Abstract
The transcription factor hypoxia-inducible factor-1 (HIF-1) is critical for erythropoietin and other factors involved in the adaptation of the organism to hypoxic stress. Conflicting results have been published regarding the role of the mitochondrial electron transport chain (ETC) in the regulation of HIF-1α. We assessed cellular hypoxia by pimonidazole staining and blotting of the O2-labile HIF-1 α-subunit in human osteosarcoma cell cultures (U2OS and 143B). In conventional, gas-impermeable cell culture dishes, ETC inhibitors had no effect on pimonidazole staining or HIF-1α abundance in a 20% O2 atmosphere; both parameters were undetectable. Pimonidazole staining and HIF activity were substantial in 0.1% O2 irrespective of ETC inhibition. At an intermediate oxygen concentration (3% O2) pimonidazole staining and HIF-α expression were detectable but strongly reduced after ETC inhibition in conventional cell cultures. All effects of ETC inhibition on HIF-1α regulation were eliminated in gas-permeable dishes. As shown in a 143B subclone deficient in mitochondrial DNA (206ρ0), genetic inactivation of the ETC led to similar responses with respect to HIF-1α regulation as ETC inhibitors. Our data demonstrate that reduction of oxygen consumption reduces the O2 gradient in conventional cell cultures, causing elevation of the cellular O2 concentration, which leads to degradation of HIF-α.
Introduction
Adaptation to low oxygen concentration leads to a series of responses through the transactivation of more than 70 target genes1 mediated by the hypoxia-inducible factor-1 (HIF-1). The hematopoietic growth factor erythropoietin (EPO) and the angiogenic peptide vascular endothelial growth factor (VEGF) are the most prominent examples of HIF-1 target genes. Actually, the HIF binding site embedded in the 3′ enhancer of the EPO gene allowed purification and characterization of HIF-1.2 HIF is also known to be involved in angiogenesis, tumor growth, and apoptosis,3,4 which makes it a challenging target for therapeutic manipulation. HIF-1 is a heterodimeric transcription factor composed of 2 subunits.2 Each HIF-1 subunit contains an N-terminal basic helix-loop-helix domain responsible for dimerization and DNA binding followed by a PAS domain (Per, aryl hydrocarbon nuclear translocator [ARNT], and Sim were the first members of this protein family) that is involved in protein interactions.5,6 Both subunits are constitutively expressed. While the β-subunit is present in the nucleus at detectable levels under normoxia and hypoxia, HIF-1α levels are affected by changes in the cellular oxygen partial pressure (pO2).7,8 In hypoxia, HIF-1α accumulates and is translocated to the nucleus where it dimerizes with HIF-1β, forming an active DNA-binding complex (reviewed by Semenza1 ). In contrast, atmospheric pO2 leads to a rapid destruction by the ubiquitin-proteasome system.9,10 Initially, HIF-1α is hydroxylated at Pro564 and Pro402 by 2-oxoglutarate–dependent dioxygenases,11,12 which have been termed “prolyl hydroxylase domain,” containing protein 1, 2, and 3 (PHD-1, 2, 3)13 or HIF-1α prolyl hydroxylase 3, 2, and 1,14 which prevent the stabilization. Furthermore, HIF-1α is hydroxylated at Asn803 by an enzyme first identified as “factor inhibiting HIF-1” (FIH-1),15 which abrogates the transactivation by preventing the binding of transcriptional coactivators such as cyclic adenosine monophosphate response-element binding protein (CREB) binding protein (CBP)/p300.16,17 Thus, the hydroxylases, which regulate HIF-1α by a direct oxygen-dependent mechanism, are oxygen sensors in vivo.
In addition to the regulation of the HIF by oxygen supply, there are different possibilities to either increase HIF-1α levels in normoxia or attenuate HIF-1α expression in hypoxia. The stabilization in normoxia can occur by inhibition of the prolyl hydroxylases by transition metals, iron chelators,18 nitric oxide,19,20 2-oxoglutarate analogs,21 or by an increased translational activity induced by growth factors, hormones, and other factors (reviewed by Bilton and Booker22 ). A destabilization in an hypoxic environment can be caused by carbon monoxide and, again, nitric oxide.23,24 It has also been reported that inhibitors of complex I of the mitochondrial electron transport chain (ETC) (eg, rotenone) can compromise normal up-regulation of HIF-1α in response to hypoxia.25,26 Interestingly, conflicting data have been published for antimycin A, an inhibitor of the downstream part of complex III of the ETC: While one group reported induction of HIF-α,26,27 other investigators observed a pronounced down-regulation of HIF-α in hypoxia.25 Consequently, the interpretation of the data was markedly different: The first group26,27 proposed that hypoxia leads to an increase in the generation of reactive oxygen species (ROSs), which in turn stabilize HIF-α. The second group25 has provided evidence for 2 important points: All inhibitors of the ETC have the same effect on HIF-1α and, furthermore, the degradation caused by inhibition of mitochondrial degradation is dependent on HIF-α prolyl hydroxylases. Thus, this group suggested that all inhibitors of the ETC cause an intracellular redistribution of molecular oxygen, which reactivates HIF-α prolyl hydroxylases. Two other groups of investigators who used almost anoxic conditions (0.1% O2) were unable to detect any effect of inhibition of the ETC on HIF-α regulation.28,29 Here, we present a unifying hypothesis that accommodates nearly all the observations reported.
Materials and methods
Reagents
The inhibitors of the respiratory chain were purchased from Sigma (Steinheim, Germany) (rotenone, myxothiazol, antimycin A) and from Merck (Darmstadt, Germany) (potassium cyanide [KCN]). Dimethyloxalyl-glycine (DMOG) was bought from Porphyrin Systems (Lübeck, Germany). The Hypoxyprobe-1 Kit (Chemicon International, Temucula, CA) for the detection of tissue hypoxia was a kind gift from Dr Klaus Wagner, University of Lübeck, Germany.
Cell culture
Human hepatoma cells (HepG2) were maintained in RPMI medium (Gibco, Karlsruhe, Germany). Two human osteosarcoma cell lines (U2OS and 143B-wt) were cultured in Dulbecco modified Eagle medium (DMEM; Gibco). All growth media were supplemented with 10% (vol/vol) fetal calf serum (FCS; Gibco), 2 mM glutamine (Gibco), and 50 IU/mL penicillin and 50 μg/mL streptomycin (Sigma). The mitochondrial DNA-deficient derivative 143B-206ρ0 of 143B-wt cells30 was provided by Prof Rudolf Wiesner, University of Cologne, Germany. For ρ0 cells DMEM was additionally supplemented with 50 μg/mL uridine. The ρ0 status was proven by uridine auxotrophy; all ρ0 cells failed to grow in the absence of uridine in the growth medium.
The studies were carried out using conventional polystyrene dishes or special gas-permeable dishes (Petriperm; Bachofer, Reutlingen, Germany) with a hydrophilic fluoroethylene-propylene (FEP) copolymer Teflon bottom membrane of 25 μm thickness on which the cells were grown. Dishes of the same type are also available from another company (Lumox; Greiner Bio-One, Frickenhausen, Germany). Cells were maintained in a saturated humidified atmosphere at 37°C, 5% CO2 and 95% air. For these culture conditions we use the term “normoxia” because we have experimentally demonstrated the absence of pimonidazole staining and of HIF activation in our cells under these conditions (see “Results”). For hypoxic stimulation, cultures were flushed in an InvivO2 400 hypoxia workstation (Ruskinn Technologies, Leeds, United Kingdom) with a gas mixture of 0.1%, 1%, or 3% O2, 5% CO2, and balance N2.
Protein extraction and analysis
Whole cell extracts for HIF-1α analysis were prepared on ice using a denaturing urea lysis buffer (8 M urea, 1% sodium dodecyl sulfate [SDS], 8.7% [vol/vol] glycerol, and 10 mM Tris [tris(hydroxymethyl)aminomethane] pH 6.8) supplemented with protease inhibitors (Protease InhibitorCocktail Set V; Calbiochem, Darmstadt, Germany). After repeated rinsing with ice-cold phosphate-buffered saline solution (PBS), cells were harvested and subjected to centrifugation (2500 rpm, 5 minutes, 4°C), lysed, and then sonicated. Twenty micrograms of protein per lane was loaded onto 7.5% SDS polyacrylamide gels, separated by electrophoresis, and blotted onto polyvinylidene fluoride (PVDF) membranes (Schleicher and Schuell, Dassel, Germany). Membranes were blocked with 5% skim milk in PBS overnight at 4°C. As primary antibody for the detection of HIF-1α protein, a monoclonal HIF-1α antibody (BD Transduction Laboratories, Heidelberg, Germany) diluted 1:1000 in 5% skim milk/PBS for 2 hours was used, followed by incubation with an anti–mouse horseradish peroxidase–coupled antibody (DAKO, Hamburg, Germany) in a dilution of 1:2000 in 5% skim milk/PBS for 1 hour. A polyclonal antibody raised against SP-1 (PEP2; Santa Cruz, Heidelberg, Germany) was diluted 1:2000 in 5% skim milk/PBS. SP-1 is not hypoxia inducible and was used to verify uniform loading and transfer. In this case a goat anti–rabbit horseradish peroxidase–coupled secondary antibody (1:2000, 5% skim milk/PBS; DAKO) was used. Antibody binding was detected using the enhanced chemiluminescence (ECL) Western Blotting Analysis system (Amersham Biosciences, Freiburg, Germany).
Assay of EPO and VEGF
For measurement of EPO and VEGF secretion into the growth medium, HepG2 cells were plated in 24-well dishes. When the cells approached confluence the dishes were incubated either in 20% O2 (“normoxia”) or in 3% O2 (“hypoxia”) for 24 hours. The growth medium was collected, and EPO secretion was analyzed by enzyme linked immunosorbent assay (ELISA; Medac, Hamburg, Germany). In the same samples VEGF secretion was assessed by ELISA (R&D Systems, Wiesbaden, Germany). Both ELISAs were done exactly as recommended by the manufacturer.
Pimonidazole staining and fluorescence microscopy
U2OS cells were grown on coverslips and stimulated with antimycin A (1 μg/mL) or rotenone (1 μM) in hypoxia (3% or 0.1% O2) or remained untreated in normoxia (20% O2) for 4 hours. Pimonidazole was administered to cells in a concentration of 200 μM for 45 minutes. Cells were fixed by methanol incubation for 10 minutes at -20°C, rehydrated in PBS, followed by blocking of nonspecific binding sites with 3% bovine serum albumin (BSA) in PBS. Coverslips were incubated with the fluorescein isothiocyanate (FITC)–labeled monoclonal Hypoxyprobe-1 monoclonal antibody 1 (mAb1) (1:100 in 3% BSA) for 45 minutes at 4°C while BSA alone served as negative control. Finally, nuclei were visualized using bisbenzimide fluorochrome (Calbiochem). After washing 3 times with PBS and distilled water, coverslips were mounted on object holders with Mowiol (Calbiochem). The immunostained cells were examined by fluorescence microscopy using an Axioplan 2 imaging microscope with Zeiss plan-Neofluar 20×/0.5 numeric aperture objective. An Axiocam camera was used to capture images, which were digitally saved using Vision Axiovision 3.1 software (Carl Zeiss Vision, Mannheim, Germany).
Oxygen consumption assay
Cells were cultured in 75 cm2 flasks to approximately 90% confluence. After trypsinization, cells were spun down (650g [2000 rpm]; 5 minutes) and resuspended in 1 mL FCS-free cell culture medium. Cells were then transferred into a temperature-controlled reaction chamber set to 37°C (Hansa-Tech Instruments, King's Lynn, United Kingdom; kindly provided by Prof Joachim Fandrey, University Essen, Germany) and stirred magnetically. Measurement of the oxygen concentration was done polarographically against a platinum electrode, and values were recorded once per second. To demonstrate maximal inhibition of the ETC, inhibitors of the respiratory chain were added directly into the reaction chamber. All experiments were repeated 4 times with cells from independent cultures. After the experiment, cells were counted and the respiration rate was calculated from the decrease of the oxygen concentration in the reaction chamber during the experiment.
Statistical analysis
The data in bar graphs are presented as means + standard deviation (SD). A significant difference between 2 group means was determined by Student t test for unpaired data. The Dunnett post hoc test was applied to compare a control mean with several treatment means. P < .05 was considered statistically significant.
Results
Mathematic statement of oxygen diffusion in monolayer cell culture
Cellular oxygen supply in conventional polystyrene cell-culture systems is a 1-dimensional diffusion process through the growth medium, and a physical description is given by the Fick First Law of Gas Diffusion:
where
is the quantity of oxygen transported in a period of time from the top layer to the bottom (ie, the cellular layer); D is the diffusion constant; and A is the area of diffusion. Assuming that the oxygen solubility in growth medium is sufficient to lead to an equilibrium between the incubator gas and the top layer of the growth medium, Cgas is the oxygen concentration defined by the composition of the incubator atmosphere. Ccell is the oxygen concentration at the bottom of the dish, and h is the diffusion distance between the top and the bottom layer of the growth medium and is defined by the volume of the growth medium used. The area A, Cgas, and h are constant over time in an individual experiment. In this setting oxygen consumption of the cells will lead to a decrease of Ccell (ie, Ccell must be lower than Cgas). The difference between Cgas and Ccell will induce oxygen diffusion through the medium. Of note, it is impossible to predict Ccell from Cgas. Clearly, Ccell depends on cell density, with dense cells leading to a more pronounced decrease of Ccell. Inhibition of oxygen consumption is expected to increase Ccell as compared with the noninhibited state; the boundary condition here is that Ccell is equal to Cgas in the absence of any oxygen consumption by the cells.
Western blot analysis of HIF-1α in extracts from human osteosarcoma cells (U2OS) treated with various mitochondrial inhibitors. Cells were cultured for 4 hours in normoxia (NOX) or hypoxia (HOX) with or without inhibitors of the ETC. The transcription factor SP-1, which is not responsive to hypoxia, served as a control for uniform loading and transfer. (A) Cells were cultured in intermediate hypoxia (3% O2) on conventional polystyrene dishes. (B) Cells were cultured in intermediate hypoxia (3% O2) on gas-permeable dishes. (C) Cells were incubated with or without antimycin A (1 μg/mL) or rotenone (1 μM) for 4 hours under strong hypoxic conditions (0.1% O2) on conventional cell culture supports.
Western blot analysis of HIF-1α in extracts from human osteosarcoma cells (U2OS) treated with various mitochondrial inhibitors. Cells were cultured for 4 hours in normoxia (NOX) or hypoxia (HOX) with or without inhibitors of the ETC. The transcription factor SP-1, which is not responsive to hypoxia, served as a control for uniform loading and transfer. (A) Cells were cultured in intermediate hypoxia (3% O2) on conventional polystyrene dishes. (B) Cells were cultured in intermediate hypoxia (3% O2) on gas-permeable dishes. (C) Cells were incubated with or without antimycin A (1 μg/mL) or rotenone (1 μM) for 4 hours under strong hypoxic conditions (0.1% O2) on conventional cell culture supports.
Effect of mitochondrial inhibitors on HIF-1α protein expression in hypoxia
Previous studies on the effects of the mitochondrial ETC on the stabilization of HIF-1 have led to controversial results.25,26,28,29 In a first set of experiments we incubated human osteosarcoma cells (U2OS) in the presence of various inhibitors of the ETC in conventional cell culture dishes and analyzed the expression of HIF-1α protein by Western blotting. Cells were subjected to the experimental conditions for 4 hours with either antimycin A (1 μg/mL), myxothiazol (1 μM), rotenone (1 μM), or potassium cyanide (1 mM). All concentrations used led to maximal inhibition of the ETC as confirmed by oxygen consumption measurements (data not shown). None of the inhibitors of the ETC induced detectable levels of HIF-1α in an incubator atmosphere composed of 20% oxygen, 5% CO2, balance N2. All substances inhibited the accumulation of HIF-1α under moderately hypoxic conditions (3% O2; Figure 1A). The effect was detectable after 4 hours and persisted after 8 hours (data not shown). Consistent with previous reports25 and in line with our mathematic analysis, all substances showed the same effect independent of the complex inhibited.
According to the mathematic statement of O2 diffusion, HIF activation in conventional cell culture dishes can be caused by an oxygen gradient over a rather long diffusion distance, which is in the range of 2 to 5 mm depending on the volume of the growth medium used. This gradient is largely reduced when cells are grown on very thin (25 μm) FEP Teflon membranes in which oxygen is soluble.31 We used these dishes in a second set of experiments to eliminate oxygen diffusion gradients. Strikingly, HIF-1α levels were no longer affected by the treatment with mitochondrial inhibitors—that is, all cells treated with inhibitors showed HIF-1α protein expression comparable to the level in control cells (Figure 1B). Again, no effect in normoxia could be observed. Importantly, the exposition time of the x-ray film of Figure 1B was significantly longer than in Figure 1A. A direct comparison between U2OS cells cultured on gas-impermeable versus gas-permeable supports showed that HIF-1α levels were markedly lower in gas-permeable dishes (Figure 2A). When cells were cultured in oxygen-impermeable dishes and the oxygen concentration in the incubator was reduced to almost anoxic conditions (0.1% O2), the destabilizing effect of the ETC inhibitors on HIF-1α was abolished (Figure 1C).
Immunoblot analysis of HIF-1α protein expression in conventional, oxygen-impermeable culture dishes versus gas-permeable dishes. (A) Human osteosarcoma cells (U2OS) were exposed to intermediate hypoxia (3% O2) for 18 hours on the indicated type of culture dish. (B) Human osteosarcoma cells (143B-wt) and their mitochondrial DNA-deficient derivative 143B-206ρ0 were grown on conventional or gas-permeable cell culture dishes and incubated in 20% O2, 1% O2, or 3% O2. Confluent 143B-wt expresses detectable levels of HIF-1α. (C) Comparison of 143B-wt and 143B-206ρ0 cells cultured on conventional versus oxygen-permeable supports at 3% oxygen.
Immunoblot analysis of HIF-1α protein expression in conventional, oxygen-impermeable culture dishes versus gas-permeable dishes. (A) Human osteosarcoma cells (U2OS) were exposed to intermediate hypoxia (3% O2) for 18 hours on the indicated type of culture dish. (B) Human osteosarcoma cells (143B-wt) and their mitochondrial DNA-deficient derivative 143B-206ρ0 were grown on conventional or gas-permeable cell culture dishes and incubated in 20% O2, 1% O2, or 3% O2. Confluent 143B-wt expresses detectable levels of HIF-1α. (C) Comparison of 143B-wt and 143B-206ρ0 cells cultured on conventional versus oxygen-permeable supports at 3% oxygen.
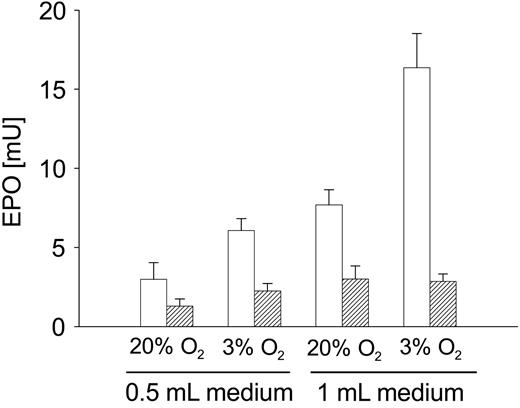
EPO secretion cells in response to variation of the diffusion distance. Human hepatoma cells (HepG2) were cultured in 24-well polystyrene dishes. When the cells approached confluence the growth medium was replaced with 0.5 mL (which corresponds to a diffusion distance of 2.5 mm) or 1 mL (diffusion distance 5 mm). The experiment was done in the absence or presence of rotenone (1 μM). EPO concentrations determined by ELISA were corrected for medium volume and are therefore given as secreted units. Bars represent mean of 3 independent cultures + SD, without rotenone (□) or with rotenone (▪). Inductions of EPO secretion by hypoxia, by an increase of the diffusion distance and by combination of both, were all statistically significant (P < .05) as calculated by the Dunnett post hoc test.
EPO secretion cells in response to variation of the diffusion distance. Human hepatoma cells (HepG2) were cultured in 24-well polystyrene dishes. When the cells approached confluence the growth medium was replaced with 0.5 mL (which corresponds to a diffusion distance of 2.5 mm) or 1 mL (diffusion distance 5 mm). The experiment was done in the absence or presence of rotenone (1 μM). EPO concentrations determined by ELISA were corrected for medium volume and are therefore given as secreted units. Bars represent mean of 3 independent cultures + SD, without rotenone (□) or with rotenone (▪). Inductions of EPO secretion by hypoxia, by an increase of the diffusion distance and by combination of both, were all statistically significant (P < .05) as calculated by the Dunnett post hoc test.
In a further experiment we used 0.5 mL or 1 mL growth medium in 24-well oxygen-impermeable culture dishes at intermediate oxygen concentration (3%) to analyze the effect of an increased diffusion distance. As a model we used HepG2 cells, which show hypoxia-inducible secretion of EPO and VEGF. As expected, the increase of the EPO secretion, which was caused by reduction of the oxygen fraction in the incubator from 20% to 3%, was statistically significant, but the induction of EPO secretion induced by an increase of the diffusion distance from 2.5 mm (which corresponds to 0.5 mL growth medium) to 5 mm (which corresponds to 1 mL) appeared even larger. In line with our hypothesis, rotenone eliminated the hypoxic induction (Figure 3). Secretion of the angiogenic peptide VEGF was affected in the same way with the exception that rotenone had a less pronounced effect on VEGF induction by hypoxia (data not shown).
HIF-1α in cells depleted of mitochondrial DNA
A direct comparison of U2OS cells grown in gas-permeable dishes to cells cultured in conventional, oxygen-impermeable supports revealed that HIF-1α levels were generally lower in gas-permeable dishes (Figure 2A). To further explore the role of a functioning mitochondrial respiratory system, we used cells that had been depleted of mitochondrial DNA (ρ0 cells) and thus lack activity of the ETC. Regarding HIF-α regulation in ρ0 cells, previous reports have come to controversial conclusions.27-29 The 206ρ0 cell line has been derived from the human osteosarcoma cell line 143B.30 With respect to cellular oxygen consumption, genetic inactivation of the ETC should have an effect comparable to pharmacologic inhibition. When we exposed 206ρ0 cells to a moderately hypoxic atmosphere of 3% or 1% O2 in polystyrene dishes, the cells showed attenuated HIF-1α protein levels in comparison to the HIF-1α induction in 143B-wt cells (Figure 2B). In oxygen-permeable cell culture supports, 206ρ0 cells displayed variable hypoxic induction of HIF-1α when the experiments were repeated under identical conditions (compare Figure 2B-C). These results suggest that parameters are involved in the regulation of HIF-1α in 206ρ0 cells that were not under control in our experimental settings.
The effect of ETC inhibition on HIF-α protein levels is antagonized by prolyl hydroxylase inhibitors
The HIF-α prolyl hydroxylases PHD1-3 catalyze hydroxylation of distinct proline residues in normoxia, which is the initial signal for von Hippel-Lindau protein (pVHL)–dependent proteasomal degradation. Enzyme activity is dependent on oxygen, ferrous iron, and 2-oxoglutarate.13 As a consequence the hydroxylases are inactive in hypoxia and in the presence of iron chelators18 or oxoglutarate analogs21 in normoxia. To assess whether activity of the PHDs has a role in HIF-α destabilization by ETC inhibitors, we studied the response of cells treated with the oxoglutarate analog DMOG and inhibitors of the respiratory chain. U2OS cells were incubated with either antimycin A (1 μg/mL) or rotenone (1 μM) or a combination of mitochondrial inhibitor and DMOG (Figure 4) in an atmosphere of 3% oxygen. We demonstrated HIF-1α accumulation in response to DMOG in normoxia and reproduced the destabilizing effect of antimycin A and rotenone in moderate hypoxia. Strikingly, the effect of ETC inhibition on HIF-1α accumulation was abrogated by simultaneous application of an inhibitor of the ETC and DMOG. This result suggests that the enhanced degradation of HIF-1α in response to mitochondrial inhibition is mediated by increased activity of the PHDs, which require molecular oxygen as a cosubstrate.
Immunohistochemical analysis with pimonidazole as an hypoxia marker
In an additional experimental approach we sought to determine cellular hypoxia using pimonidazole as a surrogate marker for hypoxia.32,33 This substance has been used previously in cell culture,34 in animal studies,35 and in tumor patients36 to detect hypoxic cells in tissues. Pimonidazole is activated by reduced nicotinamide adenine dinucleotide (NADH) or reduced nicotinamide adenine dinucleotide phosphate (NADPH) to form a nitro radical anion intermediate that is degraded in the presence of oxygen. In the absence of oxygen further reduction leads to the production of a hydroxylamine intermediate that binds to thiol groups of low molecular weight substances (eg, glutathione) and to cysteine residues in proteins. When FITC-labeled antibodies are bound to the complexes produced in hypoxia, fluorescence microscopy can be used to detect hypoxic cells. Importantly, high levels of NADH and NADPH, which are expected in hypoxia and after inhibition of the ETC, are not sufficient to form pimonidazole adducts.32 To analyze the effects of inhibitors of the mitochondrial respiratory chain, cells were treated with mitochondrial inhibitors in moderate hypoxia (3% O2) as described above, followed by the incubation with pimonidazole. Confluent and sparse cell cultures were used in the experiments because from our mathematic analysis it is predictable that sparse cells will not create a large oxygen gradient. In addition, the same experiment was conducted under almost anoxic conditions (0.1% O2). Representative results of 3 independent experiments are illustrated in Figure 5. Confluent cell cultures displayed a substantial pimonidazole signal that was abrogated by incubation with ETC inhibitors (Figure 5A). In contrast, pimonidazole staining was virtually absent in subconfluent cell cultures in moderate hypoxia (data not shown). Furthermore, treatment of sparse cells with antimycin A or rotenone did not have a detectable effect on pimonidazole staining (data not shown). These results demonstrated that inhibition of the ETC in moderate hypoxia leads to an increase in the cellular oxygen concentration.
HIF-1α protein expression in U2OS cells exposed to inhibitors of the electron transport chain in combination with PHD inhibitors. Cells were exposed to DMOG (100 μM) in normoxia, or to antimycin A (1 μg/mL) or rotenone (1 μM) in normoxia (NOX) or in hypoxia (3% O2, HOX) for 4 hours. Additionally, a combination of an ETC inhibitor and DMOG was applied to the cells.
HIF-1α protein expression in U2OS cells exposed to inhibitors of the electron transport chain in combination with PHD inhibitors. Cells were exposed to DMOG (100 μM) in normoxia, or to antimycin A (1 μg/mL) or rotenone (1 μM) in normoxia (NOX) or in hypoxia (3% O2, HOX) for 4 hours. Additionally, a combination of an ETC inhibitor and DMOG was applied to the cells.
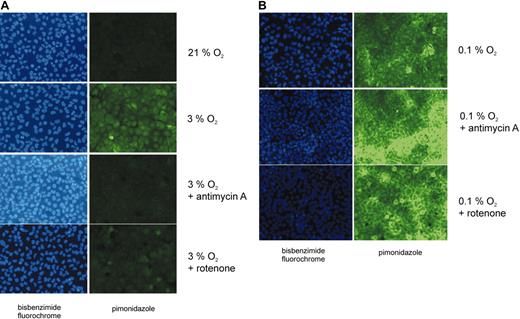
Cellular hypoxia assessed by pimonidazole staining. Human osteosarcoma cells (U2OS) were grown in oxygen-impermeable dishes and incubated in the absence or in the presence of either antimycin A (1 μg/mL) or rotenone (1 μM) in hypoxia for 4 hours. Normoxic, untreated cells served as a control. Intracellular pimonidazole complexes, which are formed under hypoxic conditions (pO2 less than 10 mm Hg), were detected by fluorescence microscopy using an FITC-labeled monoclonal antibody. Pimonidazole was applied to the cells for 45 minutes. Cell nuclei were visualized by bisbenzimide staining. Cells were exposed to (A) intermediate hypoxia (3% O2) or (B) to almost anoxic conditions (0.1% O2).
Cellular hypoxia assessed by pimonidazole staining. Human osteosarcoma cells (U2OS) were grown in oxygen-impermeable dishes and incubated in the absence or in the presence of either antimycin A (1 μg/mL) or rotenone (1 μM) in hypoxia for 4 hours. Normoxic, untreated cells served as a control. Intracellular pimonidazole complexes, which are formed under hypoxic conditions (pO2 less than 10 mm Hg), were detected by fluorescence microscopy using an FITC-labeled monoclonal antibody. Pimonidazole was applied to the cells for 45 minutes. Cell nuclei were visualized by bisbenzimide staining. Cells were exposed to (A) intermediate hypoxia (3% O2) or (B) to almost anoxic conditions (0.1% O2).
When U2OS cells were incubated under almost anoxic conditions (0.1% O2) pimonidazole staining was easily detectable in the absence and in the presence of ETC inhibitors (Figure 5B). This observation is in line with our equation, which predicts that the cellular oxygen concentration cannot exceed the oxygen concentration of the incubator atmosphere. As the HIF-α prolyl hydroxylases are inactive under these conditions, HIF-1α accumulates (Figure 1C).
Discussion
In the present work we analyzed the expression of the the α-subunit of the transcription factor HIF-1 in response to inhibition of the mitochondrial electron transport chain. The relationship between HIF-1α protein expression and oxygen concentration was established in HeLa S3 cells grown in suspension culture. The cells were exposed to defined oxygen concentrations in a tonometer.8 It was observed that maximal HIF-1α activation occurs at oxygen concentrations between 0% and 1%. Cyanide was used in this study to eliminate all oxygen gradients and, interestingly, this treatment had no effect on HIF-1α expression and DNA binding between 0% and 6% oxygen. HIF-1α is a key regulator of oxygen homeostasis known to be involved in erythropoiesis, angiogenesis, apoptosis, vasomotor control, and many other physiologic and pathologic situations (reviewed by Pugh and Ratcliffe3 and Semenza1 ). Therefore, regulation of HIF-1α is currently regarded as an objective of high biomedical relevance.4 Obviously, understanding the regulation of HIF-α was the primary focus of our study, but our results may be of importance whenever oxygen-dependent metabolic steps are to be studied.
The mitochondrial electron transport chain maintains the intracellular energy status by production of ATP while consuming most of the oxygen delivered to cells. The ETC is also a major site of ROS generation. It has been proposed that mitochondria act as O2 sensors in vivo. Specifically, it has been reported that in hypoxia complex III of the ETC produces ROSs, which stabilize HIF-1α.26,27 Within this concept inhibitors that act upstream of complex III (eg, rotenone) have been found to block ROS generation and HIF stabilization while inhibitors that affect a site downstream of complex III (eg, antimycin A) were reported to induce ROSs and HIF-1α. These differences were the rationale in our study to use various inhibitors. Importantly, all substances had the same effect in our hands, which is in line with a recent report.37 Other groups were unable to detect any effect of inhibition of the ETC on HIF-α regulation in almost anoxic conditions of 0.1% oxygen.28,29
Our project was based on the idea that oxygen consumption of cultured cells leads to a decrease of oxygen concentration in conventional polystyrene dishes, which are not permeable for oxygen.38 This drop in pO2 can cause cellular hypoxia as was first recognized for cultured hepatocytes 40 years ago.39 According to our mathematic analysis and to our data, cellular oxygen levels depend on the cell type used, the state of confluence, the metabolic activity of the cells, the diffusion distance, and the oxygen fraction of the incubator atmosphere. We have reported previously that confluent cultures with an active metabolism (eg, HepG2 cells) experience a pO2 of less than 2 mm Hg when incubated in a gas atmosphere containing 20% oxygen,40 which is usually termed “normoxia.” HepG2 cells have been used to investigate hypoxia-dependent regulation of the EPO gene.41 In the present study, an increase of the diffusion distance augmented EPO and VEGF secretion in response to hypoxia. An inverse correlation between diffusion distance and cellular oxygen concentration is well in line with the equation presented in “Results.” This effect was ablated by inhibition of the ETC. This result suggests that mitochondrial oxygen consumption can deplete the environment of oxygen. Consequently, inhibition of oxygen consumption either by pharmacologic inhibition or by elimination of mitochondrial DNA leads to an alleviation of the drop in pO2 as predicted from the mathematic description of oxygen diffusion given in “Results.”
We compared HIF-1α regulation of cells cultured in conventional dishes versus O2-permeable dishes where the growth medium as an oxygen diffusion barrier is circumvented, thus eliminating the diffusion gradient.31 In these dishes chemical inhibition of the ETC was without effect on the regulation of HIF-1α. In conventional (ie, gas-impermeable) dishes ρ0 cells showed HIF induction only at lower oxygen concentrations than wild-type cells. This difference was reduced in gas-permeable dishes in some experiments although this finding showed some degree of variation. Importantly, inhibition of the ETC in conventional dishes only had an effect at an intermediate oxygen concentration in the incubator atmosphere. At higher oxygen levels HIF-1α is not detectable; thus an increase of the cellular pO2 remains undetected. At a very low O2 concentration of 0.1% in the incubator gas, the inhibition of the ETC can only have a minor effect: The cells remain hypoxic, and HIF is active. Reassuringly, the effects of ETC inhibition on cellular oxygen concentration in conventional cell culture dishes were confirmed by pimonidazole staining of the cells. The chemical processes that lead to pimonidazole-protein complex formation in hypoxia have been discussed previously. Importantly, in this report it has been shown that pimonidazole immunofluorescence is regulated by oxygen tension but not by NADH or NADPH levels.32 Thus, pimonidazole seems suitable to monitor the oxygen supply in cell cultures. In our study, this approach was necessary because HIF-1α could not serve as the prime subject of the investigation and as an indicator of cell hypoxia at the same time.
Our analysis is in line with all reports where very low oxygen concentrations have been used.28,29,42 For intermediate concentrations all investigators have reported a down-regulation of HIF-1α caused by inhibition of the ETC. However, an alternative explanation has been given for this phenomenon: intermediate hypoxia (1.5% O2) has been reported to enhance production of ROSs, which were proposed to stabilize HIF-α. Although we have not determined ROSs in the course of our study it seems unlikely that ROSs mediate hypoxic induction of HIF-1α. Firstly, the HIF-α prolyl hydroxylases have a Michaelis constant (Km) of approximately 200 μM, which leads to an excellent regulation of enzyme turnover at low oxygen concentrations as determined experimentally.43 No other mediator was necessary for this regulation in an in vitro assay. Secondly, our results indicate that HIF-1α degradation in the presence of ETC inhibitors is PHD dependent. Clearly, these enzymes require dioxygen as a cosubstrate. Thirdly, it has been reported that ROSs can oxidatively damage the catalytic domain of the HIF-α hydroxylases (eg, as a consequence of genetic inactivation of JunD).44 If this were also the mechanism of hypoxic PHD inactivation, it would be difficult to explain how reoxygenation could lead to rapid degradation of HIF-α, especially because ROS generation is increased by reoxygenation.45-47 In addition, it was reported recently that the ROS scavenging agent Trolox eliminates fluorescence of dichlorofluorescein (DCF), which has been used as an ROS indicator but has no impact on HIF-1α protein expression,48 which is well in line with our results.
In summary, our results indicate that the cellular oxygen concentration is profoundly affected by the oxygen consumption of the cultured cells in conventional culture dishes. The oxygen gradient that results from this process can be reduced by inhibition of the mitochondrial electron transport chain. The elevation of cellular oxygen concentration leads to an increased activity of dioxygen-dependent HIF-α hydroxylases. In our view it is important that the cellular oxygen status cannot be predicted from the composition of the incubator atmosphere. When experimental confirmation is required, cellular hypoxia should be monitored by pimonidazole staining.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-03-1138.
Supported by Deutsche Forschungsgemeinschaft (SFB367-C8) (W.J., E.M.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Prof J. Fandrey, University of Essen, Germany, for provision of the equipment for oxygen consumption measurements and Prof R. Wiesner, University of Cologne, Germany, for sending us 143B-wt and 206ρ0 cells. Dr K. Wagner, University of Lübeck, is acknowledged for help with the pimonidazole staining. We are also grateful to Tanja Svensson and Patricia Rouina for excellent cell culture work and to Dorothea Brennecke for preparation of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal