Abstract
The Krüppel-like factors (KLFs) are a family of C2/H2 zinc finger DNA-binding proteins that are important in controlling developmental programs. Erythroid Krüppel-like factor (EKLF or KLF1) positively regulates the β-globin gene in definitive erythroid cells. KLF2 (LKLF) is closely related to EKLF and is expressed in erythroid cells. KLF2-/- mice die between embryonic day 12.5 (E12.5) and E14.5, because of severe intraembryonic hemorrhaging. They also display growth retardation and anemia. We investigated the expression of the β-like globin genes in KLF2 knockout mice. Our results show that KLF2-/- mice have a significant reduction of murine embryonic Ey- and βh1-globin but not ζ-globin gene expression in the E10.5 yolk sac, compared with wild-type mice. The expression of the adult βmaj- and βmin-globin genes is unaffected in the fetal livers of E12.5 embryos. In mice carrying the entire human globin locus, KLF2 also regulates the expression of the human embryonic ϵ-globin gene but not the adult β-globin gene, suggesting that this developmental-stage-specific role is evolutionarily conserved. KLF2 also plays a role in the maturation and/or stability of erythroid cells in the yolk sac. KLF2-/- embryos have a significantly increased number of primitive erythroid cells undergoing apoptotic cell death. (Blood. 2005;106: 2566-2571)
Introduction
Hematopoiesis represents a complex differentiation pathway involving many transcription factors and growth factors that interact in a concerted fashion during mammalian development.1 Transcription factors often exist as multigene families of structurally and functionally related members that are involved in the different stages of development of a particular cell lineage. For example, members of the GATA family of transcription factors are involved in both primitive and definitive erythropoiesis.2-4 Different phenotypic features seen after ablation of either the GATA1 or the GATA2 gene in mice clearly demonstrate that these factors have different but overlapping functions. The Krüppel-like factors (KLFs) are a family of DNA-binding proteins named after the Drosophila Krüppel protein. KLFs have 3 C2/H2 zinc finger domains and share conserved residues located primarily within these zinc fingers.5,6 Several of the KLFs are expressed in erythroid cells starting early in development. Erythroid Krüppel-like factor (EKLF or KLF1) was the first of 16 KLFs to be identified. EKLF-/- mice develop fatal anemia during fetal liver erythropoiesis.7,8 EKLF is responsible for positively regulating the adult β-globin gene, but it is not required for embryonic/fetal globin gene expression.9-11 Other KLF family members may be involved in the developmental control of the embryonic and fetal globin genes. A few of the KLFs, namely KLF2 and KLF5,12 and KLF11 and KLF13,13,14 have been shown to activate the fetal γ-globin gene in transient transfection assays in human erythroleukemia cell lines. So far none of these studies have been replicated in vivo. In a recent study, KLF11 (fetal Krüppel like factor, FKLF1)-null mice were found to be fertile, with normal hematopoiesis at all stages of development. There was no effect of this deletion on globin gene expression, growth and development, or on lifespan.15
From phylogenetic analysis, KLF2 (lung Krüppel-like factor, LKLF) and KLF4 (gut Krüppel-like factor, GKLF) are most similar to EKLF.5,12,16 EKLF has an overall similarity of 50.3% to KLF2 and 42% to KLF4 at the protein level. In the zinc finger domain, it has 88.9% similarity with both proteins. Moreover, EKLF and KLF2 are in close chromosomal proximity in human (chromosome 19p13.13-p13.11) and mouse (chromosome 8 C1-C2) and therefore may have diverged because of a relatively recent gene duplication event.17 As such, KLF2 may have a role similar to EKLF in erythropoiesis and globin gene regulation. In purified erythroid cells from chicken and from mouse yolk sac and fetal liver, KLF2 is expressed during development, in both primitive and definitive erythroid compartments.12,16 Two different studies have independently generated KLF2-null mice and have reported similar phenotypes of the KLF2-/- animals.18,19 Prior to embryonic day 11.5 (E11.5), KLF2-/- embryos appear to be phenotypically normal, with normal vasculogenesis and angiogenesis. At E12.5, KLF2-/- animals have normal numbers of blood vessels in the yolk sac, but the vessels are devoid of blood. They also show pale fetal livers and display growth retardation. From E12.5 onward, KLF2-/- embryos start developing severe intraembryonic hemorrhages around the cardiac outflow tract and in the abdomen.18,19 The hemorrhaging is caused by rupturing of the umbilical veins and arteries as a result of abnormal thinning of the tunica media and instability of the vessel wall.18 The KLF2-/- mice also exhibit reduced head size, possibly as a consequence of anemia. These KLF2-/- mice die between E12.5 and E14.5.18,19
Transcription factors have been studied in the context of developmental regulation of the globin genes. The major human β-like globin genes are the embryonic ϵ-globin, the 2 fetal γ-globin, and the adult β-globin genes. They are clustered on chromosome 11 in the order of their expression. In contrast, the murine locus has 2 embryonic globin genes, Ey- and βh1-globin, and 2 adult globin genes, βmaj- and βmin-globin. The adult genes are expressed beginning at fetal liver erythropoiesis. EKLF binds to the CACCC box of the β-globin gene promoter20 and is responsible for positively regulating the β-globin gene in the adult, but it is not required for embryonic or human γ-globin gene expression.9-11 In the present study, we have investigated the effect of KLF2 on primitive erythropoiesis and on the expression of the human and murine globin genes in vivo.
Materials and methods
KLF2 knockout and YAC-transgenic mice
KLF2 knockout mice were generated as described previously.19 A replacement-type targeting vector with positive-negative selection was used for gene knockout by homologous recombination. 129/Bl6/Swiss KLF2+/- male mice were bred with wild-type FVB/N female mice to generate +/- offspring. These mice were screened for the KLF2 knockout allele using primers 5′-TGCTTACAACCTCCTAAATGTTCTGA-3′ and 5′-CCTACCCGCTTCCATTGCTC-3′. KLF2+/- male and female mice were used in timed matings to generate +/+, +/-, and -/- embryos. Following dissection of pregnant females, yolk sacs were collected from E10.5 embryos, and yolk sacs and fetal livers were collected from E12.5 fetuses. The tissues were quick frozen in liquid nitrogen and stored at -70°C. Genotyping was performed using genomic DNA from tissues of the embryos and fetuses. Total RNA was isolated from the tissues by dissolving them in Trizol reagent (Sigma, St Louis, MO) and processing as per the manufacturer's protocol.
To generate KLF2 knockout mice containing the human β-like globin genes, KLF2+/- mice were bred with A85.68 FVB/N and A20.1 C57Bl6 yeast artificial chromosome (YAC) transgenic mice.21 The YAC constructs in these mice are present in one copy and have more than 140 kilobase pairs (kbp) spanning the entire human globin locus, including all of the genes and the entire locus control region. Male KLF2+/-globin+ mice were crossed with female KLF2+/- mice to generate embryos for all 3 KLF2 genotypes containing the human globin locus. Yolk sacs and fetal livers were collected and processed as described earlier.
Quantitative real-time PCR procedures and statistical analysis
cDNA was synthesized from total RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and quantitative real-time polymerase chain reactions (PCRs) were performed using the ABI-Prism 7900HT (Applied Biosystems, Foster City, CA). PCR was carried out in 96-well plates, in a 25 μL reaction volume containing a Taqman probe, forward and reverse primers, and 1 × iTaq supermix with carboxy-X-rhodamine (ROX) (Bio-Rad). Primers and probe sets were designed using PrimerExpress software (Applied Biosystems), and the 5′ to 3′ sequences of these oligonucleotides are listed in Table 1. Taqman probes were manually screened to overlap exon-exon junctions to avoid signal from DNA. Primers and probes were finally checked with the National Center for Biotechnology Information (NCBI) database using BLAST analysis,22 to ensure specificity for the intended globin gene(s). The γ-globin primer and probe set was designed to measure expression from both the Aγ- and Gγ-globin genes. Taqman probes were labeled with FAM and TAMRA as fluorophore and quencher, respectively. To ensure that changes in the amount of globin mRNA were not due to changes in the number of viable red blood cells, expression of glycophorin A (GPA) was used as an internal control. The PCR cycles for all probe and primer sets were initial heating at 95°C for 2 minutes, followed by 40 cycles of 94°C for 15 seconds and 1 minute at 60°C as per manufacturer's guide (Applied Biosystems). Each sample was subjected to 6 PCR reactions for the target gene and 6 reactions for GPA. The means were used for calculation, constituting a total of 12 reactions for each embryo. The number of embryos tested for each genotype is indicated in the figures, and ranged from 3 to 7. For each graph, the mean wild-type globin/GPA mRNA ratio was normalized to 100%, and the average of the data points of the other genotypes were calculated relative to the wild-type data. For statistical analysis, the Student t test of comparison of means was performed, and differences were considered significant at P values of less than .025.
Name of locus . | Forward primer . | Taqman probe . | Reverse primer . |
|---|---|---|---|
| Mouse Ey | CAAGCTACATGTGGATCCTGAGAA | TCAAACTCTTGGGTAATGTGCTGGTGATTG | TGCCGAAGTGACTAGCCAAA |
| Mouse βhl | AGGCAGCTATCACAAGCATCTG | AGAAACTCTGGGAAGGCTCCTGATTGTTTACC | AACTTGTCAAAGAATCTCTGAGTCCAT |
| Mouse βmaj + βmin | GTGAGCTCCACTGTGACAAGCT | CATGTGGATCCTGAGAACTTCAGGCTCCT | GGTGGCCCAGCACAATCACGATC |
| Human ϵ | GCCTTTGCTAAGCTGAGTGAG | TCAAGCTCCTGGGTAACGTGATGGTGA | TTGCCAAAGTGAGTAGCCAGAA |
| Human γG + γA | GTGGAAGATGCTGGAGGAGAAA | AGGCTCCTGGTTGTCTACCCATGGACC | TGCCATGTGCCTTGACTTTG |
| Human β | GCAAGGTGAACGTGGATGAAGT | CAGGCTGCTGGTGGTCTACCCTTGGACCC | TAACAGCATCAGGAGTGGACAGA |
| Mouse ζ | GCGAGCTGCATGCCTACAT | TGGATCCGGTCAACTTCAAGCTCCTGT | GCCATTGTGACCAGCAGACA |
| Mouse GPA | GCCGAATGACAAAGAAAAGTTCA | TTGACATCCAATCTCCTGAGGGTGGTGA | TCAATAGAACTCAAAGGCACACTGT |
Name of locus . | Forward primer . | Taqman probe . | Reverse primer . |
|---|---|---|---|
| Mouse Ey | CAAGCTACATGTGGATCCTGAGAA | TCAAACTCTTGGGTAATGTGCTGGTGATTG | TGCCGAAGTGACTAGCCAAA |
| Mouse βhl | AGGCAGCTATCACAAGCATCTG | AGAAACTCTGGGAAGGCTCCTGATTGTTTACC | AACTTGTCAAAGAATCTCTGAGTCCAT |
| Mouse βmaj + βmin | GTGAGCTCCACTGTGACAAGCT | CATGTGGATCCTGAGAACTTCAGGCTCCT | GGTGGCCCAGCACAATCACGATC |
| Human ϵ | GCCTTTGCTAAGCTGAGTGAG | TCAAGCTCCTGGGTAACGTGATGGTGA | TTGCCAAAGTGAGTAGCCAGAA |
| Human γG + γA | GTGGAAGATGCTGGAGGAGAAA | AGGCTCCTGGTTGTCTACCCATGGACC | TGCCATGTGCCTTGACTTTG |
| Human β | GCAAGGTGAACGTGGATGAAGT | CAGGCTGCTGGTGGTCTACCCTTGGACCC | TAACAGCATCAGGAGTGGACAGA |
| Mouse ζ | GCGAGCTGCATGCCTACAT | TGGATCCGGTCAACTTCAAGCTCCTGT | GCCATTGTGACCAGCAGACA |
| Mouse GPA | GCCGAATGACAAAGAAAAGTTCA | TTGACATCCAATCTCCTGAGGGTGGTGA | TCAATAGAACTCAAAGGCACACTGT |
All Taqman probes were labeled with FAM (6-carboxyfluorescein) and TAMRA (6-carboxy-tetramethylrhodamine).
Histologic analysis
E10.5 day embryos were dissected in phosphate-buffered saline pH 7.4 (PBS; Invitrogen, Carlsbad, CA), and the yolk sacs were collected and fixed in a solution containing 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer at 4°C overnight. Following genotyping of the embryos, the tissues were postfixed in 1% osmium tetroxide in 0.1 M cacodylate buffer. Tissues were then dehydrated gradually in increasing concentrations of ethanol (50%, 70%, 90%, and 100%) and embedded in PolyBed812 Epoxy Resin. For light microscopy, 1-μm thick plastic sections were cut and were stained with a solution of Toluidine Blue, Azure II, and Methylene Blue. Yolk sacs from 4 embryos of each genotype (KLF2+/+ and KLF2-/-) were used in the morphologic studies for counting cells. Cells were considered irregular if projections extended over more than 25% of the cell circumference. Only cells with visible nuclei and from areas of the yolk sac with good tissue architecture were considered for counting. For electron microscopy, ultrathin sections were cut, mounted on uncoated mesh grids, stained with uranyl acetate and lead citrate, and examined on a JEOL JEM-1230 electron microscope (JEOL, Peabody, MA). Images were acquired using an Olympus M081 microscope equipped with a 20 ×/0.40 brightfield objective lens (Olympus, Tokyo, Japan) and a Cohu 1322 camera (Cohu, San Diego, CA). Images were processed with Arc200 1.0.2 software (Arcturus Engineering, Mountain View, CA).
For the transferase-mediated dUTP (deoxyuridine triphosphate) nick end labeling (TUNEL) assays, individual yolk sacs from E10.5 embryos were dissected in PBS (pH 7.4) and fixed in 10% neutral-buffered formalin (10% commercial formalin in PBS, pH 7.4), and embedded in paraffin. Following genotyping, 5-μm paraffin sections were cut and mounted on slides. The TUNEL assays were performed using an Apoptag Plus Peroxidase in situ Apoptosis Detection kit (Chemicon, Temecula, CA) according to manufacturer's protocol.
Results
KLF2 affects expression of the murine embryonic globin genes but not the adult globin genes
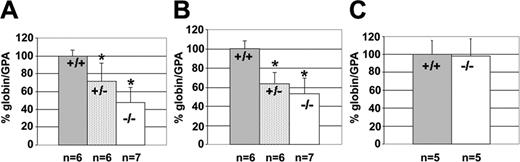
The KLF2 knockout mice used for this study have a deletion of the entire transactivation domain and part of the DNA-binding domain of the KLF2 gene. Homozygous deletion resulted in complete loss of KLF2 expression and lack of any detectable altered KLF2 mRNA.19 We studied the effect of the KLF2 knockout on endogenous β-like globin gene expression. We used quantitative real-time PCR to measure the amount of embryonic Ey- and βh1-globin mRNA in the yolk sac of E10.5 embryos, the site of primitive erythropoiesis at this stage, with amplification of GPA mRNA as an internal control. GPA is an erythroid-specific membrane marker and is abundantly expressed in differentiating primitive erythroid cells, as well as definitive cells in the fetal liver23 . The GPA/GAPDH ratio is the same in E10.5 KLF2-/- and KLF2+/+ embryos (data not shown). This marker was selected to ensure that changes in the amount of globin mRNA were not due to changes in the number of viable red blood cells, because E12.5 embryos can have internal hemorrhaging. Compared with KLF2+/+, we observed a significant reduction of Ey-globin gene expression in both KLF2+/- (value of t = 3.29; degrees of freedom (df) = 10; P < .005) and KLF2-/- (t = 6.81; df = 11; P < .001) embryos (Figure 1A). Similarly, we observed a significant reduction of the embryonic βh1-globin gene expression in both KLF2+/- (t = 6.15; df = 10; P < .001) and KLF2-/- (t = 6.39; df = 11; P < .001) embryos (Figure 1B). The Ey- and βh1-globin mRNA was reduced by approximately 50% in KLF2-/- embryos. There was no significant difference in the expression of the mouse ζ-globin gene, between KLF2+/+ and KLF2-/- embryos at E10.5 (Figure 1C). This indicates that KLF2 is required for normal mouse embryonic β-like globin gene expression but not for embryonic α-like globin gene expression.
Effect of KLF2 on endogenous murine α- and β-like globin gene expression in E10.5 yolk sac.GPA was used as an internal control. The globin/GPA mRNA ratio for the wild type is taken as 100%, and for the other genotypes it is expressed compared with 100%. n represents the number of embryos of each genotype used to determine the mean globin/GPA mRNA ratio. Error bars represent the standard deviation from the mean. *Statistically significant difference of expression in KLF2+/- or KLF2-/- embryos from the wild type; P < .025. (A) Expression of the embryonic Ey-globin gene in KLF2+/+, KLF2+/-, and KLF2-/- embryos; (B) expression of the embryonic βh1-globin gene in KLF2+/+, KLF2+/-, and KLF2-/- embryos; and (C) expression of the embryonic ζ-globin gene in KLF2+/+ and KLF2-/- embryos.
Effect of KLF2 on endogenous murine α- and β-like globin gene expression in E10.5 yolk sac.GPA was used as an internal control. The globin/GPA mRNA ratio for the wild type is taken as 100%, and for the other genotypes it is expressed compared with 100%. n represents the number of embryos of each genotype used to determine the mean globin/GPA mRNA ratio. Error bars represent the standard deviation from the mean. *Statistically significant difference of expression in KLF2+/- or KLF2-/- embryos from the wild type; P < .025. (A) Expression of the embryonic Ey-globin gene in KLF2+/+, KLF2+/-, and KLF2-/- embryos; (B) expression of the embryonic βh1-globin gene in KLF2+/+, KLF2+/-, and KLF2-/- embryos; and (C) expression of the embryonic ζ-globin gene in KLF2+/+ and KLF2-/- embryos.
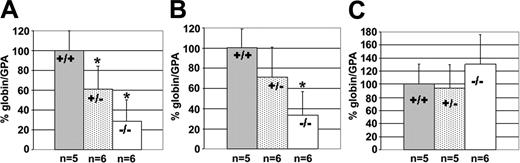
Next, we wanted to determine whether KLF2 affects the embryonic Ey- and βh1-globin and the adult βmaj- and βmin-globin genes at E12.5, when the site of erythropoiesis has shifted, and the mice predominantly express the βmaj- and βmin-globin genes in the fetal liver. Cells expressing the embryonic genes are still present in circulation in the yolk sac. Hence, we measured the expression of the Ey- and βh1-globin genes in the yolk sac of E12.5 embryos and βmaj- and βmin-globin genes in the fetal liver. A single primer and probe set was designed to detect both βmaj and βmin mRNA. We observed a significant decrease in Ey-globin gene expression in the yolk sac in both KLF2+/- (t = 2.38; df = 9; P < .025) and KLF2-/- (t = 4.57; df = 9; P < .001) embryos, compared with the wild type (Figure 2A). There was a significant reduction of the βh1-globin gene expression in KLF2-/- (t = 4.77; df = 9; P < .001) embryos but not in the KLF2+/- animals (Figure 2B). The KLF2-/- embryos expressed approximately 3-fold less mouse embryonic globin mRNA.
Effect of KLF2 on endogenous murine β-like globin gene expression at E12.5. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic Ey-globin mRNA in the yolk sac, (B) embryonic βh1-globin mRNA in the yolk sac, and (C) adult βmaj-plus βmin-globin mRNA in the fetal liver.
Effect of KLF2 on endogenous murine β-like globin gene expression at E12.5. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic Ey-globin mRNA in the yolk sac, (B) embryonic βh1-globin mRNA in the yolk sac, and (C) adult βmaj-plus βmin-globin mRNA in the fetal liver.
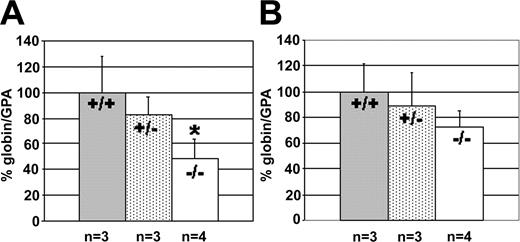
Effect of KLF2 on human β-like globin gene expression in E10.5 yolk sac in β-globin locus YAC transgenic mice. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic ϵ-globin mRNA in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+ embryos; and (B) fetal γ-globin mRNA in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+ embryos.
Effect of KLF2 on human β-like globin gene expression in E10.5 yolk sac in β-globin locus YAC transgenic mice. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic ϵ-globin mRNA in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+ embryos; and (B) fetal γ-globin mRNA in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+ embryos.
There was no significant difference in βmaj- and βmin-globin gene expression in the KLF2+/- and the KLF2-/- embryos from the wild type (Figure 2C). This indicates that KLF2 has a stage-specific role in mouse embryonic but not adult β-like globin gene expression.
KLF2-/- embryos carrying the human β-globin gene locus show reduced expression of the human embryonic ϵ-globin gene but not the adult β-globin gene
Because mice do not have a fetal globin gene, as does the human β-globin gene locus, our next goal was to observe what effect KLF2 has on the developmental regulation of the human β-globin gene locus. We mated transgenic mice carrying a YAC with the entire human β-globin gene locus21 with KLF2+/- mice, to generate KLF2+/- mice carrying more than 140 kbp of the human β-globin gene locus. The 2 parental YAC lines that were bred in these experiments, A85.68 and A20.1, have similar levels and developmental timing of human β-globin gene expression. The human embryonic ϵ- and γ-globin genes are expressed in the yolk sac at E10.5, and predominantly adult β-globin gene is expressed in the fetal liver in these animals at E12.5. Circulating erythroid cells in the yolk sac express the ϵ- and γ-globin genes at E12.5. Male YAC containing KLF2+/- mice were then crossed with female KLF2+/- mice. This ensures that the embryos are not contaminated by adult human β-globin mRNA expressed in the mother. GPA mRNA was quantitated as an internal control. At E10.5, we observed a significant reduction of the human embryonic ϵ-globin expression to about 50% in KLF2-/- (t = 2.8; df = 5; P < .025) animals but not in KLF2+/- embryos, compared with wild type (Figure 3A). This indicates that the role of KLF2 in embryonic β-like globin gene expression is conserved in mouse and man. We did not observe a significant reduction of fetal γ-globin gene expression in either KLF2+/- or KLF2-/- embryos at P values of less than .025, although a modest decrease to 70% γ-globin mRNA was observed in KLF2-/- embryos (significant at P < .05; Figure 3B).
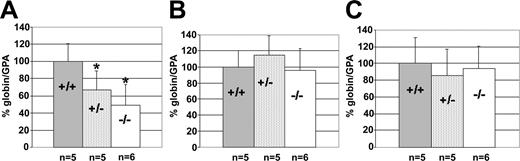
Effect of KLF2 on human β-like globin gene expression at E12.5, in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+embryos. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic ϵ-globin mRNA in the yolk sac, (B) fetal γ-globin mRNA in the yolk sac, and (C) adult β-globin mRNA in the fetal liver.
Effect of KLF2 on human β-like globin gene expression at E12.5, in KLF2+/+globin+, KLF2+/-globin+, and KLF2-/-globin+embryos. The percentage of globin/GPA mRNA ratio was determined, and statistical analyses were performed as described in the legend of Figure 1. (A) Embryonic ϵ-globin mRNA in the yolk sac, (B) fetal γ-globin mRNA in the yolk sac, and (C) adult β-globin mRNA in the fetal liver.
We measured the expression of the ϵ- and γ-globin genes in the yolk sac of E12.5 embryos and the human adult β-globin gene in the E12.5 fetal liver. We observed a significant reduction of ϵ-globin gene expression in both KLF2+/- (t = 2.81; df = 8; P < .025) and KLF2-/- (t = 3.49; df = 9; P < .005) embryos, compared with the wild type (Figure 4A). γ-Globin gene expression did not show any significant change in either KLF2+/- or KLF2-/- embryos (Figure 4B). Also, there was no significant variation in the β-globin gene expression in KLF2-/- and wild-type embryos in the E12.5 fetal liver (Figure 4C). Thus, KLF2 specifically affects the human embryonic but not adult β-like globin genes, analogous to its role in murine β-globin gene regulation.
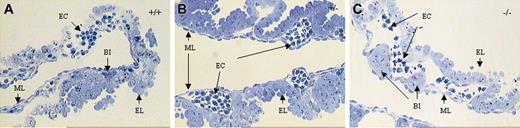
Representative photographs of 1-μm thick plastic sections of E10.5 yolk sacs. (A) KLF2+/+, (B) KLF2+/-, and (C) KLF2-/- embryos, stained with a solution of Toluidine Blue, Azure II, and Methylene Blue. EC indicates erythroid cells; BI, blood island; EL, epithelial layer; ML, mesothelial layer. Photographs were taken at 200 × magnification.
Representative photographs of 1-μm thick plastic sections of E10.5 yolk sacs. (A) KLF2+/+, (B) KLF2+/-, and (C) KLF2-/- embryos, stained with a solution of Toluidine Blue, Azure II, and Methylene Blue. EC indicates erythroid cells; BI, blood island; EL, epithelial layer; ML, mesothelial layer. Photographs were taken at 200 × magnification.
Abnormal morphology of mature erythroid cells in yolk sac vessels of KLF2-/- mice
EKLF is required for normal definitive erythroid development. In vitro assays in EKLF-/- animals show a normal number of colony-forming erythroid units (CFU-Es) and granulocytes/macrophages are present in the fetal liver, indicating that the progenitor-cell compartment is not affected.7,8 In vivo, however, mature definitive erythroid cells are phenotypically abnormal at E15.0.8 Kuo et al18 found that in vitro culture of E11.5 KLF2-/- fetal liver cells produced normal numbers of definitive erythroid and myelomonocytic cells, as well as megakaryocytes. In the present investigation, we studied the morphology of primitive erythroid cells in the yolk sac in vivo.
Representative photographs of plastic sections of E10.5 yolk sacs of KLF2+/+, KLF2+/-, and KLF2-/- embryos are shown in Figure 5A-C. Although KLF2+/+ animals show mainly round or elliptically shaped, nucleated primitive erythroid cells, we observed primarily irregular mature primitive erythroid cells in KLF2-/- animals, with pseudopodia-like appendages (Figure 5C). The KLF2+/- animals have some round or elliptically-shaped and some irregular-shaped erythroid cells, but abnormality was less evident compared with the KLF2-/- embryos. Yolk sacs from 4 individual KLF2+/+ and KLF2-/- E10.5 embryos for each genotype were studied more thoroughly. Quantitatively, in examining more than 500 cells for each genotype from yolk sac blood vessels, 68.9% (± 9.0%) of the cells were irregularly shaped in the KLF2-/- embryos, compared with 31.7% (± 11.7%) in the KLF2+/+, a difference significant at P < .001 (t = 5.62; df = 6). Qualitatively, the irregularly shaped cells in the KLF2-/- animals look far more abnormal than do those in either of the other 2 genotypes.
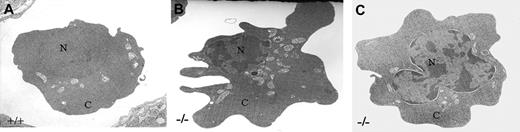
Representative electron micrographs of E10.5 yolk sacs of KLF2+/+ and KLF2-/- embryos are shown in Figure 6A-C. Electron microscopic examination of tissues similar to those studied by light microscopy confirmed that the vast majority of mature primitive erythrocytes are very irregular in shape with numerous attenuated cytoplasmic processes. The wild-type nuclei are regular with a defined nuclear membrane (Figure 6A). The KLF2-/- animals have more heterochromatic nuclei and abnormal morphology of the nuclear membrane, including enlarged perinuclear space and partial dissolution of the nuclear envelope (Figure 6B-C). Additionally, the nuclei of these cells frequently have a number of bulging projections reminiscent of blebbing, a morphologic feature consistent with apoptosis (Figure 6C).
We performed TUNEL assays to determine whether the primitive erythroid cells undergo apoptosis in the KLF2-/- embryos. Compared with wild type (5.1% ± 3.6%), the KLF2-/- embryos (27.2% ± 8.73%) show a significantly higher percentage of cell death by apoptosis (t = 5.2; df = 5; P < .005). Taken together, these experiments suggest that KLF2 is required for the final stages of maturation and/or the stability of mature primitive red blood cells.
Discussion
The KLFs belong to a family of zinc finger transcription factors that have been implicated in various cellular processes through gene regulation. EKLF, the first protein to be identified in this group, has a central role in the developmental regulation of the adult β-globin gene and is essential for the maturation and/or stability of definitive erythroid cells. Phylogenetically, KLF2 and KLF4 are the most similar to EKLF and are expressed in chicken and mouse embryos early in development.5,12,16 However, whereas KLF2-/- mouse embryos show anemia, hemorrhaging, and other phenotypes related to blood disorders, KLF4-/- mice show a defect in skin barrier function and no apparent blood-related pathology.18,19,24 These observations led us to investigate the role of KLF2 in early globin gene regulation and in primitive erythropoiesis, in knockout mouse models.
Electron micrographs of ultrathin sections of E10.5 yolk sacs. (A) KLF2+/+ and (B-C) KLF2-/- embryos, stained with uranyl acetate and lead citrate. N indicates nucleus; C, cytoplasm. Photographs were taken at 21 000 × magnification.
Electron micrographs of ultrathin sections of E10.5 yolk sacs. (A) KLF2+/+ and (B-C) KLF2-/- embryos, stained with uranyl acetate and lead citrate. N indicates nucleus; C, cytoplasm. Photographs were taken at 21 000 × magnification.
Our results show that KLF2 positively regulates the human (ϵ) and murine (Ey and βh1) embryonic globin genes at both E10.5 and E12.5, in the yolk sac, which is the site of primitive erythropoiesis. KLF2 does not affect other late-stage erythroid differentiation markers such as GPA or the embryonic ζ-globin genes in the yolk sac. KLF2 does not affect expression of the adult human β-globin gene in the fetal liver. The mouse adult (βmaj and βmin) globin genes are also not affected by the deletion of the KLF2 gene. There is a small decrease in the human γ-globin gene expression at E10.5 in the yolk sac in the KLF2-/- mouse compared with the wild type, which is significant at P < .05, but not at P < .025. This could indicate some role for KLF2 in expression of the γ-globin gene, but it is smaller than the role for KLF2 in embryonic β-like globin gene expression. Clearly KLF2 is acting in a stage-specific manner. The apparently larger decrease in the embryonic globin gene expression at E12.5 than at E10.5 in the KLF2-/- embryos, could be a result of selective apoptotic cell death of primitive but not definitive red cells.
Murine Ey- and βh1-globin gene expression is also lower in KLF2+/- compared with wild-type mice, indicating that the effect is probably due to haploinsufficiency. This is similar to EKLF+/- fetal livers, which display a decrease in transcription of the adult β-globin gene.11 However, expression of the embryonic β-like globin genes is not completely eliminated in the KLF2-/- mice. This indicates that additional factor(s) are also involved in the developmental regulation of the embryonic globin genes.
There are some similarities between the EKLF-/- and KLF2-/- mice, but during definitive and primitive erythropoiesis, respectively. At E10.5, yolk sac and blood of EKLF-/- animals have normal nucleated erythrocytes, indicating that primitive erythropoiesis is unaffected. Hematopoiesis switches to the fetal liver (definitive erythropoiesis) at around E11, and EKLF-/- embryos develop progressive anemia from E11 onward.8 Although the fetal liver contains a normal number of erythroid precursors, enucleated cells lack their full complement of hemoglobin, resulting in anemia, erythroblastosis, growth retardation, and embryonic death around E14 to E16. This indicated that EKLF is essential for the final stages in maturation and/or stability of erythroid cells, and it is unlikely to be important for early events of red-cell differentiation.7,8 Similarly, the irregularly shaped primitive red cells and accompanying apoptotic cell death that we observed in the E10.5 yolk sac seem to result from an abnormal event late in differentiation, possibly because of defects in the final stages of maturation and/or in the reduced stability of the primitive red cells. These results are consistent with the apparent reduction of blood cells in the yolk sac in E12.5 KLF2-/- mice.18,19 The aberrant cellular morphology of erythroid cells in E10.5 KLF2-/- yolk sac tissue sections was not evident in E11.5 blood smears,19 which provide less morphologic detail.
Primitive erythropoiesis is not completely abolished in KLF2-/- embryos because they survive until about E12.5. The effect of KLF2 on globin gene expression does not appear to be due solely to a defect in cellular maturation, because other late-stage differentiation markers such as ζ-globin and GPA mRNA are not reduced in KLF2-/- embryos. However, there is a strong possibility that KLF2 affects the expression of other erythroid genes in mouse erythroid cells. A reduction in Ey- and βh1-globin alone in the red cells would not necessarily be expected to result in the observed irregular projections, even though some morphologic changes do occur in peripheral red blood cells from people with β-thalassemia minor. KLF2 may also play a role in definitive erythropoiesis,19 but, in light of this new evidence for its role in primitive erythropoiesis, it is unclear whether this is a direct effect.
It has been proposed that KLF factors regulate the β-like globin genes via the 9-bp consensus sequences that includes a CACCC motif as core, located in the promoters of the genes.25 There is strong evidence that EKLF has more affinity for the proximal CACCC element in the β-globin gene but binds to both the proximal and distal CACCC elements for maximal stimulation.26 However, experimental evidence suggests that the factors do not control these genes solely by recognizing the CACCC element but on the whole promoter context of the genes.7,27,28 In transient transfection assays, it was demonstrated that EKLF could activate a β-globin gene promoter carrying a γ-globin CACCC box at a level comparable to that of the wild-type β-globin promoter, but failed to activate a γ-globin promoter carrying the β-globin CACCC box.27 Upon examination of the entire 9-bp sequence of the CACCC elements in the mouse and human β-like globin genes, it becomes apparent that KLF2 does not act on the genes based solely on recognition of the CACCC promoter elements. The sequence of the 9-bp element (CTCCACCCA) matches completely between the mouse Ey- and the human γ-globin gene, but the mouse Ey-globin gene appears much more under the control of KLF2 than does the human γ-globin gene.
In conclusion, KLF2 has a role in embryonic β-like globin gene expression and the maturation and/or stability of primitive erythroid cells. These roles mirror those of EKLF in adult β-globin gene regulation and definitive erythropoiesis. It is possible that the roles of EKLF and KLF2 may partially overlap, particularly in controlling human fetal γ-globin gene expression.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-02-0674.
Supported by the National Institutes of Health (NIH) (grants HL60080 and DK62154) (J.A.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mohua Basu and Jingmei Song for excellent technical assistance. We thank Dr Gordon D. Ginder for critical evaluation of this work and valuable suggestions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal