Abstract
Current dosing algorithms do not account for genetic and environmental factors for warfarin dose determinations. This study investigated the contribution of age, CYP2C9 and VKORC1 genotype, and body size to warfarin-dose requirements. Studied were 297 patients with stable anticoagulation with a target international normalized ratio (INR) of 2.0 to 3.0. Genetic analyses for CYP2C9 (*2 and *3 alleles) and VKORC1 (-1639 polymorphism) were performed and venous INR and plasma R- and S-warfarin concentrations determined. The mean warfarin daily dose requirement was highest in CYP2C9 homozygous wild-type patients, compared with those with the variant *2 and *3 alleles (P < .001) and highest in patients with the VKORC1 (position -1639) GG genotype compared with those with the GA genotype and the AA genotype (P < .001). Mean warfarin daily dose requirements fell by 0.5 to 0.7 mg per decade between the ages of 20 to 90 years. Age, height, and CYP2C9 genotype significantly contributed to S-warfarin and total warfarin clearance, whereas only age and body size significantly contributed to R-warfarin clearance. The multivariate regression model including the variables of age, CYP2C9 and VKORC1 genotype, and height produced the best model for estimating warfarin dose (R2 = 55%). Based upon the data, a new warfarin dosing regimen has been developed. The validity of the dosing regimen was confirmed in a second cohort of patients on warfarin therapy.
Introduction
Warfarin is the most commonly prescribed anticoagulant drug for the prophylaxis and treatment of venous and arterial thromboembolic disorders.1 The effectiveness and safety of warfarin is critically dependent on maintaining the prothrombin time, expressed as the international normalized ratio (INR), within the therapeutic range. Subgroup analyses of a number of studies have shown a sharp increase in the risk of bleeding when the INR is above the upper limit of the therapeutic range,2-5 and the risk of thromboembolic events increases when the INR falls below it.4,6
We have previously demonstrated an important contribution by age and CYP2C9 genotype to warfarin-dose requirements.7 Warfarin exerts its anticoagulant effect by reducing the regeneration of vitamin K from vitamin K epoxide in the vitamin K cycle, through inhibition of vitamin K epoxide reductase.8 This protein is encoded by the recently identified vitamin K epoxide reductase complex subunit 1 gene (VKORC1) in which rare mutations associated with clotting factor deficiencies and warfarin resistance have been identified.9 More recently, genotype for several noncoding polymorphisms in this gene has been shown to influence coumarin anticoagulant dose requirements.10,11
Patients are at greatest risk of overanticoagulation during the initiation period, with the risk of bleeding higher during the first few months than later, with the elderly and those carrying mutant CYP2C9 alleles particularly at risk12-14 These early problems are due principally to the widespread interindividual variation in response to the warfarin loading dose, explained in part by patient age and genotype.
A number of warfarin dosing algorithms15,16 and computer-generated dosing regimens17 have been developed with the aim of reducing the risk for patients of overanticoagulation during the initial days of warfarin therapy. Although many of these regimens achieve better outcomes than dosing using the original and widely used regimen reported by Fennerty et al,18 particularly among the elderly population, early achievement of target INR with avoidance of overanticoagulation remains elusive. Furthermore, patients remain, on average, within their target range for only two thirds of the time.6
The current warfarin dosing algorithms do not incorporate genetic and environmental factors that could affect warfarin-dose requirements. Knowledge of the extent to which these factors affect anticoagulation response could help in the prediction of a more individualized loading and maintenance warfarin dose for a safer anticoagulation therapy. The aim of the current study was to further characterize the effects of a number of factors that might contribute to the variability in warfarin-dose requirements, with the view to developing a novel individualized dosing regimen.
Patients, materials, and methods
Ethical permission for the study was obtained from the Joint University of Newcastle and Health Authority Ethics Committee. All patients provided written informed consent per the Declaration of Helsinki. Stable patients were recruited from anticoagulation clinics within the Newcastle upon Tyne National Health Service (NHS) Trust. A stable patient was defined as one whose warfarin-dose requirement had remained constant for at least the 3 previous clinic visits over a minimum period of 3 months, and with an INR of the prothrombin time within the range of 2.0 to 3.0. The daily maintenance dose for all the patients in the clinic is determined using the Dawn Anticoagulation Programme (4S Information Systems, Milnthorpe, United Kingdom) computer software. Patients with abnormal blood tests of hepatic or renal function and those receiving concurrent therapy known to interact with warfarin were excluded. All patients gave written consent prior to their participation in the study.
On arrival at the clinic, a blood sample (20 mL) was taken for venous INR measurement, CYP2C9 and VKORC1 genotyping, and determination of steady-state plasma warfarin enantiomer concentrations. Demographics of sex, age, weight, and height, as well as indications for warfarin therapy, additional medical problems, and concurrent medications were also recorded during the clinic visit.
The methods used for venous INR measurement, CYP2C9 genotyping, and determination of warfarin enantiomer plasma concentrations were as detailed in a previous study.7 The clearance (CL) for R- and S-warfarin was determined as follows: CL = D/2t × Css.av, in which D is the daily dose of warfarin, t is 24 hours, and Css.av is the average steady-state plasma concentration of each warfarin enantiomer, assuming that warfarin bioavailability and compliance are 100%.19
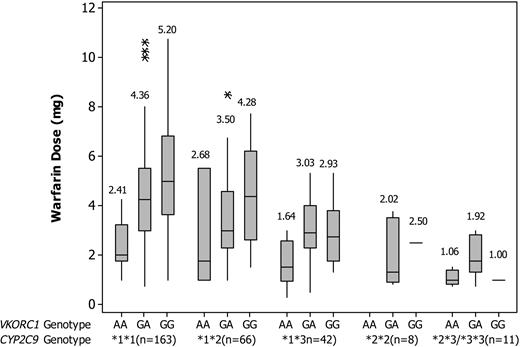
Boxplot showing the distribution of warfarin dose by CYP2C9 and VKORC1 genotype. Boxes indicate the median and interquartile ranges. Vertical lines above and below boxes indicate the minimum and maximum values. The numbers above whiskers show mean values. Each outlier is shown by an asterisk.
Boxplot showing the distribution of warfarin dose by CYP2C9 and VKORC1 genotype. Boxes indicate the median and interquartile ranges. Vertical lines above and below boxes indicate the minimum and maximum values. The numbers above whiskers show mean values. Each outlier is shown by an asterisk.
VKORC1 (–1639G>A) genotyping
Leukocyte DNA was extracted as described previously.7 DNA samples were amplified by polymerase chain reaction (PCR) in a final volume of 20 μL, each with 0.25 μM primer, 0.1 mM deoxynucleoside triphosphate (dNTP), 0.2 to 0.5 μg genomic DNA, 0.625 U Taq polymerase (Molzyme, Bremen, Germany) in 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 9.0), 1.5 mM MgCl2, 50 mM KCl, and 0.1% Triton X-100. Sequences for the forward and reverse primers were 5′-GCCAGCAGGAGAGGGAAATA-3′ and 5′-AGTTTGGACTACAGGTGCCT-3′, respectively. The PCR conditions consisted of 25 cycles of 1 minute at each of the following: 94°C, 59°C, and 72°C. Controls of known genotype as well as blank tubes without DNA added were included in each batch of samples, and the assays were validated by DNA sequencing as described previously.20 The 5′–untranslated region (UTR) PCR product (10 μL) was digested with 2 units of Msp1 in a final volume of 30 μL in the appropriate digestion buffer at 37°C for at least 16 hours. The digested products were visualized on 10% polyacrylamide gels stained with ethidium bromide.
Statistical analysis of data
Minitab version 14 (Minitab, State College, PA) was used to carry out all statistical analyses. The distributions of warfarin-dose and warfarin-clearance values were both skewed. Taking the square root of values produced a normal distribution, allowing parametric tests and regression analysis to be performed. Comparisons of age and warfarin dose were performed across the different genotypes using analysis of variance (ANOVA) and the 2-sample t test. Associations between warfarin dose, age, and plasma enantiomer concentrations were evaluated using Pearson correlation test. Stepwise regression was used to identify factors contributing to warfarin clearance followed by linear regression to obtain P values for the nonselected factors. Multivariate linear regression was performed to model the relationships of dose with other variables measured and used to develop a novel warfarin dosing algorithm. Results are presented as mean ± SD unless stated otherwise. A P value of less than .05 was taken as statistically significant.
Results
Patient characteristics
There were 176 patients who consented to take part in the study. The 121 patients with stable control of anticoagulation recruited from a previous study,7 which used the same selection criteria as the current one, were also included in the statistical analysis, giving a total of 297 patients (cohort 1). The patients' demographics, indications for anticoagulation therapy, genotype, and warfarin-dose requirements are shown in Table 1.
Characteristics of study populations
Variable . | Cohort 1, n = 297 . | Cohort 2, n = 38 . |
|---|---|---|
| Age, y, median (range) | ||
| Male | 67 (26–89) | 71 (39–91) |
| Female | 68 (23–90) | 72 (42–86) |
| Sex, n (%) | ||
| Male | 160 (54) | 19 (50) |
| Female | 137 (46) | 19 (50) |
| White, n (%) | 297 (100) | 38 (100) |
| Primary reason for anticoagulation, n (%) | ||
| AF | 191 (65) | 27 (71) |
| DVT/PE | 66 (22) | 8 (21) |
| Other | 40 (13) | 3 (8) |
| Daily warfarin dose, mg, mean (range) | 3.69 (0.3–10.75) | 3.78 (1.5–6.75) |
| CYP2C9 genotype, n (%) | ||
| *1*1 | 165 (56) | 29 (75) |
| *1*2 | 66 (22) | 4 (11) |
| *1*3 | 42 (14) | 4 (11) |
| *2*2 | 10 (3) | 0 (0) |
| *2*3 | 13 (4) | 1 (3) |
| *3*3 | 1 (<1) | 0 (0) |
| VKORC1 genotype, n (%) | ||
| GG | 74 (25) | 16 (42) |
| GA | 165 (56) | 17 (45) |
| AA | 58 (19) | 5 (13) |
Variable . | Cohort 1, n = 297 . | Cohort 2, n = 38 . |
|---|---|---|
| Age, y, median (range) | ||
| Male | 67 (26–89) | 71 (39–91) |
| Female | 68 (23–90) | 72 (42–86) |
| Sex, n (%) | ||
| Male | 160 (54) | 19 (50) |
| Female | 137 (46) | 19 (50) |
| White, n (%) | 297 (100) | 38 (100) |
| Primary reason for anticoagulation, n (%) | ||
| AF | 191 (65) | 27 (71) |
| DVT/PE | 66 (22) | 8 (21) |
| Other | 40 (13) | 3 (8) |
| Daily warfarin dose, mg, mean (range) | 3.69 (0.3–10.75) | 3.78 (1.5–6.75) |
| CYP2C9 genotype, n (%) | ||
| *1*1 | 165 (56) | 29 (75) |
| *1*2 | 66 (22) | 4 (11) |
| *1*3 | 42 (14) | 4 (11) |
| *2*2 | 10 (3) | 0 (0) |
| *2*3 | 13 (4) | 1 (3) |
| *3*3 | 1 (<1) | 0 (0) |
| VKORC1 genotype, n (%) | ||
| GG | 74 (25) | 16 (42) |
| GA | 165 (56) | 17 (45) |
| AA | 58 (19) | 5 (13) |
AF indicates atrial fibrillation; DVT, deep vein thrombosis; PE, pulmonary embolism.
VKORC1 genotyping
All samples were genotyped for the -1639G>A polymorphism, with some also genotyped for an additional intron 1 polymorphism (1173C>T), which was previously reported to affect warfarin-dose requirement.10 Analysis of genotypes for the 2 polymorphisms (data not shown) showed that G-1639 and C1173 were in strong linkage disequilibrium, and, in view of this finding, all subsequent genotyping was for the -1639G>A only. The allele frequency for the G-1639 allele was 0.57 and for A-1639 was 0.43. The observed genotype frequency showed no deviation from Hardy-Weinberg equilibrium (P = .85).
Associations with warfarin dose
Median warfarin daily dose requirements varied significantly with sex (2.9 mg females; 3.7 mg, males; P = .009). Simple correlation analysis of the data revealed that warfarin dose was highly significantly negatively correlated with age (r = -0.40; P = .001) and significantly positively correlated with body weight (r = 0.21; P = .003), height (r = 0.38; P = .001), body surface area (r = 0.21; P = .005), R-warfarin (r = 0.56; P = .001) and S-warfarin (r = 0.57; P = .001) enantiomer clearance, and total warfarin clearance (r = 0.65; P = .001).
There was no significant difference in age either between the 5 CYP2C9 or between the 3 VKORC1 genotypic groups. The mean warfarin daily dose requirement was 4.08 ± 2.13 mg in CYP2C9 homozygous wild-type patients, which was significantly higher than that in *1/*2 (3.56 ± 1.82 mg, P = .03), *1/*3 (2.7 ± 1.38 mg, P = .001), *2/*2 (1.92 ± 1.12 mg, P = .02), and *2/*3 and *3/*3 patients (1.58 ± 0.79 mg, P = .001). The mean warfarin daily dose requirement was 4.53 ± 2.22 mg in patients with the VKORC1 (-1639) GG genotype, which was significantly higher than that in the GA (3.83 ± 1.91 mg, P = .03) and AA (2.23 ± 1.26 mg, P = .001) genotype patients. Distributions of dose within the different genotypes are illustrated in Figure 1.
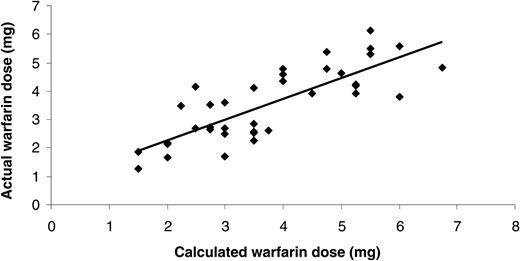
Correlation analysis of calculated warfarin dose using regression model versus actual dose in unrelated second cohort of stable patients. Warfarin dose requirements were calculated using the new regression model and correlated with their actual dose as calculated by the Dawn AC computer program. Each patient within the second cohort is represented by a diamond.
Correlation analysis of calculated warfarin dose using regression model versus actual dose in unrelated second cohort of stable patients. Warfarin dose requirements were calculated using the new regression model and correlated with their actual dose as calculated by the Dawn AC computer program. Each patient within the second cohort is represented by a diamond.
Warfarin clearance
Stepwise regression was used to evaluate the factors associated with warfarin clearance. Warfarin dose, age, body surface area, and CYP2C9 genotype significantly contributed to S-warfarin and total warfarin clearance, whereas only warfarin dose age and body size significantly contributed to R-warfarin clearance. Sex and VKORC1 genotype did not contribute to any of the 3 regression models for warfarin clearance (Table 2).
Factors affecting warfarin clearance in regression models
. | P . | . | . | ||
|---|---|---|---|---|---|
| Variable . | R-warfarin clearance . | S-warfarin clearance . | Total warfarin clearance . | ||
| Warfarin dose | .001 | .001 | .001 | ||
| Age | .001 | .04 | .001 | ||
| Body surface area | .001 | .002 | .001 | ||
| CYP2C9 genotype | .696 | .001 | .005 | ||
| VKORC1 genotype | .426 | .777 | .426 | ||
| Sex | .297 | .665 | .450 | ||
| Height | .241 | .989 | .472 | ||
| Weight | .205 | .727 | .361 | ||
. | P . | . | . | ||
|---|---|---|---|---|---|
| Variable . | R-warfarin clearance . | S-warfarin clearance . | Total warfarin clearance . | ||
| Warfarin dose | .001 | .001 | .001 | ||
| Age | .001 | .04 | .001 | ||
| Body surface area | .001 | .002 | .001 | ||
| CYP2C9 genotype | .696 | .001 | .005 | ||
| VKORC1 genotype | .426 | .777 | .426 | ||
| Sex | .297 | .665 | .450 | ||
| Height | .241 | .989 | .472 | ||
| Weight | .205 | .727 | .361 | ||
Warfarin dosing algorithm
The multivariate regression model including the variables age, CYP2C9 and VKORC1 genotype, and height produced the best model for estimating warfarin dose, having the largest R2 value (Table 3). This novel dosing algorithm was assessed in a second unrelated population of 38 patients on warfarin therapy and with stable control of anticoagulation (cohort 2; see Table 1 for details on patient characteristics and demographics). Pearson correlation analysis of data showed a very close and highly significant relationship between the calculated warfarin dose using the best regression dose model and actual dose (r = 0.80; P < .001) (Figure 2). The use of the regression model was more likely to underestimate the daily maintenance dose requirements (mean difference = -0.2 ± 0.8 mg/d).
Regression equation for modeling warfarin daily dose requirements based on age, genotype, and height
Model, x variables . | Regression equation . | P . | R2 for model, % . |
|---|---|---|---|
| Age | √D = 2.85 – 0.0137 (Age) | .001 | 16.7 |
| CYP2C9 genotype | √D = 2.01 – 0.252 (CYP*2) – 0.454 (CYP*3) | .001 | 17.5 |
| VKORC1 genotype | √D = 2.41 – 0.297 (VKORC1) | .001 | 15.0 |
| Height | √D = 2.12 + 0.0237 (Height) | .001 | 16.0 |
| Age, height, CYP2C9 genotype, VKORC1 genotype | √Dose = 0.628 – 0.0135 (Age) – 0.240 (CYP*2) – 0.370 (CYP*3) – 0.241 (VKOR) + 0.0162 (Height) | .001 | 54.2 |
Model, x variables . | Regression equation . | P . | R2 for model, % . |
|---|---|---|---|
| Age | √D = 2.85 – 0.0137 (Age) | .001 | 16.7 |
| CYP2C9 genotype | √D = 2.01 – 0.252 (CYP*2) – 0.454 (CYP*3) | .001 | 17.5 |
| VKORC1 genotype | √D = 2.41 – 0.297 (VKORC1) | .001 | 15.0 |
| Height | √D = 2.12 + 0.0237 (Height) | .001 | 16.0 |
| Age, height, CYP2C9 genotype, VKORC1 genotype | √Dose = 0.628 – 0.0135 (Age) – 0.240 (CYP*2) – 0.370 (CYP*3) – 0.241 (VKOR) + 0.0162 (Height) | .001 | 54.2 |
Age: input age in years; CYP2C9 genotype: input 0, 1, or 2 for the number of *2 and *3 alleles within the patient's genotype; VKORC1 genotype: input 1 for GG, 2 for GA, and 3 for AA; height: input height in centimeters.
Discussion
Warfarin therapy, particularly during the initiation period, is associated with a high incidence of overanticoagulation with resultant bleeding, as the inability to take into account interindividual and intraindividual variability in response to the drug makes accurate dose predication impossible. This is frequently a cause for concern. It has been estimated that almost half of AF patients who are eligible for, and would benefit from, warfarin therapy are not receiving the drug because of the associated risks and monitoring costs.15
We have previously reported on the contribution of CYP2C9 genotype, age, and other environmental factors to chronic warfarin-dose requirements.7 In this study, we have extended our previous work to show the impacts of age and CYP2C9 gene in a more representative sample of anticoagulated patients. We have also been able to assess the additional contribution from VKORC1 genotype to interindividual variability, concluding that patient age, height, and genetic polymorphism of CYP2C9 and VKORC1 account for nearly 55% of the variability in warfarin daily dose requirements.
As with previous studies,7,21-23 we found that dose requirements fell with age, decreasing by approximately 0.5 to 0.7 mg per decade between the ages of 20 to 90 years irrespective of genotype and patient height. Although the patients were not evenly spread across the 8 age decades studied, the sample reflects the distribution by age of anticoagulated patients.
Our results extend the known association between CYP2C9 genotype and daily warfarin dose. There were significant differences in mean dose requirements between each of the variant alleles compared with the wild type. Unfortunately, there was only one patient with the genotype *3/*3, despite targeting low-dose patients, and therefore the predicted doses for this genotype should be viewed with caution.
We also confirm that carriers of the VKORC1 (-1639) AA genotype require a significantly lower daily dose of warfarin than those carrying the GA or the GG genotypes. Although the VKORC1 gene has only recently been identified, a range of polymorphisms within it have now been described.9-11 Most recently, Bodin et al have demonstrated that VKORC1 genotype explains 37% of the variability in response to acenocoumarol in healthy volunteers.11 While the Bodin et al study11 looked at effects for a number of common haplotypes, all the observed variability could be explained by the tightly linked -1639G>A or 1173C>T polymorphisms only. The current data confirm the findings from earlier reports on anticoagulant response as well as demonstrate the magnitude of the effect of VKORC1 genotype on warfarin-dose requirement. The basis for the variability associated with the -1639 and 1173 polymorphisms remains unclear as neither single nucleotide polymorphism (SNP) appears functionally significant when assessed using appropriate in vitro assays.10,11 An alternative explanation is that polymorphisms situated further upstream or in the 3′-UTR but in linkage disequilibrium with G at position -1639 affect levels of VKOR.
Although we demonstrated significant positive correlations between warfarin dose and each of body surface area, weight, and height, only the latter made a significant contribution to warfarin-dose requirements in the regression model. Despite the significant difference in warfarin dose between male and female patients, sex made no significant contribution to the regression model. However, it is likely that the differences in warfarin-dose requirements are attributable to body size, with females in general smaller than males.
More than 40% of the variability in dose requirements remains unaccounted for. Factors of comorbidity and, to an extent, concurrent medication were controlled for, as these formed part of the exclusion criteria of the study. However, due to the study design, the contributions of vitamin K status and alcohol consumption to warfarin-dose requirement were not examined. One study that did examine the influence of vitamin K upon warfarin anticoagulation found that plasma vitamin K concentration was significantly negatively correlated with patient INR.24 It is unlikely that the impact of vitamin K on warfarin-dose requirements would have been realized in this cross-sectional study in patients with stable anticoagulation control since warfarin dose would have already been adjusted in the patients to control for their vitamin K status.7 It is also likely that the interindividual variability in warfarin-dose requirements is due, at least in part, to additional genetic factors, including polymorphisms in apolipoprotein E,25 multidrug resistance 1 (MDR1),26 genes encoding vitamin K–dependent clotting factors,27 and possibly genes encoding additional components of the vitamin K epoxide reductase complex.9
A significant contributor to the observed variability in dose requirements is interindividual differences in warfarin clearance. As expected, warfarin dose, age, genotype, and body surface area had a significant effect on S-warfarin clearance. These results are thus broadly in concordance with those of previous studies showing that CYP2C9 polymorphisms and age markedly affect S-warfarin clearance and thus warfarin-dose requirements.7,23,28 Warfarin dose, age, and body surface area were the only significant contributors to the interindividual variability in R-warfarin clearance. A recent detailed study found no additional contribution by CYP2C9 upstream polymorphisms to warfarin clearance,20 and, while some additional coding region polymorphisms are known to occur, these are extremely rare in whites. VKORC1 genotype had no effect on warfarin clearance and hence affects warfarin-dose requirements through a different mechanism, namely the vitamin K cycle.
In the present study, we have shown that incorporation of age, CYP2C9 and VKORC1 genotype, and height allow the best estimate of warfarin maintenance dose, none of which are taken into account in currently available algorithms. For example, using our regression model, the estimated warfarin daily dose requirement for a 170-cm tall 90-year-old patient with CYP2C9*1/*3 and VKORC1-AA genotype is over 6 times lower than that for a 30-year-old patient of the same height and with CYP2C9 wild-type and VKORC1-GG genotype (1.16 mg [95% CI: 0.91-1.44] vs 7.39 mg [95% CI: 6.71-8.11]). This clearly highlights the inadequacy of the current dosing regimens and the need to move toward a more individualized approach to warfarin therapy.
Our proposed dosing algorithm was validated in an unrelated cohort of patients on chronic therapy with warfarin. Prospective clinical studies are now required to assess dosing algorithms that incorporate the contribution of age, genotype, and height to allow individualization of warfarin dose, during both the initiation and maintenance stages of therapy. If such studies show an improved outcome of therapy, then successful implementation of the new algorithms would depend on the use of the methods that are available for rapid and easy genotyping in clinical settings.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-03-1108.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal