Abstract
The presence of activated platelets and platelet-leukocyte aggregates in the circulation accompanies major surgical procedures and occurs in several chronic diseases. Recent findings that activated platelets contribute to the inflammatory disease atherosclerosis made us address the question whether activated platelets stimulate normal healthy endothelium. Infusion of activated platelets into young mice led to the formation of transient platelet-leukocyte aggregates and resulted in a several-fold systemic increase in leukocyte rolling 2 to 4 hours after infusion. Rolling returned to baseline levels 7 hours after infusion. Infusion of activated P-selectin-/- platelets did not induce leukocyte rolling, indicating that platelet P-selectin was involved in the endothelial activation. The endothelial activation did not require platelet CD40L. Leukocyte rolling was mediated solely by the interaction of endothelial P-selectin and leukocyte P-selectin glycoprotein ligand 1 (PSGL-1). Endothelial P-selectin is stored with von Willebrand factor (VWF) in Weibel-Palade bodies. The release of Weibel-Palade bodies on infusion of activated platelets was indicated by both elevation of plasma VWF levels and by an increase in the in vivo staining of endothelial P-selectin. We conclude that the presence of activated platelets in circulation promotes acute inflammation by stimulating secretion of Weibel-Palade bodies and P-selectin–mediated leukocyte rolling.
Introduction
Platelets are the body's defense mechanism against excessive bleeding caused by endothelial damage. Activated platelets present at the site of injury provide both a prothrombotic surface and a procoagulant surface. Excessive platelet activation occurs in coronary bypass surgery and may result in thrombotic emboli and neurologic complications.1 Furthermore, many inflammatory diseases including sepsis,2,3 psoriasis,4,5 diabetes,6-8 and cystic fibrosis9 are associated with circulating activated platelets. These pathologies are also associated with endothelial inflammation.
Platelets induce leukocyte adhesion on preactivated endothelial cells in culture.10,11 In mouse models of atherosclerosis, the role of activated platelets in exacerbating lesion development has been clearly demonstrated. Activated platelets promote monocyte arrest on atherosclerotic lesions and atherosclerotic lesion growth.12 Studies in our laboratory have also demonstrated the role of platelet P-selectin, in addition to endothelial P-selectin, in atherosclerotic lesion development and maturation.13 It is important to note that P-selectin on both platelets and endothelium is expressed on the cell surface only on activation of the cells and granule secretion.14
Early endothelial inflammation is associated with the rapid release of Weibel-Palade bodies and the consequent surface expression of P-selectin and von Willebrand factor (VWF). These molecules mediate rolling of leukocytes and platelets on endothelial cells.15 This is followed by transcription and expression of molecules such as E-selectin, vascular-cell adhesion molecule 1 (VCAM-1), and other adhesion receptors.16 These receptors, in turn, mediate slow rolling and adhesion of leukocytes and have been shown to be up-regulated on treatment with activated platelets in the in vitro studies.17-20 This sequence of events leads to leukocyte rolling and extravasation at the site of injury or pathologic conditions.
Recently, vascular endothelial growth factor (VEGF) secreted from α-granules was shown to stimulate Weibel-Palade–body secretion in vitro21 and interleukin 1 (IL-1), a cytokine secreted by activated platelets, was shown to promote endothelial activation by inducing expression of intercellular adhesion molecule 1 (ICAM-1), monocyte chemoattractant protein 1 (MCP-1), and IL-6.19,20 Activated platelets cause RANTES (regulated on activation, normal T cell-expressed and secreted) deposition on IL-1β–treated endothelium. RANTES deposition, in turn, leads to increased monocyte arrest.11,12,22 In addition, the binding of platelet CD40L to its receptor CD40 on the endothelium has been shown to induce the expression of adhesion molecules, E-selectin, ICAM-1, VCAM-1, and cytokines, IL-8, MCP-1,17 and also tissue factor18 on the endothelium in vitro.
The present study was undertaken to see whether activated platelets could cause activation of intact resting endothelium in vivo, leading to inflammation. Our results show that infusion of activated platelets causes Weibel-Palade–body release that leads to P-selectin–mediated rolling of leukocytes. In addition, platelet P-selectin was crucial for this process.
Materials and methods
Reagents and antibodies
Bovine serum albumin (BSA), prostacyclin (PGI2), human thrombin, hirudin, rhodamine 6G, and 2-[N-morpholino]ethanesulfonic acid (MES) buffer were from Sigma (Saint Louis, MO). Calcein acetoxymethyl ester (calcein am), Calcein Red Orange am, 1-ethyl-3-(3-dimethyl-aminopropyl)-carbodiimide, and carboxylate-modified microspheres (1.0 μm in diameter) were from Molecular Probes (Eugene, OR). Monoclonal antibody (mAb) against mouse P-selectin mAb RB40.34 and control mAb rat IgG1 were purchased from BD Biosciences (San Diego, CA) and polyclonal antibodies against human VWF that recognize mouse VWF from Dako (Carpinteria, CA).
Animals
Male and female C57BL/6J mice wild-type (WT), CD40L-/-,23 P-selectin glycoprotein ligand-1-/- (PSGL-1-/-24; purchased from Jackson Laboratory, Bar Harbor, ME) and P-selectin-/-25 from the CBR Institute for Biomedical Research were used. Experimental procedures were approved by the Animal Care and Use Committee of the CBR Institute for Biomedical Research. Blood for platelet preparation was obtained from adult mice of all ages. Recipient mice were approximately 4 weeks old.
Blood collection and preparation of washed platelets
Mice were bled from the retro-orbital venous plexus under anesthesia. Blood was collected into polypropylene tubes containing 7.5 U/mL heparin (final concentration). Platelet-rich plasma (PRP) was prepared by centrifugation at 200g for 5 minutes at room temperature (RT). The PRP was incubated for 2 minutes with PGI2 (0.1 μg/mL) and platelets were isolated by centrifugation at 850g for 5 minutes. The resulting pellet was washed and resuspended in PIPES buffer (25 mM 1,4-piperazinediethanesulfonic acid [PIPES], 137 mN NaCl, 4 mM KCl, dextrose 0.1%, 0.35% BSA, pH 7.4). For activation, platelets were treated with human thrombin (0.2 U/mL) for 15 minutes at 37°C in presence of 2 mM EDTA (ethylenediaminetetraacetic acid) to avoid aggregation. Hirudin (1 U/mL) was added to stop the reaction. The activation was confirmed by expression of P-selectin using anti–P-selectin–fluorescein isothiocyanate (FITC) on a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ). EDTA and hirudin were also added to resting platelet preparations. Platelets were labeled using 0.25 μg/mL calcein am and infused intravenously via tail vein such that they represented approximately 10% of platelets in circulation. The releasate from activated platelets was prepared by centrifugation of the activated platelets at 850g for 5 minutes at RT followed by centrifugation at 15 000g at 4°C for 45 minutes to remove platelet microparticles.
Intravital microscopy
Leukocyte rolling was observed by intravital microscopy in mesenteric venules of 150 to 200 μm diameter with shear rates of 100 to 200 s-1 Phase-contrast intravital microscopy was done to measure leukocyte rolling per minute as described previously.25 Fluorescence intravital microscopy was done to observe platelet-leukocyte aggregates as previously described.26 Rhodamine 6G (0.67 mg/kg)27 was injected to capture rolling leukocytes using fluorescence intravital microscopy.
Images were visualized using a Zeiss IM35 inverted microscope equipped with 32×/0.40 and 10×/0.25 objective lenses (Zeiss, Jena, Germany). The microscope was connected to an SVHS Panasonic AG-6720A video recorder (Matsushita Electric, Kadoma City, Japan) using a CCD video camera (Hamamatsu Photonics Systems, Hamamatsu City, Japan). Image processing was done with Adobe Premiere 6.0 (Adobe Systems, San Jose, CA).
In vivo detection of P-selectin on endothelial surface
Yellow green (excitation/emission, 505 nm/515 nm) and red (excitation/emission, 580 nm/605 nm) carboxylate-modified microspheres (1.0 μm in diameter) were covalently coupled to anti–P-selectin mAb RB40.34 and control rat IgG1λ, respectively. The 500-μg antibody in 1 mL 50-mM MES buffer (pH 6.0) was coupled to 1.35 × 1010 microspheres according to the manufacturer's instructions (Molecular Probes). Mice were given infusions of 1 × 109 microspheres of each color and mesenteric venules observed immediately by fluorescence intravital microscopy. To control for nonspecific binding of the microspheres, the order of infusion of yellow green and red microspheres was swapped between experiments.
VWF ELISA
Plasma VWF was determined using an enzyme-linked immunosorbent assay (ELISA) technique28 with minor modification. Microtiter plates were coated overnight at 4°C with rabbit anti–human VWF, 15 μg/mL in 50 mM sodium carbonate buffer (pH 9.6). Plasma (diluted 1:20 in phosphate-buffered saline [PBS]) was incubated in the wells for 1 hour at 37°C. After 3 washes, the plates were incubated with a polyclonal anti–human VWF coupled to peroxidase (1:1000). After washing, 3,3′,5,5′-tetramethylbenzidine substrate solution (Sigma) was added to the wells and the reaction stopped with H2SO4. The number of units in each sample was determined based on the absorbance value (A450) of normal pooled plasma obtained from 3 WT mice. We defined normal pooled plasma as containing 1 U VWF antigen/mL plasma.
Statistics
The values are presented as mean ± SEM. Statistical significance was calculated using the Student t test to compare the difference between 2 groups and ANOVA followed by Tukey post hoc test was used to compare differences among multiple groups.
Results
Activated platelets in circulation stimulate leukocyte rolling.
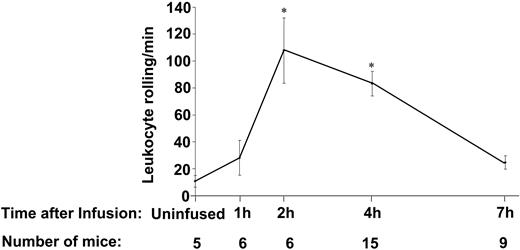
To evaluate whether the presence of activated platelets affects the adhesive properties of blood vessels, platelets from WT mice were purified, labeled with calcein, activated by thrombin, and injected via the lateral tail vein in WT recipients. Activated platelets represented about 10% of the total platelets in circulation. Leukocyte rolling was observed by intravital microscopy 1, 2, 4, and 7 hours later (Figure 1) in mesenteric venules. There was a significant increase in leukocyte rolling 2 and 4 hours after platelet infusion compared to venules of uninfused mice, P < .01 (Figure 1). The leukocyte rolling returned to baseline by 7 hours, indicating that the activating effect on endothelium is reversible. The activated platelets bound to leukocytes and platelet-leukocyte aggregates were frequently observed rolling in the mesenteric veins until 1 hour after infusion. The number of activated platelets in the aggregates decreased over time and the aggregates were hardly seen 4 hours after infusion (Table 1), whereas leukocytes free of platelets continued to roll on the vessel wall (Figure 1). The time lag of about 2 hours for endothelial activation indicates that signaling or de novo synthesis of a mediator is needed to activate the endothelium. All subsequent studies were performed 4 hours after platelet infusion.
Rolling of platelet-leukocyte aggregates after infusion of activated platelets
. | . | Number of platelet-leukocyte aggregates rolling/min . | . | |
|---|---|---|---|---|
| Time after infusion, h . | Activation of infused platelets . | Aggregates with 10 or more platelets/leukocyte* . | Aggregates with about 3 platelets/leukocyte* . | |
| 0.25 | No | 0 | 0.19 ± 0.1 | |
| 0.25 | Yes | 5.9 ± 2.4 | 4.2 ± 0.9 | |
| 1 | Yes | 5.6 ± 1.4 | 11.1 ± 1.8 | |
| 2 | Yes | 0.5 ± 0.2 | 6.9 ± 2.3 | |
| 4 | Yes | 0 | 1.6 ± 0.2 | |
. | . | Number of platelet-leukocyte aggregates rolling/min . | . | |
|---|---|---|---|---|
| Time after infusion, h . | Activation of infused platelets . | Aggregates with 10 or more platelets/leukocyte* . | Aggregates with about 3 platelets/leukocyte* . | |
| 0.25 | No | 0 | 0.19 ± 0.1 | |
| 0.25 | Yes | 5.9 ± 2.4 | 4.2 ± 0.9 | |
| 1 | Yes | 5.6 ± 1.4 | 11.1 ± 1.8 | |
| 2 | Yes | 0.5 ± 0.2 | 6.9 ± 2.3 | |
| 4 | Yes | 0 | 1.6 ± 0.2 | |
The number of rolling platelet-leukocyte aggregates per minute was observed by fluorescence intravital microscopy at the indicated time points after infusion of calcein-labeled platelets and number of fluorescent platelets per leukocyte estimated.
Average ± SEM is shown
Time course of induction of leukocyte rolling in mesenteric venules after infusion of activated platelets. The number of leukocytes rolling per minute was observed by phase-contrast intravital microscopy. Infusion of activated platelets induced a significant increase in leukocyte rolling compared with uninjected mice after 2 hours and 4 hours. Leukocyte rolling decreased by 7 hours after infusion. *P < .01, compared to uninjected mice. Average ± standard error of the mean (SEM) is shown.
Time course of induction of leukocyte rolling in mesenteric venules after infusion of activated platelets. The number of leukocytes rolling per minute was observed by phase-contrast intravital microscopy. Infusion of activated platelets induced a significant increase in leukocyte rolling compared with uninjected mice after 2 hours and 4 hours. Leukocyte rolling decreased by 7 hours after infusion. *P < .01, compared to uninjected mice. Average ± standard error of the mean (SEM) is shown.
Infusion of resting platelets or platelet releasate does not induce leukocyte rolling
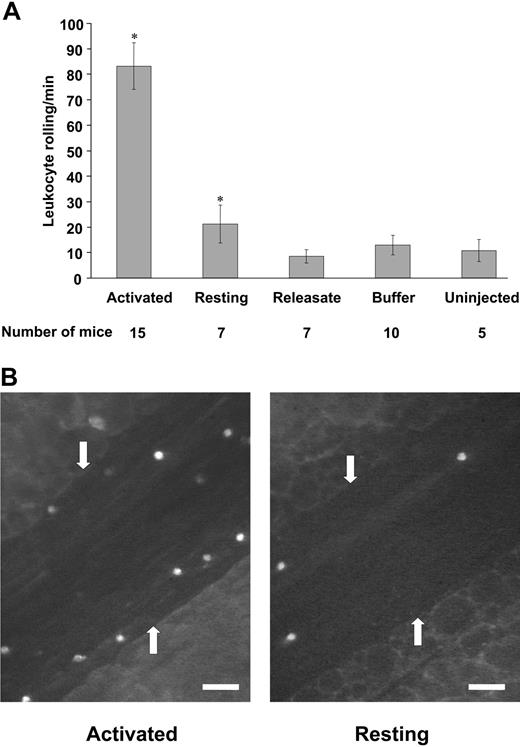
Infusion of buffer (containing thrombin, EDTA, and hirudin) did not cause an increase in leukocyte rolling over that seen in uninjected mice (Figure 2A). Whereas infusion of activated platelets significantly increased leukocyte rolling, infusion of resting platelets did not (P < .001). This indicates that only activated platelets can, in turn, activate endothelium (Figure 2A-B). The presence of activated platelets did not activate the platelets of the host animal because their P-selectin content and expression was not changed (not shown).
To determine if the observed effect of the activated platelets was due to the factors released from the activated platelets, we prepared and infused platelet releasate. Infusion of platelet releasate did not induce an increase in leukocyte rolling over that seen in uninjected mice or mice infused with buffer only (Figure 2A). This finding indicates that soluble factors released by activated platelets most likely do not contribute to the endothelial activation observed in our system.
Platelet CD40L is not involved in leukocyte rolling induced by activated platelets
Activated platelets are a major source of CD40L and CD40L was shown to activate endothelium in vitro.17 Hence, CD40L was a likely candidate mediating the endothelial activation. To test this, we infused activated CD40L-/- platelets in WT mice. Infusion of activated CD40L-/- platelets induced an about 3-fold increase in leukocyte rolling over infusion of resting CD40L-/- platelets, P < .01 (Figure 3). There was no significant difference in leukocyte rolling induced by WT or CD40L-/- platelets (P > .05). Thus, platelet CD40L apparently does not contribute to the observed endothelial activation.
Platelet and endothelial P-selectin are both critical for activated platelet-induced leukocyte rolling
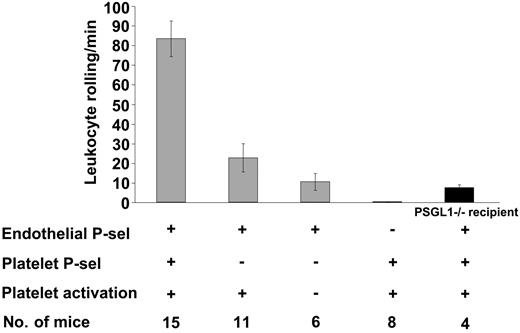
Platelet activation also leads to surface expression of P-selectin. Previous studies on chronic inflammation (atherosclerosis) indicate that platelet and endothelial P-selectin play a role in promoting monocyte arrest and lesion development.12,13 Therefore, P-selectin was the next candidate to investigate. Compared with resting platelets, activated P-selectin-/- platelets infused in WT mice did not significantly increase leukocyte rolling (Figure 4), P > .05, thus demonstrating the involvement of platelet P-selectin in this process. Furthermore, infusion of activated WT platelets in P-selectin-/- mice did not induce leukocyte rolling, indicating that on the endothelium P-selectin was the sole adhesion molecule responsible for the observed leukocyte rolling.
Leukocyte rolling in mesenteric venules 4 hours after platelet infusion. (A) The number of leukocytes rolling per minute was determined by phase-contrast intravital microscopy. Infusion of resting platelets, platelet releasate, or buffer did not induce increased leukocyte rolling as compared with uninjected controls. Infusion of activated platelets induced significantly increased leukocyte rolling compared to infusion of resting platelets (*P < .001). Average ± SEM is shown. (B) Leukocyte rolling observed by fluorescence intravital microscopy on infusion of activated (left) or resting platelets (right). Rhodamine 6G was infused to label the leukocytes. Arrows point to the vessel wall. Bar = 50 μm.
Leukocyte rolling in mesenteric venules 4 hours after platelet infusion. (A) The number of leukocytes rolling per minute was determined by phase-contrast intravital microscopy. Infusion of resting platelets, platelet releasate, or buffer did not induce increased leukocyte rolling as compared with uninjected controls. Infusion of activated platelets induced significantly increased leukocyte rolling compared to infusion of resting platelets (*P < .001). Average ± SEM is shown. (B) Leukocyte rolling observed by fluorescence intravital microscopy on infusion of activated (left) or resting platelets (right). Rhodamine 6G was infused to label the leukocytes. Arrows point to the vessel wall. Bar = 50 μm.
Leukocyte rolling in mesenteric venules 4 hours after infusion of CD40L-/- platelets. The number of leukocytes rolling per minute was determined by phase-contrast intravital microscopy. Infusion of activated CD40L-/- platelets induced increased leukocyte rolling compared with resting platelets indicating that platelet CD40L is not activating the endothelium (P < .01). Average ± SEM is shown.
Leukocyte rolling in mesenteric venules 4 hours after infusion of CD40L-/- platelets. The number of leukocytes rolling per minute was determined by phase-contrast intravital microscopy. Infusion of activated CD40L-/- platelets induced increased leukocyte rolling compared with resting platelets indicating that platelet CD40L is not activating the endothelium (P < .01). Average ± SEM is shown.
Leukocyte rolling in mesenteric venules 4 hours after platelet infusion depends on platelet and endothelial P-selectin. The number of leukocytes rolling per minute was observed by phase-contrast intravital microscopy. In WT recipients, leukocyte rolling after infusion of P-selectin-/-–activated platelets was significantly less compared to infusion of WT-activated platelets (P < .001). Infusion of WT-activated platelets in either P-selectin-/- or PSGL-1-/- (▪) recipients induced significantly less leukocyte rolling compared to infusion in WT recipients (P < .001). Average ± SEM is shown.
Leukocyte rolling in mesenteric venules 4 hours after platelet infusion depends on platelet and endothelial P-selectin. The number of leukocytes rolling per minute was observed by phase-contrast intravital microscopy. In WT recipients, leukocyte rolling after infusion of P-selectin-/-–activated platelets was significantly less compared to infusion of WT-activated platelets (P < .001). Infusion of WT-activated platelets in either P-selectin-/- or PSGL-1-/- (▪) recipients induced significantly less leukocyte rolling compared to infusion in WT recipients (P < .001). Average ± SEM is shown.
As expected, PSGL-1 was the main P-selectin ligand on leukocytes mediating adhesion to stimulated endothelium because infusion of WT-activated platelets in PSGL-1-/- recipients resulted in very few leukocytes rolling. The fact that some PSGL-1-/- leukocytes could interact with P-selectin (Figure 4) confirms the existence of additional ligands for P-selectin on leukocytes.29
Activated platelets induce release of Weibel-Palade bodies
To visualize expression of P-selectin on the vessel wall and confirm that it is elevated by the activated platelets, we have infused fluorescent microspheres coupled to anti–P-selectin antibodies 4 hours after platelet administration. The microspheres were seen binding to mesenteric venules of mice preinfused with activated but not resting platelets (Figure 5A). Isotype-coupled microspheres injected in the same mice did not bind to the mesenteric venules (not shown). Our results indicate that activated platelets indeed up-regulate P-selectin expression on mesenteric venules. P-selectin is stored in Weibel-Palade bodies in endothelial cells and is released to the cell surface on Weibel-Palade–body secretion. Therefore, it appears that activated platelets stimulate Weibel-Palade body secretion. To confirm whether Weibel-Palade bodies were released, we decided to determine plasma levels of VWF, another important adhesion molecule stored in Weibel-Palade bodies. Because platelets also contain and secrete VWF, we infused platelets obtained from VWF-/- mice.28 There was a 27% increase in circulating VWF 4 hours after infusion of activated VWF-/- platelets as compared with infusion of resting VWF-/- platelets (Figure 5B). The significant increase in plasma VWF levels confirms our hypothesis that endothelium was stimulated to release Weibel-Palade bodies.
Discussion
Our results indicate that activated platelets in circulation can initiate an acute inflammatory response in that they induce P-selectin expression on the endothelium and consequently stimulate leukocyte rolling on the vessel wall. Our study is different from previous studies in which activated platelets were shown to promote leukocyte rolling on preactivated/inflamed endothelium (already expressing leukocyte adhesion molecules),12,22,30 because we have investigated the effect of activated platelets on mesenteric venules of healthy young mice. The activation of endothelium was reversible because leukocyte rolling returned to baseline levels after 7 hours (Figure 1). We hypothesize that in the absence of a significant injury/infection accompanying platelet activation, the endothelium is able to return to its resting state.
Because the soluble factors released by activated platelets in vitro did not cause endothelial activation when infused in vivo (Figure 2A), we considered biologic activities of transmembrane molecules that are expressed on platelets only on activation. Both CD40L and P-selectin mediate cellular interactions, induce signaling, and are subsequently shed from activated platelets.31,32 Plasma CD40L levels increase in pathologies such as rheumatoid arthritis,33 sickle cell disease,34 and systemic lupus erythematosus,35 all of which are associated with platelet activation.36,37 However, contrary to the in vitro studies demonstrating endothelial activation,17,18 platelet CD40L was not responsible for endothelial activation in our in vivo system (Figure 3). It is possible that CD40L is shed rapidly from activated platelets in vivo and it has been reported that, on cleavage from platelets, CD40L loses its proinflammatory activity.31
Weibel-Palade–body secretion induced by infusion of activated platelets. (A) In vivo P-selectin expression in mesenteric venules. Fluorescent microspheres (1 μm) coupled to anti–P-selectin antibody were infused in mice given injections with activated or resting platelets 4 hours prior to intravital microscopy. Arrows point to the venule wall. Many microspheres were observed binding to mesenteric venules of a mouse preinfused with activated platelets (left) compared to venules of a mouse given an infusion of resting platelets (right). No microspheres decorated the arterioles (☆). Bar = 100 μm. Representative images are shown. (B) Plasma VWF levels after infusion of VWF-/- platelets. Plasma VWF was determined using ELISA 4 hours after infusion of activated or resting VWF-/- platelets in WT mice. VWF level of a pool of plasma of WT mice was defined as 1 U/mL. There was a significant increase in circulating VWF after infusion of activated platelets as compared to resting platelets (P < .02). Average ± SEM is shown.
Weibel-Palade–body secretion induced by infusion of activated platelets. (A) In vivo P-selectin expression in mesenteric venules. Fluorescent microspheres (1 μm) coupled to anti–P-selectin antibody were infused in mice given injections with activated or resting platelets 4 hours prior to intravital microscopy. Arrows point to the venule wall. Many microspheres were observed binding to mesenteric venules of a mouse preinfused with activated platelets (left) compared to venules of a mouse given an infusion of resting platelets (right). No microspheres decorated the arterioles (☆). Bar = 100 μm. Representative images are shown. (B) Plasma VWF levels after infusion of VWF-/- platelets. Plasma VWF was determined using ELISA 4 hours after infusion of activated or resting VWF-/- platelets in WT mice. VWF level of a pool of plasma of WT mice was defined as 1 U/mL. There was a significant increase in circulating VWF after infusion of activated platelets as compared to resting platelets (P < .02). Average ± SEM is shown.
Considering the important role of platelet P-selectin in the chronic inflammatory processes,12,13,30 we next investigated whether platelet P-selectin was important for stimulation of leukocyte rolling. Indeed, P-selectin on activated platelets was important for stimulating leukocyte rolling (Figure 4). Platelet P-selectin has been shown to be crucial in monocyte activation and firm adhesion.12,30 However, we did not see any firm adhesion of leukocytes in the healthy venules we examined, making it unlikely that the integrins of the rolling leukocytes became activated by chemokines in the process.
We have established that the leukocyte rolling was solely supported by the expression of endothelial P-selectin (Figure 4). How is endothelial P-selectin expression up-regulated? This could be accomplished by certain cytokines such as IL-4 and oncostatin M that increase P-selectin mRNA synthesis and cause prolonged surface expression of P-selectin.38 In addition or alternatively, preformed P-selectin could be released from Weibel-Palade bodies. To evaluate the hypothesis that elevated P-selectin expression on the cell surface was associated with Weibel-Palade–body release, VWF levels in plasma were determined. Indeed, concomitantly with the increased expression of P-selectin on the vessel wall, a 27% increase in circulating VWF was found 4 hours after infusion of activated VWF-/- platelets (Figure 5). A similar increase in plasma VWF levels, indicative of endothelial activation, has been seen in hypertensive patients.39,40 We have no evidence of systemic platelet activation in mice infused with activated platelets. Platelet P-selectin is neither up-regulated on endogenous platelets nor lost, that is, it can be detected on ex vivo activation. This indicates that the increase in plasma VWF could not be due to the activation of endogenous platelets. Thus, our results indicate that activated platelets can initiate acute inflammation by stimulating the release of Weibel-Palade bodies. In addition, elevation of plasma VWF by Weibel-Palade–body release could increase the thrombotic potential of the animal.
Infusion of activated platelets leads to rapid formation of platelet-leukocyte aggregates and this process is dependent on P-selectin.5,41 Such aggregates can be seen rolling on endothelium.5,26 In this study, rolling aggregates were frequently observed during the first hour after platelet infusion but decreased thereafter (Table 1). In contrast, it was at the later time points, when a majority of platelets dissociated from leukocytes perhaps as a consequence of P-selectin shedding,42,43 that the most leukocyte rolling was observed (Figure 1). It is our hypothesis that it was the formation of platelet-leukocyte aggregates that induced Weibel-Palade body secretion. There are several possible candidates for the Weibel-Palade–body secretagogue produced by P-selectin–mediated interaction of platelet with leukocytes: (1) leukocyte microparticles,44,45 (2) chemokines,46 and (3) transcellular metabolites.47 Leukocyte microparticles activate endothelium by up-regulating IL-6, MCP-1, and tissue factor.48 Tissue factor leads to thrombin and fibrin formation both of which are secretagogues for Weibel-Palade bodies.49 Platelets have been shown to stimulate leukocytes to produce cytokines such as IL-8,46 which induces Weibel-Palade–body release.50 Transcellular metabolism between activated platelets and neutrophils can generate leukotrienes LTC4 and LTD4 by platelets from neutrophil-derived LTA4.51,52 Interestingly, LTC4 and LTD4 stimulate Weibel-Palade–body release in cultured endothelial cells.47 The rolling aggregates of platelets and leukocytes could be bringing the secretagogue in proximity to the endothelium causing subsequent Weibel-Palade–body release.
In conclusion, the current study highlights a new pathway of platelet-induced inflammation. It shows that activated platelets stimulate secretion of Weibel-Palade bodies in vivo and thus induce P-selectin–mediated leukocyte rolling. The elucidation of the exact molecular mechanism through which activated platelets induce inflammation will help devise more effective therapy for use during major surgery such as coronary bypass surgery and the treatment of diseases that produce platelet activation.
Prepublished online as Blood First Edition Paper, June 14, 2005; DOI 10.1182/blood-2005-04-1530.
Supported by grants HL41002 and HL66105 from the National Institutes of Health, National Heart, Lung and Blood Institute.
An Inside Blood analysis of this article appears at the front of the issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ulrich von Andrian for advice and Lesley Cowan for assistance in preparing the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal