Abstract
Prostaglandins, a family of lipidic molecules released during inflammation, display immunomodulatory properties in several models. One use includes exposure of monocyte-derived dendritic cells (DCs) to a cocktail of cytokines that contains prostaglandin E2 (PGE2) for purposes of maturation; such cells are currently being used for cancer immunotherapy trials. Our analysis of the transcription profile of DCs matured in the presence of tumor necrosis factor α (TNFα) and PGE2 revealed a strong up-regulation of indoleamine 2-3 dioxygenase (IDO), an enzyme involved in tryptophan catabolism and implicated in both maternal and T-cell tolerance. Using quantitative assays to monitor levels of IDO mRNA, protein expression, and enzyme activity, we report that PGE2 induces mRNA expression of IDO; however, a second signal through TNF receptor (TNF-R) or a Toll-like receptor (TLR) is necessary to activate the enzyme. Interestingly, use of TNFα, lipopolysaccharide, or Staphylococcus aureus Cowan I strain (SAC) alone does not induce IDO. The effect of PGE2 is mediated by activation of adenylate cyclase via the Gs-protein-coupled receptor E prostanoid-2 (EP2). A better understanding of these regulatory mechanisms and the crosstalk between TNF-R/TLR and EP2 signaling pathways will provide insight into the regulation of T-cell activation by DCs and may help to improve existing immunotherapy protocols.
Introduction
Prostaglandin E2 (PGE2) is a catabolite of arachidonic acid and is generated by the sequential activity of cyclo-oxygenase (COX) and prostaglandin E synthase.1 Produced during inflammation, PGE2 is believed to act as a counter-inflammatory agent, modulating inflammatory responses and helping to restore tissue homeostasis. For example, PGE2 has been reported to suppress T-cell proliferation,2,3 inhibit macrophage and dendritic-cell cytokine production (eg, interleukin 12 [IL-12] and tumor necrosis factor α [TNFα]4,5 ), and modulate antigen presentation by down-regulating expression of major histocompatability complex (MHC) II.6 Additionally, PGE2 may skew CD4+ T cells toward a T helper cell type 2 (TH2) phenotype7,8 and B cells toward immunoglobulin E (IgE) production.5 In other studies, PGE2 has been shown to play a role in T-cell development9 and may be an inhibitor of apoptosis in double-positive thymocytes.10 Consistent with these reports, expression of various prostaglandin biosynthetic enzymes and receptors has been detected in the thymus.11
Dendritic cells (DCs) are considered to be the only antigen-presenting cell (APC) capable of priming naive T cells, and they are also potent stimulators of recall responses.12 Briefly, DCs exist in the periphery as immature cells where they serve as “sentinels,” responsible for capturing antigen. Upon maturation, DCs migrate to the draining lymphoid organs, where they may initiate immune responses. This ability to traffic out of peripheral tissue with captured antigen and enter the afferent lymph is unique to the DCs, making them the appropriate carrier of tissue-restricted antigen to lymphoid organs for the initiation of immunity.12 Their role in priming T cells has also prompted much interest in discovering strategies to efficiently use DCs carrying tumor antigen for adoptive transfer and immunization of tumor-reactive T cells. Our understanding of DC biology, however, has not resulted in over-whelming success; few DC-based studies have reported an effect greater than the 10% rate achieved by Coley.13-15 Given that DCs are capable of mediating both T-cell priming as well as T-cell inactivation (tolerance), a deeper understanding of how to counter DC tolerogenic programs may advance our efforts to achieve tumor immunity. One area of active investigation has been the definition of maturation programs for DCs, in an attempt to obtain large numbers of stimulatory DCs for purposes of immunotherapy.
On the basis of careful studies analyzing T-cell stimulatory capacity, cell yield, DC migration studies, and safety concerns, the majority of DC-based trials use Good Manufacturing Practice (GMP)-grade cytokine cocktails (eg, TNFα, IL-1β, IL-6, and PGE2) for the ex vivo maturation of monocyte-derived immature DCs. The principal reason for the addition of PGE2 is based on the observation that it regulates the migratory capacity of DCs by sensitizing CC chemokine receptor 7 (CCR7) to its ligands, CC chemokine ligand 19 (CCL19) and CCL21.16,17 Additionally, PGE2 enhances the effect of the maturation cocktail, making it possible to use 20 to 100 times less TNFα.18 In further support of its use, DCs matured in the presence of PGE2 show no defect in the generation of phenotypically mature DCs, the priming of naive allogeneic T cells, or the stimulation of antigen-specific CD4+ and CD8+ T-cell responses.19,20 In prior studies, we have used TNFα and PGE2 to mature DCs. Such cells possess the ability to both prime and tolerize CD8+ T cells, depending on the immune context and the route by which antigen is processed and presented.21
Indoleamine 2,3-dioxygenase (IDO) is an enzyme involved in tryptophan catabolism. Its role in antimicrobial resistance is well described; by actively depleting tryptophan, essential for the growth of microorganisms, both within the infected cell and in the surrounding milieu, IDO serves to suppress growth of invasive bacteria. More recently, it has been established that IDO regulates maternal tolerance and possibly more general aspects of T-cell tolerance.22-26 IDO expression has been reported in placental trophoblasts and interferon γ (IFNγ)-activated APCs (including macrophages and DCs), reflecting its counter-inflammatory role. An exciting advance for this field has been the discovery that IDO is initially expressed as a proenzyme. Although little is known regarding the biochemical signals responsible for transcriptional activation of the IDO gene, it has been shown that reverse signaling via B7 (CD80/CD86), as well as engagement of CD200R, serve to regulate IDO enzyme activity.27-29
Herein, we characterize the mechanism by which IDO is up-regulated in monocyte-derived DCs. We report that that PGE2 induces mRNA expression of IDO; however, the enzyme remains inactive. Only in response to a second signal via TNF receptor (TNF-R) or Toll-like receptor (TLR) do we observe bioactive IDO. Surprisingly, use of TNFα, lipopolysaccharide, or Staphylococcus aureus Cowan I strain (SAC) alone does not induce IDO. This study offers new insights into the immune-modulatory effects of PGE2 as well as provides a better understanding as to how IDO expression is achieved in inflammatory situations. Importantly, this work will impact the design of future DC-based immunotherapy trials.
Materials and methods
Human subject materials
Human blood components were obtained from healthy donors (Etablissement Français du Sang [EFS], Rungis, France). Materials were stripped of patient identifiers and shipped to Institut Pasteur in accordance with institutional policy (no. HS2003-5720) and the tenets of the Helsinki protocol.
Reagents
TNFα (Endogen, Boston, MA) was used at a concentration of 100 ng/mL and PGE2 (Sigma, St Louis, MO) at 5 μM, unless otherwise stated. Lipopolysaccharide (LPS), serotype 055:B5 (Sigma) was sonicated and used at 50 ng/mL, SAC was used at 0.0025% wt/vol (Pansorbin, Calbiochem-Behring, La Jolla, CA). l-tryptophan, l-kynurenine, and forskolin (Sigma) were used as described in “Results”; prostaglandin E receptor EP1 subtype (EP1), EP3, and EP4 agonists (L-335677, L-826266, and L-161982, respectively) were provided by Merck Frost & Co (Québec, Canada) and used at a concentration of 50 μM; butaprost and 19(R)-OH PGE2 (EP2 agonists), sulprostone (EP1>>3 agonist) were obtained from Cayman Chemicals, Ann Arbor, MI, and used at 0.5 to 250 μM. The adenylate cyclase inhibitor SQ22536 (Biomol International, LP, Plymouth Meeting, PA) was titrated, and the optimal concentration to inhibit forskolin was found to be 1 mM. H-89 (Sigma) was used at 10 to 50 μM for the inhibition of protein kinase A (PKA).
Isolation and preparation of dendritic cells
Monocyte-derived DCs were prepared as described. Briefly, buffy coats were obtained from healthy donors, and peripheral blood mononuclear cells (PBMCs) were isolated by sedimentation over Ficoll-Hypaque (Pharmacia Biotech, Piscataway, NJ). CD14-enriched and CD14-depleted fractions were prepared using CD14 Miltenyi microbeads followed by magnetic cell sorting according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Immature DCs were prepared from the CD14+ fraction by culturing cells in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF; Berlix, Seattle, WA) and IL-4 (R&D Systems, Minneapolis, MN) for 7 days. GM-CSF (1000 U/mL) and IL-4 (500 U/mL) were added to the cultures on days 0, 2, and 4. To generate mature DCs, the cultures were transferred to fresh wells on day 6 or 7, and the indicated maturation stimulus was added for an additional 1 to 2 days. At days 6 to 7, greater than 95% of the cells were CD14-CD83-HLA-DRlo DCs. After maturation, on days 8 to 9, 70% to 95% of the cells were of the mature CD14-CD83+HLA-DRhi phenotype (data not shown).
Quantitative analysis of IDO mRNA expression
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA), and cDNA was synthesized from 1 to 2 μg RNA using oligo-dT (Roche, Indianapolis, IN) and Superscript reverse transcriptase (Invitrogen) according to manufacturers' instructions. IDO-specific mRNA is quantified relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or TATA box binding protein (TBP) using the following primers: IDO forward, 5′-AGAGTCAAATCCCTCAGTCC-3′; IDO reverse, 5′-AAATCAGTGCCTCCAGTTCC-3′; GAPDH forward, 5′-ACTCCACGACGTACTCAGCG-3′; GAPDH reverse, 5′-GGTCGGAGTCAACGGATTTG-3′; TBP forward, 5′-GCACAGGAGCCAAGAGTGAA-3′; and TBP reverse, 5′-TCACAGCTCCCCACCATATT-3′. Primers for EP receptors are described in Kamphuis et al.30 Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using the SYBR Green JumpStart Taq ReadyMix (Sigma) according to the manufacturer's instructions. The reactions were run on a PTC200 equipped with a Chromo4 detector (MJ Research, Boston, MA). The analyses were performed with the Opticon Monitor software version 2.03 (MJ Research). All the measures were performed in duplicate and validated when the difference in threshold cycle (Ct) between the 2 measures was less than 0.3. Amplification and dissociation curves of increasing amounts of total PBMC cDNA allowed validation of our assay; dissociation curves displayed a single peak, ruling out the presence of primer dimers or parasitic products (Supplemental Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). The ratio of gene of interest-housekeeping gene was calculated according to the formula: ratio = 2-dCt (dCT = mean Ct gene - mean Ct housekeeping). GAPDH and TBP were used to normalize for IDO and EP-receptor mRNA expression, respectively.
Detection of IDO protein expression
Cell lysates were prepared from 106 DCs using RIPA buffer (20 mM Tris [tris(hydroxymethyl)aminomethane] pH 7.5, 150 mM NaCl, 10% glycerol, 1% Nonidet P-40 [[Octylphenoxy]polyethoxyethanol], Complete [Roche; protease inhibitor cocktail]). One third of the total protein lysate was separated on 12% or 14% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to polyvinylidene difluoride (PVDF) membranes, protein loading was monitored using Ponceau Red staining. IDO protein was detected using a rabbit polyclonal antibody (Ab) preparation (generous gift of David Munn, Medical College of Georgia, Augusta, GA) and anti-rabbit IgG-horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA), and visualized by chemiluminescence (ECL; Amersham, Piscataway, NJ).
Determination of IDO enzymatic activity
To monitor enzyme activity, DCs were washed and resuspended in Hanks Buffer (HBSS) containing 100 μM tryptophan (Life Technologies, Grand Island, NY) and incubated for 4 hours. Supernatants were harvested and assayed for the presence of kynurenine, the first stable catabolite downstream of IDO. Kynurenine was detected by either high-pressure liquid chromatography (HPLC) or using a modified spectophotometric assay. HPLC was performed according to Yong and Lau31 with minor modifications. Briefly, 40 μL clarified sample was injected into an Amersham reverse phase C2/C18 column and eluted with KH2PO4 buffer (0.01 M KH2PO4 and 0.15 mM EDTA [ethylenediaminetetraacetic acid], pH 5.0) containing 10% methanol at a flow rate of 1.0 mL/min. The spectrophotometer was set at 254 nm to detect both kynurenine and tryptophan. Retention time was determined empirically using standard solutions of kynurenine and tryptophan. IDO activity is reported as the concentration of kynurenine produced. Alternatively, kynurenine were measured spectrophotometrically.32,33 The amount of 50 μL of 30% trichloroacetic acid was added to 100 μL culture supernatant, vortexed, and centrifuged at 8000g (10 000 rpm) for 5 minutes. Volume (75 μL) of the supernatant was then added to an equal volume of Ehrlich reagent (100 mg P-dimethylbenzaldehyde, 5 mL glacial acetic acid) in a microtiter plate well (96-well format). Optic density was measured at 492 nm, using a Multiskan MS (Labsystems, Helsinki, Finland) microplate reader. A standard curve of defined kynurenine concentration (0-100 μM) permitted analysis of unknowns.
Results
IDO expression and activity are up-regulated during DC maturation
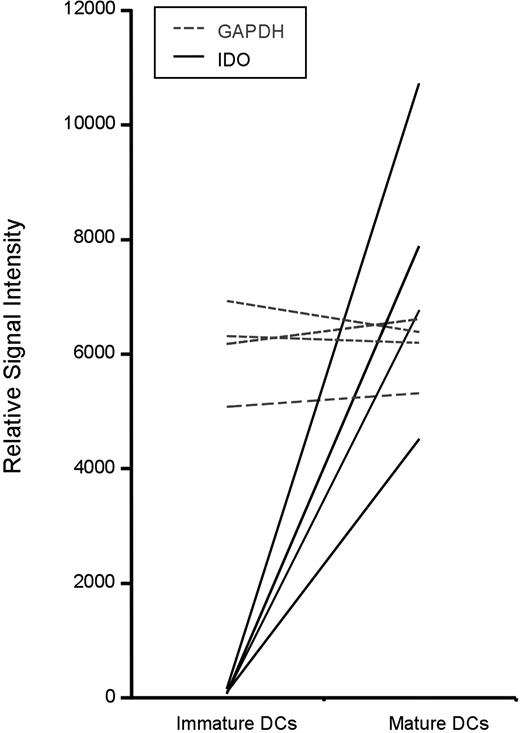
The phenotypic and functional changes that occur during DC maturation are critical for generating MHC/peptide complexes and engaging T cells; however, the molecular definition of distinct maturation programs has only recently been considered. As previously reported by others,34 we analyzed the transcriptional profile of monocyte-derived DCs at the various stages of differentiation using Affymetrix gene arrays (R.S.L. and M.L.A., unpublished data collected 2003-2004). Strikingly, we observed a greater than 100-fold increase in expression of IDO as a result of DC maturation. This has been reproduced in 4 of 4 donors, and the relative signal intensity of IDO mRNA expression is shown, using GAPDH expression as an internal reference (Figure 1).
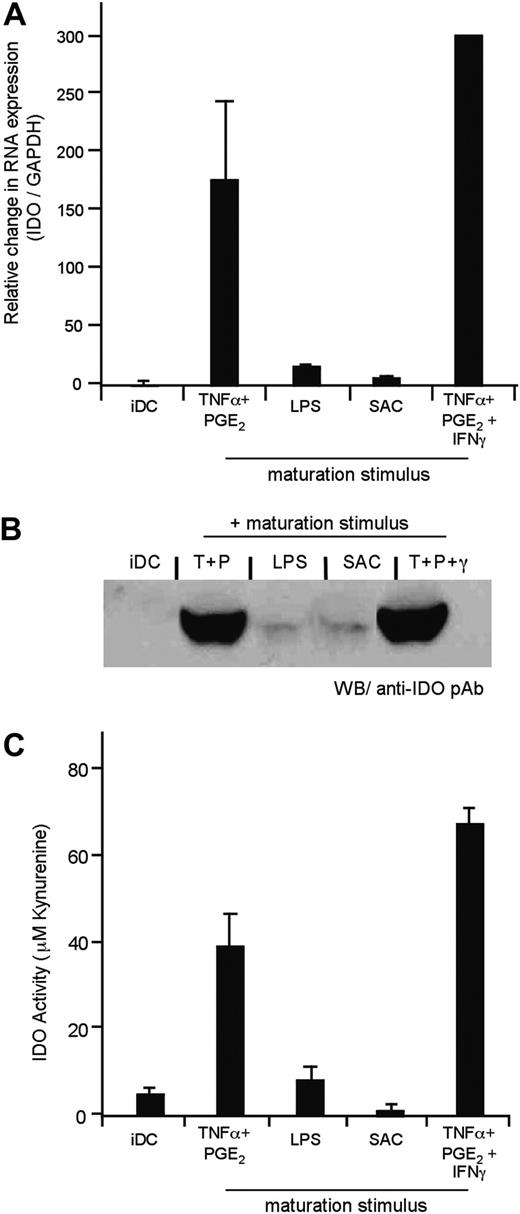
On the basis of IDO's proposed role in immune tolerance, we evaluated the effect of different DC maturation stimuli on IDO expression. Despite some published data in this area,35-37 a thorough assessment of the expression and activity of IDO as influenced by DC maturation had yet to be performed. We first established assays to monitor IDO mRNA expression using real-time quantitative RT-PCR (q-PCR), and Western blot. RNA was extracted from iDCs exposed to distinct maturation stimuli, and IDO expression was quantified as described in “Materials and methods.” Consistent with our gene array studies, iDCs matured with TNFα and PGE2 up-regulated IDO mRNA (Figure 2A). In contrast, iDCs exposed to LPS or SAC did not express measurable levels of IDO mRNA. Evaluation of cell lysates using an IDO rabbit pAb (generously provided by Dr David Munn, University of Georgia) showed good correlation between protein expression and the mRNA levels (Figure 2B).
In addition, it was important to monitor IDO enzymatic activity, because there has been a reported discrepancy between IDO expression and activity, suggesting possible posttranslational regulation of the enzyme.35,38 IDO activity may be assayed by quantifying tryptophan catabolism as well as the generation of kynurenine (the first catabolite in the metabolic pathway). After 36 to 48 hours of exposure to the distinct maturation stimuli, the DCs were cultured in HBSS containing 100 μM tryptophan. After 4 hours, the concentration of tryptophan and kynurenine was determined by HPLC. As shown, the 2 products can be easily separated, and the area under the curve correlates with the amount of the respective analyte (Supplemental Figure S2A). Notably, we observed robust enzyme activity in DCs matured with TNFα and PGE2, but no evidence of tryptophan catabolism was detected in iDCs or in LPS- or SAC-matured DCs (Figure 2C). In our initial studies, IFNγ-exposed DCs served as a positive control for IDO expression and activity. DCs matured from multiple individuals permitted evaluation of donor variability (Table 1).
IDO expression and activity
. | IDO mRNA (qPCR)* . | . | . | . | IDO activity (μM kynurenine, HPLC) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions . | n . | Average . | Range . | SEM . | n . | Average . | Range . | SEM . | ||||||
| iDC | 7 | 0.017 | 0.09-0.06 | 0.007 | 6 | 4.3 | 2.8-7.7 | 0.8 | ||||||
| TNFα + PGE2 | 7 | 3.3 | 1.01-8.17 | 1.01 | 6 | 38 | 19.3-68.9 | 7.6 | ||||||
| LPS | 4 | 0.24 | 0.09-0.39 | 0.06 | 3 | 7.6 | 2.8-14 | 3.3 | ||||||
| SAC | 3 | 0.05 | 0-0.13 | 0.04 | 1 | 4.3 | N/A | N/A | ||||||
| TNFα + PGE2 + IFN-γ | 1 | 5.1 | N/A | N/A | 3 | 66.5 | 60.4-74 | 4.0 | ||||||
. | IDO mRNA (qPCR)* . | . | . | . | IDO activity (μM kynurenine, HPLC) . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions . | n . | Average . | Range . | SEM . | n . | Average . | Range . | SEM . | ||||||
| iDC | 7 | 0.017 | 0.09-0.06 | 0.007 | 6 | 4.3 | 2.8-7.7 | 0.8 | ||||||
| TNFα + PGE2 | 7 | 3.3 | 1.01-8.17 | 1.01 | 6 | 38 | 19.3-68.9 | 7.6 | ||||||
| LPS | 4 | 0.24 | 0.09-0.39 | 0.06 | 3 | 7.6 | 2.8-14 | 3.3 | ||||||
| SAC | 3 | 0.05 | 0-0.13 | 0.04 | 1 | 4.3 | N/A | N/A | ||||||
| TNFα + PGE2 + IFN-γ | 1 | 5.1 | N/A | N/A | 3 | 66.5 | 60.4-74 | 4.0 | ||||||
N/A indicates not applicable.
Reported as a ratio of relative mRNA expression IDO/GAPDH. Non-normalized data are presented
Dendritic-cell maturation induces IDO expression. Extracted data from Affymetrix gene array studies (U133A chips) is shown for the 4 experiments. Immature dendritic cells were differentiated from monocyte precursors using GM-CSF and IL-4. These cells were then exposed to TNFα and PGE2 for 36 hours to generate mature dendritic cells. The raw signal intensity reflects the relative expression of IDO (solid line) and GAPDH (dotted line). Each line indicates an independent donor.
Dendritic-cell maturation induces IDO expression. Extracted data from Affymetrix gene array studies (U133A chips) is shown for the 4 experiments. Immature dendritic cells were differentiated from monocyte precursors using GM-CSF and IL-4. These cells were then exposed to TNFα and PGE2 for 36 hours to generate mature dendritic cells. The raw signal intensity reflects the relative expression of IDO (solid line) and GAPDH (dotted line). Each line indicates an independent donor.
Because of our interest in monitoring IDO functional activity in several conditions for DC stimulation, we took advantage of a medium-throughput (96-well assay) colorimetric assay for monitoring kynurenine.33 To validate this approach, we established a standard curve for quantifying kynurenine and compared the experimental values obtained using the colorimetric assay with those from the HPLC analysis. A strong correlation was observed (Supplemental Figure S2B-D), thus allowing us to use the colorimetric assay for monitoring IDO activity.
Prostaglandin E2 is responsible for the expression of IDO mRNA
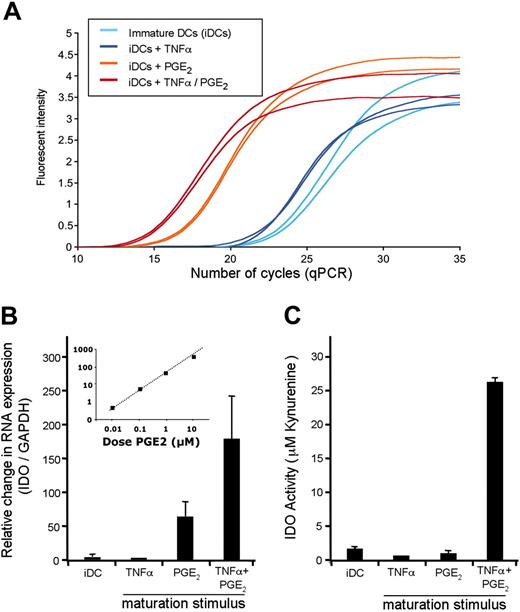
In an attempt to define the stimuli responsible for IDO expression, we revealed a surprising result. Similar to treatment of iDCs with LPS or SAC, when used alone TNFα did not up-regulate IDO expression. Instead, it was exposure of PGE2 that accounted for expression of IDO mRNA (Figure 3A-B). Although we observed a 50- to 200-fold increase in transcription of IDO, PGE2-treated DCs lacked measurable IDO activity (Figure 3C).
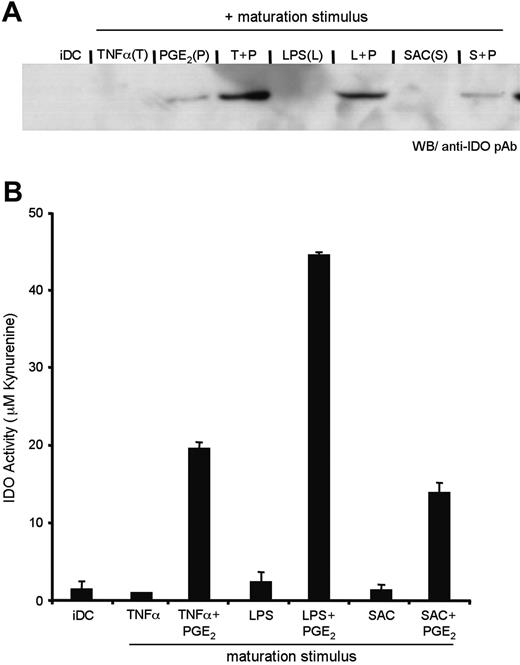
This finding suggests that, although PGE2 induces transcription of the IDO gene, a second signal, such as exposure to TNFα, is required to achieve active IDO enzyme. Similar to TNF-R engagement, TLR ligation also induced IDO activity when used in combination with PGE2 (Figure 4A-B). Characterization of the pAb used suggests that it may be selective for active enzyme (data not shown); consequently, we are unable to determine whether TNF-R/TLR regulates a transcriptional or posttranscriptional event. What is clear from our data, based on Figures 2 to 4, is that there exists a two-step regulation of IDO activity during DC maturation.
The maturation stimulus used influences IDO expression and activity. Human monocyte-derived iDCs were cultured in media alone or exposed to distinct maturation stimuli as indicated. (A) The relative expression levels of IDO were measured using quantitative RT-PCR. IDO expression is represented as a ratio of IDO/GAPDH as compared with iDCs (set to a value of 1.0 to normalize the data). Each bar corresponds to the mean of all donors assayed (n = 1-7). Error bars indicate standard error of the mean (SEM). Non-normalized data are reported in Table 1. (B) IDO protein was detected by Western blot in cell extracts using a polyclonal rabbit anti-human IDO Ab. The cell number from which the protein was derived was normalized prior to loading the gel; and Ponceau Red staining of the membrane confirmed that equivalent protein content was being analyzed (data not shown). (C) Following the different culturing conditions, DCs were washed well and incubated for 4 hours in HBSS containing 100 μM tryptophan. Supernatants were harvested, and the concentration of kynurenine was determined. The mean concentration of kynurenine, as measured by HPLC, is represented (n = 1-6). Error bars indicate SEM. Numeric values and the range observed in different donors are reported in Table 1.
The maturation stimulus used influences IDO expression and activity. Human monocyte-derived iDCs were cultured in media alone or exposed to distinct maturation stimuli as indicated. (A) The relative expression levels of IDO were measured using quantitative RT-PCR. IDO expression is represented as a ratio of IDO/GAPDH as compared with iDCs (set to a value of 1.0 to normalize the data). Each bar corresponds to the mean of all donors assayed (n = 1-7). Error bars indicate standard error of the mean (SEM). Non-normalized data are reported in Table 1. (B) IDO protein was detected by Western blot in cell extracts using a polyclonal rabbit anti-human IDO Ab. The cell number from which the protein was derived was normalized prior to loading the gel; and Ponceau Red staining of the membrane confirmed that equivalent protein content was being analyzed (data not shown). (C) Following the different culturing conditions, DCs were washed well and incubated for 4 hours in HBSS containing 100 μM tryptophan. Supernatants were harvested, and the concentration of kynurenine was determined. The mean concentration of kynurenine, as measured by HPLC, is represented (n = 1-6). Error bars indicate SEM. Numeric values and the range observed in different donors are reported in Table 1.
PGE2 triggers the transcription of IDO mRNA. (A-B) IDO mRNA expression in DCs stimulated with TNFα, PGE2, or a combination of both stimuli was monitored by qRT-PCR. The PCR amplification curves from a representative experiment, performed in duplicate, are displayed (A). The same data are reported as a ratio of IDO/GAPDH, again normalized to expression in iDCs (set to a value of 1.0). (B) The inset represents the level of IDO mRNA as a function of the dose of PGE2. (C) DCs exposed to the conditions described above were monitored for their IDO activity. Kynurenine production was measured using a colorimetric assay; known concentrations were used for the establishment of a standard curve (Supplemental Figure S2B). Values are the mean of triplicate wells, and error bars indicate standard deviation. Data in Figure 3 are representative of 4 experiments.
PGE2 triggers the transcription of IDO mRNA. (A-B) IDO mRNA expression in DCs stimulated with TNFα, PGE2, or a combination of both stimuli was monitored by qRT-PCR. The PCR amplification curves from a representative experiment, performed in duplicate, are displayed (A). The same data are reported as a ratio of IDO/GAPDH, again normalized to expression in iDCs (set to a value of 1.0). (B) The inset represents the level of IDO mRNA as a function of the dose of PGE2. (C) DCs exposed to the conditions described above were monitored for their IDO activity. Kynurenine production was measured using a colorimetric assay; known concentrations were used for the establishment of a standard curve (Supplemental Figure S2B). Values are the mean of triplicate wells, and error bars indicate standard deviation. Data in Figure 3 are representative of 4 experiments.
Signaling via EP2 triggers IDO expression
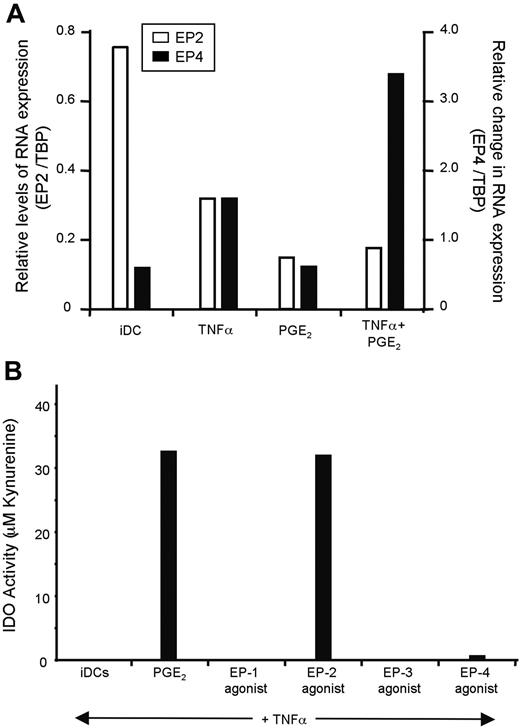
Eight human prostanoid receptors have been described, of which 4 bind PGE2 with high affinity (dissociation constant (Kd) in the low-nM range). Of these 4 receptors, EP1 and EP3 are coupled to inhibitory G proteins; whereas EP2 and EP4 signal via stimulatory G proteins. On the basis of our microarray data and published studies,39 only EP2 and EP4 are expressed by monocyte-derived DCs (data not shown). We validated these findings in our culture system using quantitative RT-PCR. As shown, iDCs express higher levels of EP2 and low levels of EP4. After exposure to TNFα and PGE2, the pattern of expression is reversed (Figure 5A). Of note, PGE2 seems to be responsible for the down-regulation of EP2, whereas both TNFα and PGE2 are required to observe up-regulation of EP4.
TNF-R and TLR engagement triggers IDO enzymatic activity. DCs were prepared as above, with maturation stimuli consisting of TNFα, LPS, or SAC, either in the presence or absence of PGE2. (A) IDO protein expression was detected by Western blot, and (B) enzymatic activity was quantified by the colorimetric assay for kynurenine, as described in the “Materials and methods.” Error bars indicate SEM.
TNF-R and TLR engagement triggers IDO enzymatic activity. DCs were prepared as above, with maturation stimuli consisting of TNFα, LPS, or SAC, either in the presence or absence of PGE2. (A) IDO protein expression was detected by Western blot, and (B) enzymatic activity was quantified by the colorimetric assay for kynurenine, as described in the “Materials and methods.” Error bars indicate SEM.
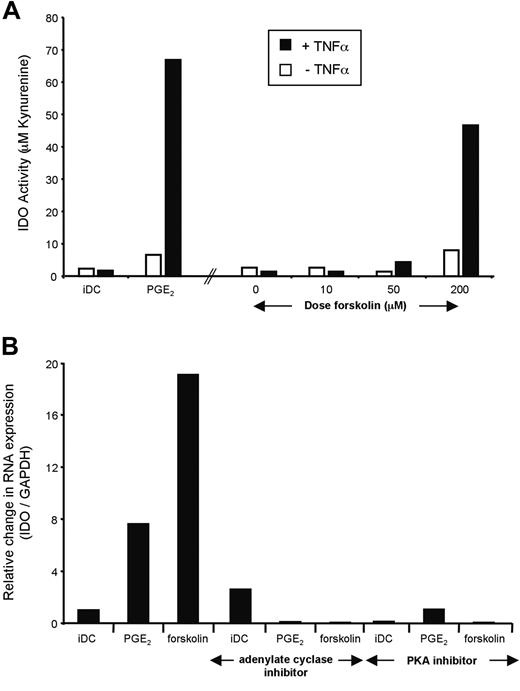
Next, we evaluated which EP receptor mediates IDO expression. This was done functionally, by exposing iDCs to agonists specific for EP1 to EP4 in the presence of TNFα, and IDO activity was measured as described above. In 3 of 3 individuals, only the EP2 agonist butaprost resulted in the induction of IDO activity (Figure 5B). As EP2 is a Gαs-coupled receptor, we tested whether ectopic activation of adenylate cyclase would mimic the effects of a receptor agonist. When iDCs were treated with the adenylate cyclase activator forskolin in combination with TNFα, the IDO activity was equivalent to that in TNFα- and PGE2-matured DCs (Figure 6A). We next directly tested the effect of adding either an inhibitor of adenylate cyclase or the cAMP-triggered kinase PKA. As shown, both inhibitors decreased the PGE2- and forskolin-induced expression of IDO. Together, these results suggest that during DC maturation, PGE2 acts through the EP2 receptor expressed on iDC, which in turn activates adenylate cyclase leading to PKA activation and an increase in the transcription of the gene coding for IDO. The presence of a TNF-R or TLR agonist enhances expression, and, importantly, this second signal facilitates IDO enzymatic activity.
Discussion
A novel mechanism by which PGE2 modulates the immune system
Like many effectors of immune regulation, the actions of PGE2 are complex and in some instances at apparent odds. With respect to its effect on T cells, the data indicate that PGE2 inhibits proliferation and skews responses toward TH2 differentiation.2,7,40 And although inhibition of TNFα, IL-6, and IL-12p35 in antigen-presenting cells would be consistent with a suppressive role for PGE2,4,41 recent studies indicate a proinflammatory activity of PGE2, based on the induction of bioactive IL-23.42,43 PGE2 has also been shown to act as a survival signal for thymocytes and may be critical for T-cell development.10 Furthermore, PGE2 is critical both for the generation of migratory DCs: monocyte-derived DCs demonstrate poor migration potential to CCR7 ligands unless matured in the presence of PGE216 ; in vivo, it has been demonstrated that mouse Langerhans-cell migration to the draining lymph node is dependent on PGE2 signaling via EP4.44 The stimulation of DC migration has been interpreted as proinflammatory, because migratory DCs are required for the trafficking of antigen to the T-cell area of lymph nodes, thus permitting the initiation of an immune response.43
PGE2 acts via EP2 to stimulate IDO expression. (A) We monitored EP2 and EP4 mRNA expression in DCs exposed to TNFα, PGE2, or a combination of both stimuli. TBP was used as a reference for our qPCR studies because its expression levels matched that of the EP-Rs. Of note, no message could be detected for EP1 and EP3 by qPCR (data not shown). (B) PGE2 was replaced by different EP agonists during DC maturation, and IDO activity was assessed after 48 hours. L-335677, Butaprost, L-826266, and L-161982 were used to stimulate EP1 to EP4, respectively. All were used at a concentration of 50 μM. In similar experiments, sulprostone was used as an EP1>> EP3 agonist and 19R-hydroxy PGE2 as an EP2 agonist, with similar results (data not shown).
PGE2 acts via EP2 to stimulate IDO expression. (A) We monitored EP2 and EP4 mRNA expression in DCs exposed to TNFα, PGE2, or a combination of both stimuli. TBP was used as a reference for our qPCR studies because its expression levels matched that of the EP-Rs. Of note, no message could be detected for EP1 and EP3 by qPCR (data not shown). (B) PGE2 was replaced by different EP agonists during DC maturation, and IDO activity was assessed after 48 hours. L-335677, Butaprost, L-826266, and L-161982 were used to stimulate EP1 to EP4, respectively. All were used at a concentration of 50 μM. In similar experiments, sulprostone was used as an EP1>> EP3 agonist and 19R-hydroxy PGE2 as an EP2 agonist, with similar results (data not shown).
We report a novel mechanism by which PGE2 acts to counter inflammatory responses. Our study indicates that PGE2 induces mRNA expression of IDO by signaling via the Gs-protein-coupled receptor EP2. This in turn activates adenylate cyclase, catalyzing the formation of cAMP and activating PKA. Evidence to support this mechanism is provided by the use of EP-receptor analogs and the demonstration that only the EP2 agonist, butaprost, was able to mimic the effects of PGE2 (Figure 5). Additionally, we demonstrate that direct stimulation of adenylate cyclase triggered IDO expression; whereas, treatment with an adenylate cyclase inhibitor or a PKA inhibitor blocked EP2 signaling and inhibited PGE2-mediated induction of IDO (Figure 6). Although this pathway accounted for a 50- to 200-fold increase in IDO expression, to our surprise, PGE2-treated DCs displayed no active IDO enzyme as monitored by the catabolism of tryptophan. Only when combined with a second signal via TNF-R or a TLR did we observe the production of kynurenine (Figures 2, 3, 4). Although use of TNFα, lipopolysaccharide, or SAC alone does not induce IDO, they are required to trigger enzymatic activity in PGE2-treated DCs. These findings offer the first innate effector pathway for the activation of IDO and suggest a link between DC migration and the acquisition of mechanisms for immune tolerance. Indeed, such a connection pushes one to consider the possibility that migratory DCs are programmed so that its default pathway is tolerance.
PGE2 signals via cyclic adenosine monophosphate (cAMP) and PKA to trigger IDO. (A) The adenylate cyclase activator forskolin mimics PGE2 activation of EP2/4 and induces IDO activity in the presence of TNFα. Monocyte-derived DCs (MoDCs) were matured during 48 hours in the presence of increasing doses of forskolin with or without TNFα. IDO activity was assessed spectrophotometrically after 4 hours at the end of the culture. (B) The adenylate cyclase inhibitor SQ22536 and the PKA inhibitor H-89 block IDO expression. Monocyte-derived DCs were cultured in the presence of PGE2 or forskolin, in addition to TNFα for 48 hours. The effect of inhibiting adenylate cyclase during this culture using SQ22536, as well as the effect of the PKA inhibitor H-89, were assessed on IDO mRNA levels.
PGE2 signals via cyclic adenosine monophosphate (cAMP) and PKA to trigger IDO. (A) The adenylate cyclase activator forskolin mimics PGE2 activation of EP2/4 and induces IDO activity in the presence of TNFα. Monocyte-derived DCs (MoDCs) were matured during 48 hours in the presence of increasing doses of forskolin with or without TNFα. IDO activity was assessed spectrophotometrically after 4 hours at the end of the culture. (B) The adenylate cyclase inhibitor SQ22536 and the PKA inhibitor H-89 block IDO expression. Monocyte-derived DCs were cultured in the presence of PGE2 or forskolin, in addition to TNFα for 48 hours. The effect of inhibiting adenylate cyclase during this culture using SQ22536, as well as the effect of the PKA inhibitor H-89, were assessed on IDO mRNA levels.
Activation of indoleamine 2,3-dioxygenase
The actions of IDO offers an intriguing mechanism for achieving T-cell tolerance (reviewed in Mellor and Munn23 ); it catabolizes the essential amino acid tryptophan, resulting in the generation of a putative proapoptotic agent, kynurenine, as well as other downstream immunomodulatory metabolites.45,46 IDO expression by human monocyte-derived DCs and macrophages potentiates the ability of these cells to inhibit T-cell proliferation. Furthermore, a role for IDO has been shown critical in achieving maternal tolerance, suppression of T cells reactive to haplotype-mismatched allografts, and the control of autoreactive T cells.23 More recently, it was demonstrated that the ectopic expression of IDO in tumor cells confers immune evasion.47
Although there is strong support for IDO's role in mediating T-cell tolerance, less is known about the regulation of IDO expression and the mechanism of enzyme activation. The only characterized trigger of IDO expression is IFNγ. It has been demonstrated in some cell types that IFNγ-induced gene expression can be enhanced or antagonized when added in combination with other cytokines. For example in eosinophils, it has been demonstrated that GM-CSF synergizes with IFNγ, whereas IL-3 antagonizes IFNγ-mediated IDO expression.48 With respect to stimulators of IDO activity, they include cytotoxic T-lymphocyte antigen 4 (CTLA-4) and CD28, which have been shown to transmit signals to the DCs by crosslinking the costimulatory molecules B7-1/B7-2.27 Of note, IFNγ also appears capable of achieving active IDO enzyme; however, its contribution may need to be reevaluated in light of data for B7-reverse signaling.49 If we are to interpret the findings that IFNγ and B7-reverse signaling are critical for IDO activation, it appears that stimulation of the adaptive immune system is required to achieve tolerance of the adaptive immune system. In identifying PGE2 as a trigger for IDO expression, we offer a novel means by which the innate immune system may regulate T-cell immunity.
Although previous studies have demonstrated the possibility of posttranslational control for IDO,48,50 we are unable to conclude whether our observation that TNF-R and TLR ligands are required for achieving enzymatic activity is reflective of translational control or a still undefined posttranslational event. Nonetheless, our results help to clarify the signals responsible for IDO expression and activity in monocyte-derived DCs. Given the interest in using DCs for clinical immunotherapy and the sensitivity of IDO expression to the culturing conditions,51,52 we hope that our findings will help clarify some of the differences observed in the various systems that are currently in use.
Implications for DC-based immunotherapy
With respect to DC-based immunotherapy protocols, it is important to note that many use maturation cocktails containing PGE2. As discussed above, this is in part due to its role in sensitizing DCs to CCR7 ligands, thus enhancing DC migration to the draining lymph nodes. Our finding that PGE2 is also sensitizing DCs to TNFα-mediated IDO activation predicates caution, as we may be inducing the opposite of what is intended. One consideration would be to segregate the distinct effects of PGE2. Migration appears to be mediated by EP4, whereas IDO gene expression occurs in response to EP2 stimulation. Use of EP4-specific agonists may allow such a separation to be achieved. Alternatively, it may be possible to include an IDO inhibitor in the DC maturation cocktail, thus generating a mature, CCR7-responsive DC with an inactive IDO enzyme.
In sum, our findings illustrate a novel role for PGE2 and important insight into the regulation of IDO. With a better understanding of these regulatory mechanisms and the crosstalk between TNF-R/TLR and EP2 signaling pathways, we hope to uncover new insight into the regulation of T-cell activation by DCs and the interplay between tumors and the immune system.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-03-0979.
Supported in part by grants from Fondation pour la Recherche Médicale (D.B.); the National Institutes of Health (NIH) Medical Scientist Training Program (MSTP) (grant GM07739) and The Albert Cass Fellowship (R.S.L.); Ligue Nationale Contre le Cancer, Association pour la Recherche contre le Cancer, and The Doris Duke Charitable Foundation (M.L.A.).
An Inside Blood analysis of this article appears at the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Olivier Lantz and Michael Lotze for their review of the manuscript and helpful scientific discussions, and Dr Nathalie Blachére and Elisabeth Fontan for their assistance with the HPLC.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal