Abstract
The monocyte population in blood is considered a possible source of endothelial precursors. Because endothelial-specific receptor tyrosine kinases act as regulators of endothelial cell function, we investigated whether expression of the vascular endothelial growth factor receptor-2 (VEGFR-2) on monocytes is important for their endothelial-like functional capacity. Peripheral-blood monocytes expressing vascular endothelial growth factor receptor-2 (VEGFR-2), or CD14+/VEGFR-2+, were isolated, and their phenotypic, morphologic, and functional capacities were compared with those of monocytes negative for this marker (CD14+/VEGFR-2-). CD14+/VEGFR-2+ cells constituted approximately 2% ± 0.5% of the total population of monocytes and 0.08% ± 0.04% of mononuclear cells in blood. CD14+/VEGFR-2+ cells exhibited the potential to differentiate in vitro into cells with endothelial characteristics. The cells were efficiently transduced by a lentiviral vector driving expression of the green fluorescence protein (GFP). Transplantation of GFP-transduced cells into balloon-injured femoral arteries of nude mice significantly contributed to efficient reendothelialization. CD14+/VEGFR-2- did not exhibit any of these characteristics. These data demonstrate that the expression of VEGFR-2 on peripheral blood monocytes is essential for their endothelial-like functional capacity and support the notion of a common precursor for monocytic and endothelial cell lineage. Our results help clarify which subpopulations may restore damaged endothelium and may participate in the maintenance of vascular homeostasis.
Introduction
Angiogenesis is a process of new blood vessel development (neovascularization) from preexisting vasculature, whereas vasculogenesis refers to blood vessel formation from endothelial progenitors that differentiate in situ. Until recently, angiogenesis was considered the only means of adult neovascularization, and vasculogenesis was thought to be limited to embryologic development.1 However, the existence of circulating endothelial progenitor cells (EPCs) has provided evidence that postnatal vasculogenesis also occurs in adults.2 The potential of EPCs as therapeutic tools for rescue of tissues from ischemic damage has been suggested.2 Among the number of different factors implicated in the regulation of the angiogenic response, vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen and a potent inducer of vascular permeability, is perhaps the most important player.3-5
VEGF acts through specific tyrosine kinase receptors that include VEGFR-1 (flt-1), VEGFR-2 (flk-1/KDR), and VEGFR-3/Flt-4, which convey signals that are essential for embryonic angiogenesis and hematopoiesis. Although VEGF binds to all 3 receptors, most biologic functions are mediated through VEGFR-2, and the role of VEGFR-1 is unknown.6 VEGFR3/Flt4 signaling is known to be important for the development of lymphatic endothelial cells, and VEGFR3 signaling may confer lymphatic endothelial-like phenotypes to endothelial cells.7 VEGFRs relay signals for processes essential in the stimulation of vessel growth, vasorelaxation, induction of vascular permeability, endothelial cell migration, proliferation, and survival.8 Endothelial cells express all the VEGFRs. It has been reported that during embryogenesis, a single progenitor cell, the hemangioblast, can give rise to both the hematopoietic and the vascular systems.9 Work addressing the origin of endothelial progenitor lineage in adult peripheral blood has demonstrated that monocytes also coexpress endothelial lineage markers such as VEGFR-2 and Ac133 and have the capacity to differentiate into adherent mature endothelial cells and to form cordlike structures in Matrigel.10,11 Rehman et al12 recently reported that peripheral-blood endothelial-like cells are derived from monocytes/macrophages and secrete angiogenic growth factors. Furthermore, Urbich et al13 demonstrated that CD14+ and CD14- populations in peripheral blood have the capacity to improve neovascularization after hindlimb ischemia.
Given that VEGFR-2 is an endothelial-specific receptor that controls many aspects of vascular growth and angiogenic responses,14,15 we hypothesized that expression of this endothelial-specific tyrosine kinase receptor on CD14+ monocytes may be important for their ability to function as EPCs. We, therefore, isolated circulating monocytes with and without expression of this receptor and studied their functional capacity, including their ability to contribute to reendothelialization in balloon-injured femoral arteries of nude mice.
Patients, materials, and methods
Approval for these studies was obtained from the Karolinska University Hospital institutional review board. Informed consent was provided in accordance with the Declaration of Helsinki.
Isolation of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells from peripheral blood
Peripheral-blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation with Ficoll (Fresenius Kabi, Oslo, Norway) from the blood of healthy human volunteers according to standard procedure. CD14+/VEGFR-2+ cells were isolated through fluorescence-activated cell sorting. CellQuest 3.3 software (BD Biosciences, Palo Alto, CA) was used to acquire images; Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA), to process them. For staining, PBMCs were initially incubated with anti-CD3 antibody-coated magnetic particles (Dynal, Oslo, Norway) to remove T lymphocytes, in accordance with the procedure described by the manufacturer. Remaining cells were distributed into several tubes, each containing 20 × 106 cells. Fifteen microliters of anti-VEGFR-2 (20 μg/mL; Reliatech, Mascheroder, Germany) antibodies was added to each tube and incubated for 30 minutes at room temperature. After washing once with phosphate-buffered saline (PBS), the cells were labeled with 20 μL of a 1:10 diluted secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse monoclonal antibody (Serotec, Stockholm, Sweden) and 20 μL phycoerythrin (PE)-conjugated anti-CD14 antibodies (4 μg/mL; BD Biosciences, San Jose, CA). During cell sorting, using a stringent gate, highly purified CD14+/VEGFR-2+ and the CD14+/VEGFR-2- fractions were collected for further analysis.
Western blotting
Lysates of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells (1 × 106 cells for each population) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and immunoblotted with anti-VEGFR-2 antibodies (1:200). Horseradish peroxidase-conjugated F(ab')2 fragments of goat anti-mouse secondary antibodies (1:2000) (Jackson ImmunoResearch, Bar Harbor, ME) were used together with the enhanced chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL) to identify the bound antibody.
Phenotyping and cell cultivation
After sorting with the fluorescence-activated cell sorter (FACS), cells were placed on glass slides using cytospin. Immunocytochemistry was performed with the use of enzyme staining. Cells were stained with the following antibodies: anti-CD133, anti-CD141 (thrombomodulin), anti-CD144 (VE-cadherin) (all from Becton Dickinson, San Jose, CA), von Willebrand factor (VWF; Serotec), and antibodies to endothelial nitric oxide synthase (e-NOS) (all antibodies were used at a dilution of 1:50) for 1 hour at room temperature, washed 3 times with PBS, and stained with secondary biotinylated goat anti-mouse antibodies (1:500). The avidin-peroxidase procedure was carried out using the Vectastain Elite ABC kit (ImmunKemi, Stockholm, Sweden) according to the manufacturer's instructions. The ACE (gives red staining) and DAB-Nickel (gives brown/black staining) substrate (Immunkemi, Stockholm, Sweden) kits were used as color developers.
Each population was further characterized by flow cytometry, as described earlier,16 using an array of antibodies to specific markers expressed on monocytes and endothelial cells (Table 1). FITC-conjugated acetylated low-density lipoprotein (AcLDL) (Molecular Probes, Eugene, OR), antibodies to VWF (DakoPatts, Glostrup, Denmark), α-actin, fibroblast (Serotec), and ulex europaeus (Sigma, Stockholm, Sweden) were used. All other antibodies were purchased from Becton Dickinson. Corresponding control isotypes were used for evaluation of nonspecific binding of monoclonal antibodies (mAbs). Cells were analyzed on a Becton Dickinson flow cytometer (FACSorter).
Phenotypic characteristics of freshly isolated CD14+/VEGFR-2– cells and CD14+/VEGFR-2+ cells from peripheral blood
Antibody . | CD14+/VEGFR-2+ cells, % . | CD14+/VEGFR-2– cells, % . | HAECs, % . |
|---|---|---|---|
| Stem/progenitor/mature endothelial cell markers | |||
| VEGFR-2 | 100 | 0.005 ± 0.001 | 5 |
| VEGFR-1 | 78 ± 1.5 | 81 ± 1.33 | 3 |
| Tie-2 | 100 | 23 ± 2.5 | 0 |
| CD34 | 3 ± 2.1 | 0.01 ± 0.007 | 2 |
| CD123 | 0 | 28 ± 2.1 | 0 |
| CD133 | 2 ± 0.6 | 0.05 ± 0.002 | 0 |
| CD90 | 0 | 0 | 0 |
| AcLDL | 100 | 100 | 100 |
| CD31 | 100 | 20 ± 2.6 | 100 |
| CD144 | 0.2 ± 0.01 | 0 | 100 |
| VWF | 100 | 0 | 100 |
| Ulex europaeus | 100 | 100 | 100 |
| Monocyte/macrophage markers | |||
| CD14 | 98 ± 0.2 | 97 ± 2.2 | 0 |
| CD15 | 9 ± 0.02 | 8 ± 3.2 | 0 |
| CD11b | 95 ± 7.2 | 92 ± 2.4 | 0 |
| CD11c | 6 ± 5.6 | 84 ± 2.7 | 0 |
| CD68 | 0 | 3 ± 1.5 | 0 |
| CD83 | 0 | 2.7 ± 1.4 | 0 |
| Control markers | |||
| α-actin | 0 | 0 | 0 |
| Fibroblasts | 0 | 0 | 0 |
| CD45 | 78 ± 10.1 | 100 | 0 |
| CD3 | 0 | 0 | 0 |
| CD56 | 0 | 0 | 0 |
| CD16 | 95 ± 4.8 | 92 ± 0.4 | 0 |
| CD19 | 0 | 0 | 0 |
Antibody . | CD14+/VEGFR-2+ cells, % . | CD14+/VEGFR-2– cells, % . | HAECs, % . |
|---|---|---|---|
| Stem/progenitor/mature endothelial cell markers | |||
| VEGFR-2 | 100 | 0.005 ± 0.001 | 5 |
| VEGFR-1 | 78 ± 1.5 | 81 ± 1.33 | 3 |
| Tie-2 | 100 | 23 ± 2.5 | 0 |
| CD34 | 3 ± 2.1 | 0.01 ± 0.007 | 2 |
| CD123 | 0 | 28 ± 2.1 | 0 |
| CD133 | 2 ± 0.6 | 0.05 ± 0.002 | 0 |
| CD90 | 0 | 0 | 0 |
| AcLDL | 100 | 100 | 100 |
| CD31 | 100 | 20 ± 2.6 | 100 |
| CD144 | 0.2 ± 0.01 | 0 | 100 |
| VWF | 100 | 0 | 100 |
| Ulex europaeus | 100 | 100 | 100 |
| Monocyte/macrophage markers | |||
| CD14 | 98 ± 0.2 | 97 ± 2.2 | 0 |
| CD15 | 9 ± 0.02 | 8 ± 3.2 | 0 |
| CD11b | 95 ± 7.2 | 92 ± 2.4 | 0 |
| CD11c | 6 ± 5.6 | 84 ± 2.7 | 0 |
| CD68 | 0 | 3 ± 1.5 | 0 |
| CD83 | 0 | 2.7 ± 1.4 | 0 |
| Control markers | |||
| α-actin | 0 | 0 | 0 |
| Fibroblasts | 0 | 0 | 0 |
| CD45 | 78 ± 10.1 | 100 | 0 |
| CD3 | 0 | 0 | 0 |
| CD56 | 0 | 0 | 0 |
| CD16 | 95 ± 4.8 | 92 ± 0.4 | 0 |
| CD19 | 0 | 0 | 0 |
Data are expressed as mean ± SD. n = 5 CD14+/VEGFR-2+ cells; n = 5 CD14+/VEGFR-2–2 cells. HAECs were the primary cell line.
For some experiments, sorted CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells were cultivated on fibronectin (20 μg/mL)-coated tissue culture plates, in endothelial-selective medium (EndoCult; Stem Cell Technologies, Vancouver, BC, Canada). These cultures were observed for growth and morphology. Proliferative capacity of the 2 cell types was tested with the addition of 5-bromodeoxyuridine (BrdU). Assay was performed as described earlier.14 After 1 week in culture, the cells were cytospun on glass and stained for anti-CD144 expression.
Human aortic endothelial cells (HAECs) were purchased from Clonetics (San Diego, CA) and were cultivated in recommended medium. HAECs were used as controls in the phenotypic analysis and the tubule formation assay.
Transmission electron microscopy
Electron microscopic analysis was performed at the core facility unit for electron microscopy at Karolinska University Hospital. CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells were grown separately on membrane filters in 24-well plates. Membranes were fixed in 2% glutaraldehyde, briefly rinsed in distilled water, placed in 70% ethanol for 10 minutes and then in 99.5% ethanol for 15 minutes, all at 4°C, and dried. After fixation, the membrane was cut free and was fixed for 1 hour at 4°C in a buffer containing 0.15 M sodium cacodylate, 1% osmium tetraoxide, and 3 mM CaCl2, pH 7.4. Subsequently, the wells were rinsed briefly in 0.15 M sodium cacodylate buffer, dehydrated in ethanol as described, and embedded in Spurr resin (Agar Scientific, Essex, England). Sections were contrasted with uranyl acetate followed by lead citrate and were examined at 80 kV under a transmission electron microscope (Leo 906; Tecnai, Oberkochen, Germany).
Migration assay
Freshly isolated CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells (5 × 104) were added to 4 fibronectin-coated Transwell inserts (Becton Dickinson, Stockholm, Sweden), each with a 3.0-μm membrane pore size. Cell migration was assayed in the absence and presence of 50 ng/mL vascular endothelial growth factor (VEGF) in the lower chamber or 300 ng/mL monocyte chemotactic factor-1 (MCP-1) in medium containing 5% fetal calf serum. Cells were allowed to migrate for 22 hours. Migrated cells were collected and counted in a hemocytometer. Each experiment was performed in triplicate wells and was repeated 4 times.
Angiogenic growth factor secretion by CD14+/VEGFR-2+ and CD14+/VEGFR-2- cell populations
To assess growth factor secretion, freshly isolated cells were cultivated in growth factor- and serum-free medium for 72 hours. Supernatants from all cultures were collected and stored at -70°C until further analysis. Conditioned medium was assayed for the angiogenic growth factors, VEGF, granulocyte-colony-stimulating factor (G-CSF), granulocyte macrophage-colony-stimulating factor (GM-CSF), fibroblast growth factor (FGF), and stromal derived-factor-1 (SDF-1). Growth factors were assayed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
In vitro angiogenesis assay
The formation of capillary tubule-like structures by freshly isolated CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells was assessed in a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma, frequently used for the evaluation of in vitro angiogenesis. Twenty-four-well plates were coated with 200 μL Matrigel (pregelled for 30 minutes at 37°C in 5% CO2), and the cells were seeded on the polymerized matrix at a density of 5 × 104 cells/well. Resultant tubulelike structures were examined using a phase-contrast light microscope after cells were cultivated for 1 week at 37°C in 5% CO2. HAECs were used as positive control cells.
Lentiviral transduction of FACS-sorted CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells
The recombinant lentiviral vector was produced using a 3-plasmid expression system, as described earlier.17 For transduction, FACS-sorted cells were transferred to 10-mL tubes with 1-mL culture medium and were incubated with concentrated pHR′EF1α GFPSIN virions at a multiplicity of infection (MOI) of 0.1 at 37°C in a 5% CO2 atmosphere overnight. After virus incubation, cells were washed once in PBS and were directly injected in the denuded femoral artery of nude mice (see “Mice”). To detect GFP expression, and following the same transduction procedure, transduced cells were cultured in 6-well plates and were observed on day 6 after transduction by fluorescence microscopy.
Mice
Balloon injury of the right femoral artery was performed as follows: nude C57 black mice (Taconic M&B, Bomholtvej, Denmark), each weighing 20 to 25 g, were anesthetized with isoflurane, and the right femoral artery was exposed to the level of bifurcation through a transabdominal incision. Microclamps (S&T, Geneva, Switzerland) were placed on lower aorta, left iliac artery, and the distal part of the right femoral artery. A 2-F Fogarty balloon catheter (Baxter, Deerfield, IL) was introduced into the right femoral artery, inflated, and withdrawn 3 times with rotation. Inflation was performed with the cannula and 1 mL Ringer solution with free outflow through the microincision. Cells (5 × 105 per animal) in 100 μL MCDB medium (CD14+/VEGFR-2+, n = 10; CD14+/VEGFR-2-, n = 10) from Invitrogen (Stockholm, Sweden) were instilled through the same cannula and incubated in the freshly injured arterial bed for 15 to 20 minutes, whereas 6 control mice that underwent transplantation received only culture medium. After incubation, unbound cells were aspirated, the catheter was removed, and the microincision was sutured with 11-0 nylon (S&T) interrupted suture. After removal of the microclamps, the blood flow was restored. All animal procedures were performed in accordance with institutional guidelines and conformed to the Guide for the Care and Use of Laboratory Animals at Huddinge University Hospital. Animals were killed at 2 and 4 weeks after human cell transplantation, and each right femoral artery was harvested for histopathologic examination. Vessels were embedded in optimum cutting temperature medium (OCT; Histolab, Gothenburg, Sweden) and frozen in liquid nitrogen.
Immunohistochemistry
To localize human cells in the mouse artery, 5-μm cryosections were stained with an anti-GFP antibody (1:50). Immunoperoxidase was performed using the Vectastain Elite ABC kit (ImmunKemi), according to the manufacturer's instructions. The ACE (gives red staining) substrate kit was used as a color developer, and hematoxylin was used for counterstaining. Sections were examined under a Nikon TE300 light microscope (Nikon, Stockholm, Sweden) equipped with an apochromatic 40×/0.60 objective lens. In addition, a mouse anti-human nucleus monoclonal antibody (Chemicon, Temecula, CA) was used to detect human cells in the mouse artery by immunofluorescence.18 The phenotype of the transplanted human cells was detected by double staining with antibodies to human nuclei (1:50) and anti-VE-cadherin (1:100). This was followed by staining with secondary anti-mouse subclass-specific FITC or Texas Red-conjugated antibodies (1:500). Endothelialization was calculated as the percentage of the surface covered by human ACE-positive cells and the total luminal surface. For histomorphology (6 animals per group, 6 sections per femoral artery), cross-sections of the artery were stained with hematoxylin/eosin and were examined for vessel diameter, media area, and intima-to-media (I/M) ratio. Sections were examined under a fluorescence confocal microscope (Leica TCS SP2; Leica Microsystems, Stockholm, Sweden) equipped with an oil-immersion 40×/0.90 objective lens. Images were acquired with LCS 2.61 software, and were processed with Adobe Photoshop 7.0 software. Images were acquired with ACT-1 v.2 software (Nikon) and processed with Adobe Photoshop 7.0 software.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from 3 human-mouse chimeric femoral arteries using the Micro-FastTrack RNA isolation kit (Invitrogen, Groningen, The Netherlands). In addition, RNA was extracted from the femoral artery of one mouse that had received CD14+/VEGFR-2- cells and one mouse that had undergone sham transplantation 4 weeks earlier. Human-specific primers were used to detect human CD31 and e-NOS in the human cell-transplanted mouse arteries. Freshly isolated CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells were also tested for the expression of e-NOS. ABL was used as an endogenous reference gene. Primer sets were commercially synthesized using CyberGene (Huddinge, Sweden). Primer sequences were as follows: CD31 forward (F), 5′-CCA CTG CAG AGT ACC AGG TGT TGG-3′; CD31 reverse (R), 5′-ATC GAG AAG GAG CGT TTC T-3′; expected product size, 248 bp; e-NOS-F, 5′-CTG TAT GGC TCC GAG ACC-3′; e-NOS-R, 5′-GCT GTT GAA GCG GAT CTT ATA AC-3′; expected product size, 279 bp; ABL-F, 5′-CGG CTC TCG GAG GAG ACG ATG A-3′; ABL-R, 5′-CCC AAC CTT TTC GTT GCA CTG T-3′; expected product size, 385 bp.
Polymerase chain reaction (PCR) reaction was carried out as described earlier.19
Statistical analysis
Where applicable, results are presented as mean ± SD. The Mann-Whitney U test was used for comparisons between the groups. P < .05 was considered significant.
Results
Characterization of CD14+/VEGFR-2+ and CD14+/VEGFR-2-
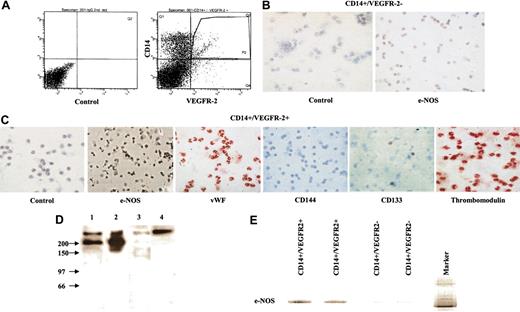
We found that CD14+/VEGFR-2+ cells constituted approximately 2% ± 0.5% (Figure 1A) of the total population of monocytes and 0.08% ± 0.04% of PBMCs in blood. Immunocytochemical analysis demonstrated that the freshly isolated CD14+/VEGFR-2- cell fraction did not express e-NOS (Figure 1B). However, freshly isolated CD14+/VEGFR-2+ cells expressed e-NOS, VWF, and thrombomodulin, and some cells expressed CD133 (Figure 1C) but not VE-cadherin. Expression of the VEGFR-2 receptor on CD14+/VEGFR-2+ cells was further supported by the fact that lysates of this population, when immunoblotted with anti-VEGFR-2 antibodies, gave the expected band of approximately 200 kDa, which was absent or faintly expressed in the CD14+/VEGFR-2- cell population (Figure 1D). Furthermore, the e-NOS mRNA was strongly expressed in CD14+/VEGFR-2+ cells, whereas in the VEGFR-2- fraction, only a faint e-NOS mRNA band was visible (Figure 1E). We cannot rule out the fact that this faint band consisted of a small number of contaminating VEGFR-2+ cells in the CD14+/VEGFR-2- cell population. Phenotypic markers expressed by the 2 populations are shown in Table 1. Both populations expressed the CD45 marker, indicating the hematopoietic origin of these cells.
Characterization of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Flow cytometry sorting of CD14+/VEGFR-2+ and CD14+/VEGFR-2- from PBMCs depleted of T lymphocytes. (B) CD14+/VEGFR-2- cells did not express e-NOS. (C) CD14+/VEGFR-2+ cells expressed several endothelial-specific markers such as e-NOS (black), VWF (red), and thrombomodulin (red), and some cells expressed CD133 (red) but not CD144. Cells were counterstained with hematoxylin. Magnification, 40 ×. (D) Western blotting of the lysates from CD14+/VEGFR-2+ cells with antibodies to VEGFR-2 gave the expected bands of approximately 200 kDa (lanes 1-2), whereas CD14+/VEGFR-2- cells (lane 3) and the control secondary antibody did not (lane 4). (E) mRNA expression for e-NOS was detected strongly in CD14+/VEGFR-2+ cells and weakly in CD14+/VEGFR-2- cells. N indicates nucleus.
Characterization of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Flow cytometry sorting of CD14+/VEGFR-2+ and CD14+/VEGFR-2- from PBMCs depleted of T lymphocytes. (B) CD14+/VEGFR-2- cells did not express e-NOS. (C) CD14+/VEGFR-2+ cells expressed several endothelial-specific markers such as e-NOS (black), VWF (red), and thrombomodulin (red), and some cells expressed CD133 (red) but not CD144. Cells were counterstained with hematoxylin. Magnification, 40 ×. (D) Western blotting of the lysates from CD14+/VEGFR-2+ cells with antibodies to VEGFR-2 gave the expected bands of approximately 200 kDa (lanes 1-2), whereas CD14+/VEGFR-2- cells (lane 3) and the control secondary antibody did not (lane 4). (E) mRNA expression for e-NOS was detected strongly in CD14+/VEGFR-2+ cells and weakly in CD14+/VEGFR-2- cells. N indicates nucleus.
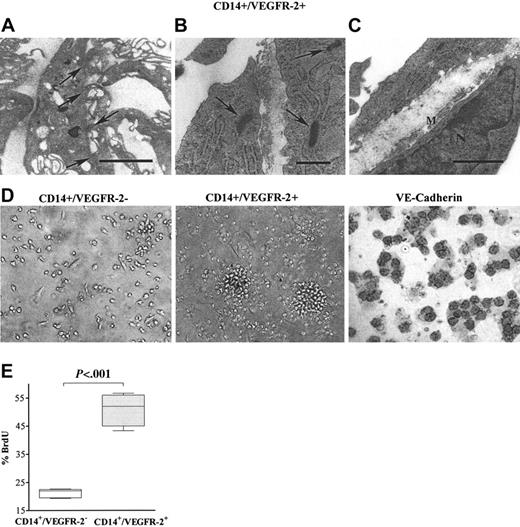
Electron microscopic analysis of CD14+/VEGFR-2+ cells cultured on Transwell tissue culture inserts demonstrated the formation of a basement membrane, the presence of Weibel-Palade bodies, and tight junctions characteristic of endothelial cells (Figure 2A-C), whereas the negative fraction did not exhibit these features.
CD14+/VEGFR-2+ cells, when cultivated on fibronectin-coated culture wells, formed colonies after 3 days in culture. During the next few days, the morphology of these colonies changed from a center of densely packed round cells to seemingly mature flattened, elongated cells. On the other hand, the CD14+/VEGFR-2- population remained adherent as slightly rounded cells for 2 weeks but did not form clusters (Figure 2D). Cultivated CD14+/VEGFR-2+ cells expressed VE-cadherin after 1 week in culture (Figure 2D), but the VEGFR-2- fraction did not express this marker at any time point during cultivation. Significantly higher numbers of BrdU+ cells were observed in the CD14+/VEGFR-2+ cell population than in the CD14+/VEGFR-2- population (Figure 2E), indicating higher proliferative capacity in the CD14+/VEGFR-2+ cell population. Nevertheless, this population could be subcultured for only 4 to 5 passages.
In vitro morphologic characterization of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Transmission electron micrographs illustrate that CD14+/VEGFR-2+ cells demonstrated the presence of tight junctions typical for endothelial cells (arrows; bar = 2 μm) and (B) Weibel-Palade bodies (arrow; bar = 0.5 μm) and (C) produced basement membrane (M; bar = 1μm). (D) CD14+/VEGFR-2- cells in culture did not form colonies, whereas the CD14+/VEGFR-2+ cells formed colonies initially consisting of rounded cells that, after 1 week, grew out as single elongated cells. After 1 week in culture, only the CD14+/VEGFR-2+ cells expressed VE-cadherin (black surface staining). (E) Significantly higher numbers of CD14+/VEGFR-2+ cells were BrdU+ compared with CD14+/VEGFR-2- cells.
In vitro morphologic characterization of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Transmission electron micrographs illustrate that CD14+/VEGFR-2+ cells demonstrated the presence of tight junctions typical for endothelial cells (arrows; bar = 2 μm) and (B) Weibel-Palade bodies (arrow; bar = 0.5 μm) and (C) produced basement membrane (M; bar = 1μm). (D) CD14+/VEGFR-2- cells in culture did not form colonies, whereas the CD14+/VEGFR-2+ cells formed colonies initially consisting of rounded cells that, after 1 week, grew out as single elongated cells. After 1 week in culture, only the CD14+/VEGFR-2+ cells expressed VE-cadherin (black surface staining). (E) Significantly higher numbers of CD14+/VEGFR-2+ cells were BrdU+ compared with CD14+/VEGFR-2- cells.
In vitro functional capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- peripheral-blood cells
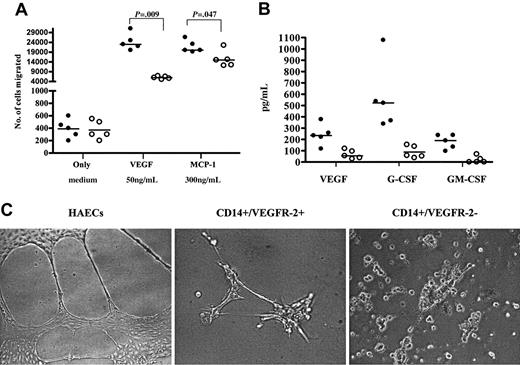
Significantly higher numbers of freshly isolated CD14+/VEGFR-2+ cells migrated toward VEGF and MCP-1 compared with CD14+/VEGFR-2- cells (Figure 3A) (P = .009 and P = .047, respectively). Angiogenic growth factors produced by the 2 populations did not vary significantly. Both populations produced VEGF, G-CSF, and GM-CSF but not SDF-1 or FGF. However, growth factor levels were higher in the CD14+/VEGFR-2+ population than in the CD14+/VEGFR-2- population (Figure 3B). In Matrigel, 3-day cultivated cells showed that only CD14+/VEGFR-2+, but not CD14+/VEGFR-2-, cells could form tubulelike structures (Figure 3C). HAECs were used as controls. Thus, the in vitro data indicated that freshly isolated CD14+/VEGFR-2+ cells demonstrated endothelial-like characteristics compared with CD14+/VEGFR-2- cells.
CD14+/VEGFR-2+ cells differentiated to endothelial cells and efficiently repopulated denuded mouse femoral arteries
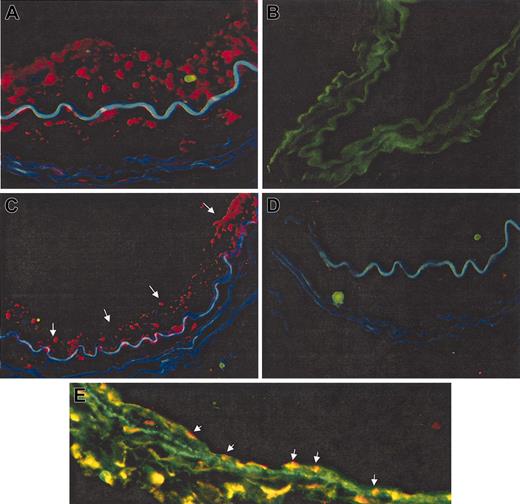
Exposure of the 2 cell populations to lentiviral particles expressing GFP led consistently to transduction efficiencies greater than 70%. Screening of artery sections for human cells demonstrated that because of high autofluorescence, visualization of GFP+ cells was difficult. Therefore, we performed enzyme-based immunohistochemical staining with anti-GFP antibody to detect GFP+ cells. Using this antibody we found no GFP+ areas in mice after sham transplantation or after CD14+/VEGFR-2- transplantation (Figure 4A-C). However, coverage of the intimal surfaces of arteries with GFP+ cells (red) was already seen at 2 weeks after transplantation in mice receiving CD14+/VEGFR-2+ cells (Figure 4D). The GFP+ area was significantly increased in vessels harvested 4 weeks after seeding (Figure 4E-F). In addition, human cells were also identified by an anti-human nucleus antibody through immunofluorescence. The specificity of the human nucleus antibody was first determined by using normal human and mouse arteries. Human cell nuclei stained red (Figure 5A). The control mouse artery did not stain from the human nucleus antibody (Figure 5B). Four weeks after transplantation, a high degree of repopulation was seen, and approximately 70% ± 8% of the lesion was covered with human cells (red) in mice receiving CD14+/VEGFR-2+ human cells (Figure 5C) but not in mice receiving CD14+/VEGFR-2- cells (Figure 5D). Animals receiving CD14+/VEGFR-2- cells did not demonstrate the presence of these cells in the denuded arteries, spleen, or lungs or in the circulation 2 or 4 weeks after transplantation (data not shown). The endothelial cell phenotype was confirmed in vivo by staining with antibodies to VE-cadherin (green), and each section was double-stained with the anti-human nucleus antibody to detect human cells (red). Double-stained cells are yellow (Figure 5E).
Cross-sections of injured arterial segments were examined at 2 and 4 weeks after transplantation for histomorphologic changes. Balloon injury in the mouse femoral artery resulted in dilation of the lesioned arterial segment so that the inner vessel diameter was significantly increased from 0.301 ± 0.041 mm in uninjured control arteries to 0.601 ± 0.043 and 0.631 ± 0.028 mm in injured vessels incubated with media alone or with CD14+/VEGFR-2-, respectively (P < .001). This widening was not found in femoral arteries of mice that received CD14+/VEGFR-2+ cells (0.404 ± 0.08 mm). A reduction in cross-sectional media area from 0.028 ± 0.010 mm2 to 0.012 ± 0.007 mm2 was observed in injured arteries. Thinning of the media area was not seen in CD14+/VEGFR-2+ cell-transplanted arteries (0.033 ± 0.013). Reendothelialization mediated by these cells did not result in neointima formation in femoral arteries at 2 or 4 weeks after injury. The I/M ratio was not significantly higher in CD14+/VEGFR-2+-transplanted vessel segments (normal uninjured artery, 0.03 ± 0.009; media control, 0.02 ± 0.008; CD14+/VEGFR-2-, 0.02 ± 0.009; CD14+/VEGFR-2+, 0.04 ± 0.02; P = NS).
In vitro migratory and tubule-forming capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) In a migration assay in the absence and presence of 50 ng/mL concentration of VEGF and 300 ng/mL MCP-1, a significantly higher number of CD14+/VEGFR-2+ cells (•) than CD14+/VEGFR-2- (○) cells migrated toward VEGF and MCP-1. (B) The CD14+/VEGFR-2+ cell population produced higher levels of the angiogenic growth factors, VEGF, G-CSF, and GM-CSF than the CD14+/VEGFR-2- population. (C) CD14+/VEGFR-2+ but not CD14+/VEGFR-2- cells formed capillarylike tubules in Matrigel. HAECs were used as control cells.
In vitro migratory and tubule-forming capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) In a migration assay in the absence and presence of 50 ng/mL concentration of VEGF and 300 ng/mL MCP-1, a significantly higher number of CD14+/VEGFR-2+ cells (•) than CD14+/VEGFR-2- (○) cells migrated toward VEGF and MCP-1. (B) The CD14+/VEGFR-2+ cell population produced higher levels of the angiogenic growth factors, VEGF, G-CSF, and GM-CSF than the CD14+/VEGFR-2- population. (C) CD14+/VEGFR-2+ but not CD14+/VEGFR-2- cells formed capillarylike tubules in Matrigel. HAECs were used as control cells.
Transcription of human endothelial-specific genes in mice receiving CD14+/VEGFR-2+ cells
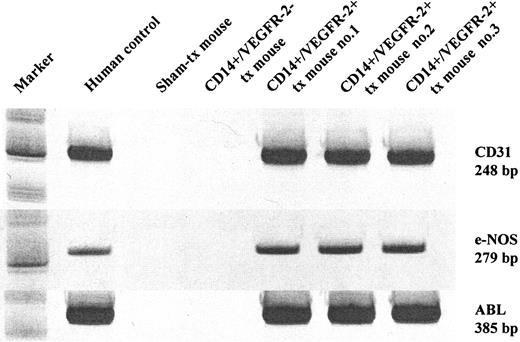
We further confirmed the engraftment of the transplanted cells by determining the expression of human genes in mice after transplantation. One month after transplantation, we analyzed femoral arteries of the mice killed with human cells through RT-PCR using primers specific for the human endothelial-specific gene CD31 and e-NOS. The CD31 and e-NOS primers were species specific for humans because they did not amplify the respective mouse gene (Figure 6A). These results were obtained with arteries from mice receiving CD14+/VEGFR2+ cells but not in those receiving CD14+/VEGFR-2- cells. These data demonstrate that the transplanted human CD14+/VEGFR-2+ cells engrafted the damaged mouse artery.
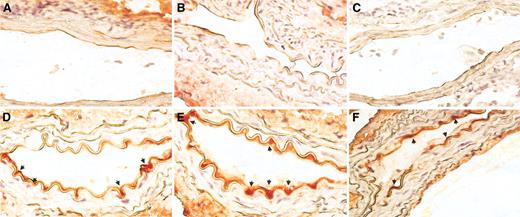
In vivo engraftment capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. Immunohistochemical staining of sections from balloon-injured mouse femoral artery with an anti-GFP antibody showed (A) no localization of GFP+ areas in sham-transplanted and (B) CD14+/VEGFR-2--transplanted cells on the luminal surface of the seeded artery. (C) Staining with control secondary antibody. (D) Significant number of GFP+ (red) CD14+/VEGFR-2+ cells (arrows) at 2 weeks and (E-F) 4 weeks were found in the animals that received these cells. Original magnification, 60 ×.
In vivo engraftment capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. Immunohistochemical staining of sections from balloon-injured mouse femoral artery with an anti-GFP antibody showed (A) no localization of GFP+ areas in sham-transplanted and (B) CD14+/VEGFR-2--transplanted cells on the luminal surface of the seeded artery. (C) Staining with control secondary antibody. (D) Significant number of GFP+ (red) CD14+/VEGFR-2+ cells (arrows) at 2 weeks and (E-F) 4 weeks were found in the animals that received these cells. Original magnification, 60 ×.
Discussion
Controversy exists with respect to the identification and the origin of EPCs. EPC and endothelial-like cell populations can be grown from cells expressing hematopoietic markers,2,20,21 myeloid cells,10,12,13,22 side population cells,23 and circulating mature endothelial cells.24 Thus, the circulating cell population that contributes to postnatal neovascularization is heterogenous and displays variable morphologic growth characteristics. Moreover, within these populations, it may be that only a small, specific fraction of cells has this capacity. Earlier studies have demonstrated that circulating monocytes can develop an endothelial phenotype10,22 and that they have the ability to take part in neovascularization.13 A recent study demonstrated that peripheral-blood endothelial-like cells are derived from monocytes/macrophages.12 Published results indicate that only a subpopulation of monocytes may have endothelial-like characteristics. Thus, although there is convincing evidence that endothelial-like cells generated from the monocytic lineage improve neovascularization, the exact phenotype and characterization of the monocyte subpopulation capable of generating endothelial-like cells is unknown.
Immunofluorescence staining for detection of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells after transplantation. Human nucleus antibody stained cells (red) in normal human artery (A) but not in control-injured, sham-transplanted mouse artery (B). (C) Balloon-injured mouse femoral artery showed the localization of transplanted human CD14+/VEGFR-2+ cells on the luminal surface of the seeded artery (red cells, arrows) at 4 weeks. (D) No localization of transplanted CD14+/VEGFR-2- cells was observed at 4 weeks. (E) The endothelial cell phenotype of transplanted human cells was confirmed by double staining with human nucleus antibody and antibodies to VE-cadherin (double-positive cells are stained yellow; arrows). Original magnification, 60 ×.
Immunofluorescence staining for detection of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells after transplantation. Human nucleus antibody stained cells (red) in normal human artery (A) but not in control-injured, sham-transplanted mouse artery (B). (C) Balloon-injured mouse femoral artery showed the localization of transplanted human CD14+/VEGFR-2+ cells on the luminal surface of the seeded artery (red cells, arrows) at 4 weeks. (D) No localization of transplanted CD14+/VEGFR-2- cells was observed at 4 weeks. (E) The endothelial cell phenotype of transplanted human cells was confirmed by double staining with human nucleus antibody and antibodies to VE-cadherin (double-positive cells are stained yellow; arrows). Original magnification, 60 ×.
In the present study, we demonstrate that circulating CD14+ monocytes that express the endothelial-specific tyrosine kinase receptor VEGFR-2 have EPC capacity, whereas CD14+ cells not expressing this marker lacked this capacity. The CD14+/VEGFR-2+ cell population demonstrated features required by cells involved in revascularization. This population demonstrated the ability to migrate toward the angiogenic growth factors VEGF and MCP-1, formed capillarylike tubules, and demonstrated the presence of Weibel-Palade bodies and tight junctions and the production of basement membrane. These cells responded to vascular injury by restoring the endothelial lining of damaged arteries within 2 to 4 weeks. This finding may have implications for treating vascular proliferative disease because restenosis after balloon angioplasty remains a major problem in vascular therapy. Ex vivo expansion of these cells may have implications for cell-based therapies for vascular diseases. Our results showed that under the growth culture conditions used in the present study, this population demonstrated limited proliferative capacity in vitro, albeit higher than in the CD14+/VEGFR-2- population. We are working out conditions that may allow even greater expansion of these cells in vitro. It is likely that these cells in vivo may attain proliferative capacity because the balloon injury may provide a microenvironment that is conducive for the proliferation of EPCs by producing several factors, including growth factors, cytokines, and other proteins. EPCs have extensive clonogenic and proliferative capacity but lack expression of CD45 and CD14.25,26 On the other hand, because CD14+/VEGFR-2+ cells express CD45 with limited proliferative capacity, the possibility remains that they may be terminally differentiated cells and not progenitor cells. Our ongoing studies using this population will help answer some of these questions in the future. Nonetheless, our observations in this study indicate that circulating CD14+/VEGFR-2+ cells can be recruited to sites of vascular damage and may take part in reendothelialization during vascular injury.
In vivo engraftment and functional capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Transcription of human endothelial-specific genes in the mouse artery. Human CD31 and e-NOS were detected in balloon-injured mouse femoral arteries that received human CD14+/VEGFR-2+cells but not in mice that underwent sham transplantation or that received CD14+/VEGFR-2 cells. ABL was used as the housekeeping gene.
In vivo engraftment and functional capacity of CD14+/VEGFR-2+ and CD14+/VEGFR-2- cells. (A) Transcription of human endothelial-specific genes in the mouse artery. Human CD31 and e-NOS were detected in balloon-injured mouse femoral arteries that received human CD14+/VEGFR-2+cells but not in mice that underwent sham transplantation or that received CD14+/VEGFR-2 cells. ABL was used as the housekeeping gene.
On the other hand, the CD14+/VEGFR-2- population did not demonstrate any of these functional properties. They exhibited the classic monocyte phenotype and expressed high levels of the VEGFR-1 marker. Thus, although CD14+/VEGFR-2- cells expressed VEGFR-1, which is also a high-affinity tyrosine kinase receptor for VEGF, they did not demonstrate high migratory capacity toward VEGF and did not take part in reendothelialization. VEGFR-1 expression, though considered restricted to endothelial cells, has been found on monocytes and macrophages.27 Furthermore, this population expressed little or no e-NOS. e-NOS is essential for neovascularization.28 Not only is it important in the mobilization of stem and progenitor cells, it determines their angiogenic capacity in ischemic tissue. e-NOS-deficient cells are impeded by a marked reduction in homing and incorporation into microvessels.28 Thus, the lack of VEGFR-2 receptor and e-NOS expression on the CD14+/VEGFR-2- cells and the weak signaling abilities of VEGFR-18 may contribute to the nonendothelial character and the ineffectiveness of the CD14+/VEGFR-2- cells in the process of reendothelialization.
Both cell populations (CD14+/VEGFR-2+ and CD14+/VEGFR-2-) were amenable to transduction by lentiviral vectors with high efficiency, implicating their potential use as vehicles for the delivery of therapeutic genes at sites of injury. However, only CD14+/VEGFR-2+ cells engrafted and were found in the seeded grafts. Therefore, this may be a good candidate population for therapeutic applications. CD14+/VEGFR-2- cells were not detected at the site of injury or in other organs at 2 or 4 weeks after transplantation. We are performing additional work to monitor the fate of these cells immediately and up to 1 week after transplantation.
A common progenitor to endothelial and smooth muscle cells has been proposed.29 Studies have demonstrated the presence of functional VEGF receptors (VEGFR-2 and VEGFR-4) on vascular smooth muscle cell.30 Unlike our previous study14 in which VEGFR-2+ cells differentiated into both endothelial and smooth muscle cells in vivo, after transplantation in the present study CD14+/VEGFR-2+ cells were found only on the luminal surfaces of the seeded arteries but not in the media, indicating that a different subpopulation of cells in the blood expressing VEGFR-2 may be precursors of smooth muscle cells.
Data suggest that monocytes are necessary for arteriogenesis and possibly neovascularization.31,32 However, until now a possible direct role for these cells in the reendothelialization process has not been reported. Reendothelialization with CD14+/VEGFR-2+ cells restored vessel diameter and media thickness in balloon-injured mouse femoral arteries. Furthermore, no neointima formation was observed in animals that underwent transplantation with these cells. It is likely that the efficiency of reendothelialization may not solely be attributable to the incorporation of these cells in the denuded arteries but may also be influenced by the release of proangiogenic factors. Thus, the CD14+/VEGFR-2+ population may improve reendothelialization by production of the growth factors. Indeed CD14+/VEGFR-2+ cells in cultivation produced higher levels of VEGF, G-CSF, and GM-CSF than did CD14+/VEGFR-2- cells. In all studies to date using CD14+ monocytes as the starting population to generate EPCs, the investigators first generated these cells in vitro to endothelial cells by cultivation in medium favoring endothelial differentiation. However, in this study, we demonstrated that there exists a subpopulation of monocytes in the circulation that already expresses many endothelial cell markers and, therefore, that this population may be recruited during reendothelialization. This may be the most likely population recruited to regenerate low-grade endothelial damage induced by risk factors for coronary artery disease.30 Hence, this population may have an important role as an endogenous repair mechanism to maintain the integrity of the endothelial monolayer by replacing denuded parts of the artery.
In summary, we report a novel finding that the expression of the tyrosine kinase receptor VEGFR-2 on CD14+ monocytes is important for their endothelial-like functional capacity. Freshly isolated circulating CD14+/VEGFR-2+ cells, when transplanted, exhibited functional competence to improve reendothelialization in balloon-injured femoral arteries. By restoring an intact endothelium, these cells may participate in the maintenance of vascular homeostasis.
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2005-04-1407.
Supported by grants from the Lars Erik Gelins Foundation and the Swedish Research Council (no. K2002-06X-14004-02B) (S.S.-H.).
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal