, respectively, using β-actin as a standard. The histogram represents the level of ferritin expression after normalization for β-actin. Error bars indicate standard deviation.
Reduced ferroportin expression in the spleen of IRP2-deficient mice. (A) Ferroportin expression was analyzed by fluorescent immunostaining on spleen sections. The red pulp (RP) and the white pulp (WP) are indicated. Macrophages of the red pulp were revealed with an anti-F4/80 antibody. Nuclei were stained with Dapi. Pictures were acquired using a wide field Axiovert 200M microscope and a × 25 immersion objective from Zeiss. (B) Ferroportin expression was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting. The histogram depicts the levels of ferroportin expression after normalization for β-actin. Error bars indicate standard deviation. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each).
Reduced ferroportin expression in the spleen of IRP2-deficient mice. (A) Ferroportin expression was analyzed by fluorescent immunostaining on spleen sections. The red pulp (RP) and the white pulp (WP) are indicated. Macrophages of the red pulp were revealed with an anti-F4/80 antibody. Nuclei were stained with Dapi. Pictures were acquired using a wide field Axiovert 200M microscope and a × 25 immersion objective from Zeiss. (B) Ferroportin expression was analyzed in groups of 4 +/+, +/-, and -/- mice by Western (top panels) and Northern (bottom panels) blotting. The histogram depicts the levels of ferroportin expression after normalization for β-actin. Error bars indicate standard deviation. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each).
Discussion
Cell culture and in vitro studies demonstrated the importance of the IRP/IRE regulatory network in cellular iron metabolism.1,14-16 We recently generated mice with loss-of-function alleles for IRP1 or for IRP218 to extend and complement phenotypic studies on mice with constitutive deletions of IRP1 and/or IRP2.13,17 The detailed analyses of mice lacking IRP2 expression reported here have uncovered a previously unrecognized function of IRP2 in securing physiologic iron distribution among the duodenum, the liver, and the spleen, and the need for IRP2 expression for normal erythropoiesis.
Altered iron metabolism in IRP2-deficient mice
IRP2-deficient mice display mild microcytosis with reduced hematocrit and serum hemoglobin values, associated with altered iron distribution in the central iron recycling system, the liver-bone marrow-spleen axis, and iron deposits in the duodenal mucosa. Of note, these alterations occur without detectable change in hepcidin mRNA levels. However, the lack of IRP2 does not disrupt the physiologic regulation of hepcidin in severely anemic mice following PHZ injection. This result indicates that the reduced hematocrit of Irp2-/- animals may not have reached the threshold required for hepcidin to respond. Similarly, the iron accumulation in the liver of Irp2-/- mice may not be sufficient to trigger hepcidin expression, or it may fail to be sensed as iron overload as a result of its cellular or subcellular distribution. Conceivably, the antagonistic effects of microcytosis and liver iron loading on hepcidin expression could neutralize each other.
Reduced TfR1 expression in the bone marrow of IRP2-deficient mice. (A) TfR1 mRNA levels in the bone marrow were assayed by RNase protection in groups of 4 wild-type versus 4 Irp2-/- mice. β-Actin was used as a standard. The bands corresponding to full-length probes (TfR1: 490 nt, β-actin: 276 nt) and to the β-actin (250 nt) and TfR1 (371 nt) mRNAs are indicated by arrows. The signals were quantified using a phosphorimager. The histogram represents the levels of TfR1 mRNA after normalization for β-actin expression. Error bars indicate standard deviation. TfR1 mRNA levels are significantly reduced in mutant mice compared with wild-type mice (Student St test). (B) TfR1 protein levels (middle panel) were analyzed in 4 wild-type versus 4 Irp2-/- mice (top panel) by Western blotting using β-actin as a standard (bottom panel).
Reduced TfR1 expression in the bone marrow of IRP2-deficient mice. (A) TfR1 mRNA levels in the bone marrow were assayed by RNase protection in groups of 4 wild-type versus 4 Irp2-/- mice. β-Actin was used as a standard. The bands corresponding to full-length probes (TfR1: 490 nt, β-actin: 276 nt) and to the β-actin (250 nt) and TfR1 (371 nt) mRNAs are indicated by arrows. The signals were quantified using a phosphorimager. The histogram represents the levels of TfR1 mRNA after normalization for β-actin expression. Error bars indicate standard deviation. TfR1 mRNA levels are significantly reduced in mutant mice compared with wild-type mice (Student St test). (B) TfR1 protein levels (middle panel) were analyzed in 4 wild-type versus 4 Irp2-/- mice (top panel) by Western blotting using β-actin as a standard (bottom panel).
The reasons for the altered iron distribution in Irp2-/- mice are not fully resolved. While liver iron loading and macrophage iron deficiency are reminiscent of type 1 hemochromatosis, the normal serum Tf saturation and the enterocyte iron loading are not.40 Furthermore, the IronChip analysis revealed unchanged HFE mRNA expression in various organs (Supplemental Figure S1). The phenotypic manifestations of IRP2 deficiency have several other interesting implications. The increased expression of ferritin in enterocytes on the one hand and the unaffected expression of duodenal iron transport molecules, the normal duodenal transfer of 59Fe, and the normal serum transferrin saturation on the other are striking in terms of the mucosal block hypothesis proposed by Crosby.41 This hypothesis states that duodenal ferritin serves to withhold iron from entering into circulation and thus as a means to negatively regulate iron transfer. Irp2-/- mice with an 8- to 9-fold increase in ferritin and seemingly normal iron transfer appear to uncouple the 2 events in a way that is not predicted by the mucosal block scenario. One critical issue is whether IRP2-deficient mice are microcytic because of iron mismanagement within the iron-recycling pathway. Normal serum iron levels and transferrin saturation values suggest that this is not the case. As mice with haploinsufficiency for TfR1 suffer from microcytosis,38 the approximately 1.6-fold reduction of TfR1 mRNA expression accompanied by diminished TfR1 protein expression in erythroid cells of Irp2-/- mice can plausibly explain the microcytosis. We did not find increased ferritin expression in total bone marrow samples that could withhold a fraction of the iron needed for erythropoiesis. However, changes in ferritin levels in erythroid precursors might be masked by the contribution of ferritin from the other cells of the bone marrow. Indeed, Cooperman et al recently reported a small increase in ferritin expression in erythroid precursors sorted from the bone marrow of a distinct IRP2-deficient line.42 Although Western blotting detected no changes in eALAS accumulation in total bone marrow extracts from our Irp2-/- mice (data not shown), Cooperman et al42 found increased de novo synthesis of eALAS in sorted erythroid precursors after metabolic labeling, associated with accumulation of free protoporphyrin IX. Altogether, these data suggest that microcytosis in Irp2-/- mice is likely caused by an intrinsic defect of intracellular iron availability in erythroid cells.
Using a gene replacement vector to target the Irp2 locus nonconditionally, LaVaute et al13 described the development of a progressive neurodegenerative disease with locomotor impairment in Irp2-/- mice older than 6 months. We have not observed any overt signs of neuropathology such as tremor or any obvious postural abnormality in young adults or in 8-month-old Irp2-/- mice (data not shown). While both IRP2-deficient mouse lines display duodenal and hepatic accumulation of iron and ferritin, we did not observe increased ferroportin and DMT1 expression in the duodenum as reported by LaVaute et al.13 Several factors could explain such discrepancies. While both mouse lines are on similar mixed C57BL6/Sv129 genetic backgrounds, they were analyzed at a different age (8-10 weeks in the present study versus 4 months and older in LaVaute et al13 ). However, we tested but did not detect significantly increased duodenal ferroportin expression in 4- and 8-month-old Irp2-/- mice either (data not shown). Perhaps of more importance, the targeting strategies are distinct. While LaVaute et al13 used a gene replacement vector bearing a PGK-Neo cassette, we inserted a promoter-less βGeo gene-trap construct into an early intron of the irp2 gene.18 Artifacts arising from selection markers are well documented.43 Therefore, we crossed our Irp2-/- line bearing the βGeo cassette with a Cre deletor strain to derive an IRP2-null line that lacks any selection marker inserts and that differs from the wild type only by the absence of exon 3. A preliminary analysis of this IRP2Δ/Δ line also displays a complete loss of IRP2 expression and shows the same phenotype as the one reported here, including the misdistribution of iron and microcytosis, and excluding increased duodenal ferroportin expression or overt symptoms of neuropathology up to 6 months of age (B.G., D.F., M.W.H.; unpublished results, 2005). This demonstrates that the phenotypic traits reported here result from IRP2 deficiency, per se, and are not technical artifacts arising from the presence of the gene trap construct.
Molecular basis of altered iron metabolism in IRP2-deficient mice
IRP2 deficiency leads to iron accumulation in the liver and in the duodenum, with a concomitant posttranscriptional increase in ferritin protein expression. The mechanisms underlying iron deposition in those tissues remain to be elucidated. We did not observe any increase in hepatic TfR1 expression in mutant animals. A second Tf receptor with about 25-fold lower affinity than TfR1, TfR2, is expressed on hepatocytes.44 However, the IronChip analysis did not reveal any change in TfR2 mRNA expression either. Hence, a potential increase in liver iron uptake could potentially involve Tf-independent pathways. A reduction in iron efflux may also account for iron accumulation, although there is no detectable change in hepatic ferroportin expression (data not shown). Still unknown ferroportin-independent iron export pathways might be affected, or ferroportin function could be decreased posttranslationally.
Contrary to what was observed in the liver and in the duodenum, the iron and ferritin content of splenic macrophages is lower in Irp2-/- animals. Curiously, Irp2-/- macrophages from the spleen and bone marrow display reduced ferroportin expression, a finding that was not reported before.13,42 This is unexpected, since ferroportin down-regulation should result in iron retention. Hypothetically, an increase in the iron transport activity of ferroportin may overcome the reduction of its expression, or an as-yet-unknown iron export pathway may be activated. In that case, ferroportin down-regulation might be secondary to iron deficiency and may result from translational repression of the IRE-containing ferroportin mRNA by IRP1 whose IRE-binding activity would be increased in iron-deficient cells.
A recent study revealed that hepcidin binds ferroportin and augments its turnover in human cell lines.8 This mechanism does not explain the observed reduction in ferroportin expression in Irp2-/- macrophages, because hepcidin expression in the liver and ferroportin expression in other tissues are not detectably affected. Splenic macrophages acquire iron mainly from senescent erythrocytes. As the reduction of the red-cell mass (Table 2) cannot account for the decreased iron content of the spleen (Table 1), a possible reason for the iron deficiency of macrophages could be a quantitatively modest impairment of erythrophagocytosis.
Roles of IRP1 and of IRP2 in iron homeostasis
An intriguing feature of IRP2-deficient mice is the remarkable preservation of mRNA expression patterns in the brain, duodenum, liver, and spleen, assessed with the IronChip (Supplemental Figure S1). These data imply broad functional redundancies between IRP1 and IRP2. Constitutive inactivation of both Irp1 and Irp2 genes in the mouse results in embryonic lethality19 (B.G. et al, unpublished data), showing that the IRP/IRE regulatory system is essential for life. IRP1-deficient animals are almost normal and display none of the phenotypic traits of our IRP2-deficient mouse line17 (B.G. et al, manuscript in preparation). These findings suggest that the 2 IRPs can largely replace each other. Thus, at least under laboratory conditions, IRP2 can fully compensate for the lack of IRP1, whereas IRP1 can largely but not fully compensate for the absence of IRP2. The strong redundancy of the 2 IRPs combined with the embryonic lethality of doubly deficient mice poses a challenge to dissect and understand the physiologic roles of the IRP/IRE regulatory system in vivo. The possibility of inactivating one or both of the Irp genes in a time- and tissue-specific manner using conditional alleles18 provides an experimental system to further uncover the role of the IRP/IRE regulatory system in mammalian physiology.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-04-1365.
Supported by a European Molecular Biology Organisation (EMBO) long-term fellowship (ALF199-212; B.G.) and by funds from the Gottfried Wilhelm Leibniz Prize (M.W.H.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Patrick Hundsdoerfer and Karen Brennan for helpful discussions and to Yevhen Vainshtein for help with handling of the microarray data. We thank the EMBL transgenic mouse service and the staff of the EMBL animal house for their contribution to this work.

![Figure 2. Expression of DMT1 and ferroportin, and 59Fe transport in the duodenum of IRP2-deficient mice. (A) DMT1 expression was analyzed by RNase protection assay. The antisense RNA probe matching a sequence common to the 2 3′ variants of the DMT1 mRNA isoforms plus a domain specific for the noIRE form is depicted (top). Total RNA (5 μg) was cohybridized with the DMT1 probe together with a β-actin probe as an input control. Arrows indicate the signals corresponding to full-length probes (DMT1: 500 nucleotide [nt], β-actin: 276 nt), to β-actin (250 nt), and to the IRE (320 nt)/no-IRE (400 nt) DMT1 isoforms. Total RNA from control and iron-deficient mice was used as a positive control for regulation of DMT1 expression. The histogram shows the levels of the DMT1 mRNA isoforms after normalization for β-actin. Error bars indicate standard deviation. These figures are representative of data obtained with 3 independent lots of mice (including 4 +/+ and 4 -/- animals each). (B) Ferroportin expression was analyzed by Western blotting (top panels) and by Northern blotting (bottom panels). Equal loading was checked by detection of β-actin. The expression of ferroportin and of the DMT1 mRNA 3′ splice variants was determined by Western blotting (D) and RNase protection assay (C), respectively, in wild-type and Irp2-/- mice injected with phenylhydrazine (PHZ) versus a saline as a control (ctr). β-Actin mRNA was used as a standard. (E) Measurement of duodenal 59Fe transfer in vivo. Twelve-week-old males and females were fasted overnight and 59Fe transfer was analyzed as described in “Materials and methods.” The size (n) of the samples is indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-04-1365/6/m_zh80190584860002.jpeg?Expires=1769196866&Signature=AFhqyA3mXH41sMGyYOybNrHh7telniUQ8HtwgsLyvmjxi3sJkE0SccgFs3a20d9sRchGmDaTckv7PF-UryDHrfy-QS44hCtA1rTwgxEnlaAcdXQyE88Us2R6SvpGJhzRaumQ-JCwQrkuLFp7lnsXKeMWxdDPBNDUpLFD9QKATK6l04YMlgDZ2fUY0RjZnGtnHxcxlU9kYgfk9Klq7L-rDafiMZXAqelfVb0lF9a0EOGNPC86NhawvLL7v4jO-J6IRwJXkaQP4iUdUOsCXHDi6B0KOwel8wBkvBKe~XHdLWicta-3gY2ApVMIbomoMwjbr-m4TFFSqjuCx4A4~m3J8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal