Abstract
Some cutaneous T-cell lymphomas, (CTCLs) clonal T cells are deficient in interferon signaling, making them promising targets for viral oncolysis. We evaluated cytopathic effects of measles virus (MV) in CTCL. CTCL cell lines and infiltrating lymphocytes in CTCL expressed MV receptors CD150 and CD46. In a phase 1 dose escalation trial a total of 16 injections of live MV, Edmonston-Zagreb vaccine strain, were given intratumorally to 5 patients with CTCL. Patients had antimeasles-serum antibodies and were pretreated with interferon-α to prevent uncontrolled virus spread. The well-tolerated treatment with MV resulted in clinical responses. Evaluation of biopsies, before and at 11 days after injection, by immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR) demonstrated local viral activity with positive staining for MV nucleoprotein (NP), an increase of the interferon γ (IFN-γ)/CD4 and IFN-γ/CD8 mRNA ratios and a reduced CD4/CD8 ratio. All patients demonstrated an increased antimeasles antibody titer after therapy. The data demonstrate that CTCLs are promising targets for an MV-based oncolytic therapy.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are the second most common extranodal lymphomas and are characterized by an accumulation of clonal T lymphocytes in the skin. In early stages, most CTCL variants do not reduce life expectancy. However, they have a significant impact on the quality of life of affected patients since patches, plaques, and tumors are often disfiguring and itchy. Topical steroids, PUVA (psoralen and U-VA), locally applied cytostatics such as 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), or radiotherapy are used for treatment in the earlier stages. The more advanced stages require systemic therapy such as combination of PUVA with retinoids or recombinant interferon-α (IFN-α). In patients with blood involvement, extracorporal photopheresis, in combination with IFN-α or retinoids seems to be effective. Palliative chemotherapy can also be an option in the treatment of advanced stages of CTCL.1 However, it has no proven effect on survival time2 and leads to a further immunosuppression with an increased risk of infection.
We and others have shown that the clonal CD4+ tumor-cell populations display a T helper 2 (TH2) cytokine profile.3,4 The TH2 tumor-cell population should be inhibited by IFN-γ (IFN-γ) and/or IFN-α. This natural inhibition mechanism seems to be switched off in CTCL-derived tumor cells.4,5 These tumor-specific defects in the interferon signal transduction pathway6 can be selectively targeted in Sézary syndrome-derived clonal T cells with interferon-sensitive viruses such as vesicular stomatitis virus (VSV) in vitro in the presence of IFN-α. This approach was also successfully used in melanoma-bearing nude mice resulting in selective oncolysis.7
Beneficial effects of viral infections on lymphoma patients have been known for decades. Viral oncolysis and/or virus-induced antitumor immune responses are the proposed mechanisms explaining so-called “spontaneous” tumor regressions of Hodgkin disease8-10 and Burkitt lymphoma11,12 after measles infections. Consequently, live attenuated measles virus (MV) induced regression of human B-cell lymphoma xenografts in immunodeficient mice.13 In various animal models, the replication-competent attenuated Edmonston B vaccine strain of MV was successfully used to treat multiple myeloma, ovarian cancer, and glioma xenografts after either intratumoral, intraperitoneal, or intravenous administration.14-16 These observations suggest a substantial antineoplastic potential for MV.
MV is a negative strand RNA virus, belonging to Paramyxoviridae family, genus Morbilli virus. The MV genome encodes 6 proteins, 3 of which participate in the formation of the viral envelope [the matrix (M), hemagglutinin (H), and fusion (F) proteins]. Both H and F proteins are determinants for virus-to-host attachment and fusion. Upon infection, cells expressing F and H proteins become highly fusogenic with neighboring uninfected cells leading to cell-cell fusion and virus propagation.17,18 The receptors of MV are well defined. The wild-type MV strains bind to CD150 (signaling lymphocytic activation molecule; SLAM)19 and with low affinity to CD46, whereas the live-attenuated vaccine strains interact with CD46, a complement regulatory protein.20,21 Expression of CD46 by CTCL T cells would thus render them susceptible to the measles vaccine virus that possesses an excellent safety record. CD46 was found to be overexpressed on some cancer cell lines rendering them a preferred target for MV.17 Replication of MV is inhibited by interferon by various mechanisms, including cleavage of viral mRNA, inhibition of virus translation and inhibition of cell growth.18-20 IFN treatment in conjunction with MV would thus restrict virus replication to interferon-resistant tumor cells, such as CTCL cells, deficient in interferon signaling, including pathways necessary to induce antiviral molecules. In this study we have used attenuated vaccine strain MV-Edmonston Zagreb (MV-EZ) in patients with CTCL in a clinical trial. Intratumoral injection of MV-EZ after systemic pretreatment with IFN-α caused local MV-EZ infection that resulted in tumor regression. This effect was not abrogated by the preexisting antimeasles antibodies in these patients and yet was well tolerated.
Patients, materials, and methods
Cells, viruses, antibodies for immunohistochemistry, and FACS staining
Hematoxylin and eosin staining and immunohistochemistry for CD4, CD8 (all antibodies from DAKO, Glostrup, Denmark), and Mx21 (Immunotech, Marseille, France) immunostaining was carried out on paraffin-embedded sections. On the cryostat sections stainings for CD46 (Pharmingen, San Diego, CA), CD150/SLAM (kindly provided by Dr J. Schneider-Schaulies, Würzburg, Germany), and anti-MV nucleoprotein (NP) against nucleoprotein (kindly provided by Dr F. Wild, France) were performed. Immunostainings were visualized by Alcalic Phosophatase-Antialcalic Phosphatase Complex or peroxidase-antiperoxidase. A negative control without the specific primary antibody was carried out in parallel to all stainings. To establish the MV NP staining, control slides (Chemicon via Juro, Lucerne, Switzerland) as positive and negative control and umbilical cord biopsies as negative control tissue were used. The slides were assessed patient by patient on serial sections, including CD4, CD8, CD46, and CD150. Three T-lymphoma cell lines—HUT 78, SeAx, and MyLa (kindly provided by K. Kaltoft, Aahus, Denmark)22,23 —were analyzed for expression of CD46 and CD150 by fluorescence-activated cell sorting (FACS), with appropriate isotype controls. The viruses used in this study were the authentic vaccine strain, Edmonston Zagreb, a product of Berna Biotech LTD, Bern, Switzerland.
T-lymphoma cell lines
The T-lymphoma cell lines, SeAx, HUT 78, and MyLa, were cultivated in RPMI 1640 medium (GIBCO BRL, Karlsruhe, Germany) supplemented with 10% heat inactivated fetal calf serum (Berna Biotech AG), 1% penicillin/streptomycin (GIBCO BRL; catalog no. 15140-148), 1% l-glutamine (Berna Biotech AG), and 1% sodium pyruvate (GIBCO BRL, catalog no 11360-039).
Study design and patients
The clinical trial was an open-label, nonrandomized, single-center phase 1 dose escalation trial approved by the ethical committee of the University Hospital Zurich. Patients were eligible if they had histologically documented cutaneous T-cell lymphoma IIb or higher, resistant to or relapsing after conventional therapies. The primary objective was to assess toxicity and tolerability of treatment. Secondary objectives were to evaluate the local effect on tumors injected with measles vaccine virus after systemic pretreatment with interferon.
All of the patients had clinically measurable disease and had anti-measles virus antibodies prior to inclusion into the protocol. The study population consisted of 5 white patients, 4 men and 1 woman, between 53 and 64 years (mean age, 57 years). Mean Karnofsky performance index was 88%. All patients were followed until tumor progression.
Tumor treatment
Patients were injected with the measles-virus vaccine intratumorally after IFN-α had been administered subcutaneously at a dose of 9 Mio units twice according to the treatment scheme in Table 1.
Treatment regimen for each cycle
. | Screening . | day 1 . | day 3 . | day 4 . | day 14 . | day 16 . | day 17 . | day 28 . |
|---|---|---|---|---|---|---|---|---|
| IFN-α 9 Mio U subcutaneously | NA | x | x | ND | x | x | ND | ND |
| Measles virus intratumorally | NA | ND | ND | x | ND | ND | x | ND |
| Punch biopsy | x | ND | ND | ND | ND | ND | ND | x |
. | Screening . | day 1 . | day 3 . | day 4 . | day 14 . | day 16 . | day 17 . | day 28 . |
|---|---|---|---|---|---|---|---|---|
| IFN-α 9 Mio U subcutaneously | NA | x | x | ND | x | x | ND | ND |
| Measles virus intratumorally | NA | ND | ND | x | ND | ND | x | ND |
| Punch biopsy | x | ND | ND | ND | ND | ND | ND | x |
Each treatment cycle consisted of 2 intratumoral injections of the measles virus (day 4 and day 17). Injections of IFN-α (9 Mio U subcutaneously) were administered 72 hours and 24 hours before each of the measles-virus injections. At screening and on day 28 a tumor biopsy was taken.
NA indicates not applicable; ND, not done.
Each treatment cycle consisted of 2 intratumoral injections of the measles virus (day 4 and day 17). Injections of IFN-α (9 Mio U subcutaneous) were administered 72 hours and 24 hours before each of the measles-virus injections. At screening and on day 28 a tumor biopsy was taken.
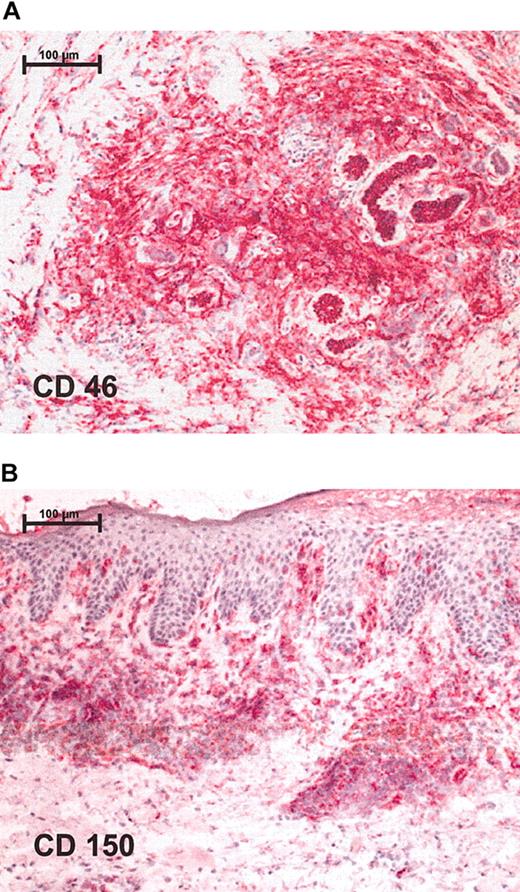
Immunohistochemistry shows positive staining for measles receptors in CTCL lymphoma biopsies. Red staining represents lymphocytes that express respective receptor (A) CD46 and (B) CD150. Stainings were performed on cryostat sections and were assessed together with contiguous slices stained for CD4 and CD8. Original magnification, × 100 (scale bar indicates 100 μm). Images were visualized under an Axiophot light photomicroscope (Zeiss, Jena, Germany) equipped with a 10 × objective lens and a 10 × ocular.
Immunohistochemistry shows positive staining for measles receptors in CTCL lymphoma biopsies. Red staining represents lymphocytes that express respective receptor (A) CD46 and (B) CD150. Stainings were performed on cryostat sections and were assessed together with contiguous slices stained for CD4 and CD8. Original magnification, × 100 (scale bar indicates 100 μm). Images were visualized under an Axiophot light photomicroscope (Zeiss, Jena, Germany) equipped with a 10 × objective lens and a 10 × ocular.
MV injections contained live Edmonston-Zagreb measles vaccine virus (MV-EZ) at 100, 500, and 1000 tissue culture infective dose 50 (TCID50). To achieve maximum safety, a dose-escalating protocol was chosen. Patients were assigned to 2 dose groups. The lower dose group started first (starting dose, 100 TCID50, day 4 and 17 of cycle 1). After treatment in the first cycle was well tolerated, dosage was increased for the second cycle to 500 TCID (day 4 and 17 of cycle 2, day 32 and 45). The higher dose group started after the lower dose group had finished one cycle (day 28). Starting dose of the higher dose group was 500 TCID50 and was escalated according to the dose escalation scheme up to a maximum dose of 1000 TCID50. Within the study a total of 8 cycles with 16 injections (3 patients with 2 cycles, 2 patients with 1 cycle each) were applied.
Initially, staging with measurements of all tumors was performed, and the lesion to be injected was chosen based on its capacity to be measured. The injected lesion was assessed at baseline, at all study visits, and after completion of the treatment cycle. The same injection site was used for administration of both doses of a cycle. Only when lesions disappeared after treatment was a different site used for injection. The patients' general condition was monitored before and after each injection.
Safety parameters
Patients were monitored for adverse events throughout the study. Vital signs were checked at each visit and a physical examination was carried out, including examination for inflammatory reactions at the injection site. At the beginning of each visit patients were thoroughly asked for any experienced adverse events. Adverse events were graded according to the World Health Organization (WHO) classification (mild = WHO grade 1, moderate = WHO grade 2, severe = WHO grade 3, life threatening = WHO grade 4) and relationship to treatment was assessed by the investigator (not related, remotely, possibly, or probably related). Standard laboratory parameters were assessed at baseline and on days 4, 5, 10, 17, 24, and 28 of each treatment cycle.
Biopsies
Biopsies for histologic evaluation, immunohistochemistry, and reverse transcriptase-polymerase chain reaction (RT-PCR) were collected before treatment and on day 28, 11 days after the last injection of measles vaccine viruses to detect changes induced by virus spread. Biopsy specimens were divided and half of the tissue was snap-frozen, while the other half was formalin-fixed.
RNA extraction and cDNA synthesis from biopsies
Total RNA was isolated from 14 frozen samples using TRIzol reagent (Invitrogen AG, Basel, Switzerland) according to the manufacturer's protocol. Approximately 1 μg RNA was reverse transcribed using an oligo(dT)15 priming and avian myeloblastosis virus reverse transcriptase (first-strand cDNA synthesis kit for RT-PCR; Roche, Mannheim, Germany) at 42°C for 1 hour. cDNAs were then stored at -20°C until further use. Real-time quantitative PCR analyses were conducted on a Light Cycler (Hoffmann-LaRoche, Basel, Switzerland) machine using amplification primer mixes for analysis of CD4, CD8, and IFN-γ (Search-LC, Heidelberg, Germany) as described previously.24 Ratios of IFN-γ/CD4, IFN-γ/CD8, and CD4/CD8 were used to monitor immune response.25
Immunologic parameters
Blood samples were taken at screening, and on days 4, 5, 10, 17, 24, and 28. Serum was cryopreserved and analyzed for IL-2, IL-12, and IFN-γ by enzyme-linked immunosorbent assay (ELISA). For antimeasles antibody evaluation ELISA optical density (OD) values of anti-measles immunoglobulin G (IgG) from serum samples before and after treatment were compared. Blood samples for antimeasles antibodies were taken at baseline and during follow-up 5 to 9 months after start of the treatment patient 2 [p2], 8; p3, 7; p4, 5; and p5, 9 months). Additionally CD4/CD8 ratio was determined by FACS analysis.
Response assessment
A simple, practical, and also objective method for monitoring disease activity in CTCL is based on a weighted body surface area measurement. The tumor mass measurement is done by means of determination of a Tumor Burden Index (TBI). This system has been shown to have prognostic significance and correlates with other biologic parameters.26 TBI was determined at baseline and at the end of the treatment.
The determination of the TBI involves the depiction of skin lesions using an estimation of the affected surface area by the rule of 9. Specific skin lesions were differentiated in patches (flat lesions with a diameter larger than 1 cm), plaques (flat or slightly elevated lesions with increased consistency with a diameter larger than 1 cm), and tumors (large nodular lesions with a diameter larger than 1 cm).
The TBI was calculated according to the following formula: TBI = (tumor) X% × 4 + (plaque) X% × 2 + (patch) X% ×, where X% = surface of involved skin (in %). TBI ratio is defined as follows: TBI ratio = Postbaseline TBI / Baseline TBI.
Response was judged according to the following criteria: complete clinical response, TBI ratio of 0 (complete disappearance of all cutaneous disease [not histologically verified]); partial response, TBI ratio of 0.5 or less (more or at least 50% reduction in TBI from baseline); stable disease, TBI ratio greater than 0.5 and but less than 1.25 (< 50% reduction [from baseline] and < 25% increase in TBI); and progressive disease, TBI ratio greater than 1.25 (more than 25% increase in TBI compared with the smallest TBI since treatment started).
Results
CTCL cells express measles-virus receptors
To test the susceptibility of CTCL tumors to infection with the measles virus, expression of measles-virus receptors CD150 and CD46 on the surface of CTCL cell lines and on biopsies from patients with lymphoma was assessed by FACS staining and immunohistochemistry, respectively. All 3 lymphoma cell lines— HUT 78, SeAx, and MyLa—expressed CD150 and CD46, but at different levels (data not shown). Biopsies from patients with lymphoma also tested positive for measles-virus-receptor expression. Positive staining for CD46 (Figure 1A) and CD150 (Figure 1B) was detected in all 5 study patients in a total of 8 different biopsies. Keratinocytes were negative for CD46 and CD150. Besides lymphocytes, blood vessels and Langerhans cells showed positive staining for measles-virus receptor CD46.
Intralesional injection of measles vaccine virus after IFN-α treatment is well tolerated and leads to regression of tumor lesions
To test feasibility of CTCL treatment with MV, a total of 16 MV-EZ injections were given to 5 patients with CTCL; each injection was preceded by treatment with IFN-α (Table 1). Patient characteristics and receptor status of their tumor lesions are shown in Table 2. All biopsies were positive for CD150 and CD46. Assessment of standard laboratory parameters at baseline and on days 4, 5, 10, 17, 24, and 28 of each treatment cycle showed no abnormal changes. Treatment as applied according to the dose-escalating scheme was extremely well tolerated. At none of the doses (100, 500, and 1000 TCID50) did any of the patients experience any adverse events more than grade 1 (see Table 2). In peripheral blood an increase of total lymphocytes and CD4 and CD8 lymphocytes was seen after each of the MV-EZ injections. These changes however were not statistically significant. Mean CD4/CD8 ratio remained unchanged throughout the treatment (Figure 2).
Patient characteristics, MV treatment procedure, and outcome
Patient no.; sex; age, y; diagnosis . | IHC for MV receptors . | . | . | Dose/injection sites/time of injection . | . | Response of distant lesions* . | Overall response, TBI ratio . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| . | CD 46 . | CD 150 . | Previous treatment . | . | Local response* . | . | . | Adverse reactions . | Comments . | |
| 1; M; 65; MF with mucinosis follicularis stage IIb | + | ++ | Tumor excisions, IFN-α, radiotherapy, pegylated interferon, PUVA, topical steroids | 100 TCID50/cervical left/day 4, cycle 1; 100 TCID50/cervical left/day 17, cycle 1; 500 TCID50/cervical left/day 4, cycle 2 = day 32; 500 TCID50/cervical left/day 17, cycle 2 = day 45 | Partial regression of the treated plaque (4×4×0.3 cm) (Figure 3A-B) | Partial regression of distant lesions (Figure 3C-D) | 6.5/21 = 0.3 (PR) | Slight erythema at injection site, itching, stabbing sensation in left shoulder after the first MV injection | none | |
| 2; M; 54; MF stage IIb | ++ | ++ | UV therapy, radiotherapy, PUVA, IFN-α, methotrexate, radiotherapy, topical steroids | 100 TCID50/right thigh/day 4, cycle 1; 100 TCID50/right thigh/day 17, cycle 1; 500 TCID50/right plantar/day 4, cycle 2 = day 32; 500 TCID50/right plantar/day 17, cycle 2 = day 45 | Complete regression of the treated plaque (3×3×0.3 cm) (Figure 3E-F) | Distant tumor (1×1×0.5 cm) became injected lesion and also responded (Figure 3G-H) | 17/18 = 0.94 (SD) | Slight erythema at injection site, dizziness on the day after the first MV injection | Change of injected lesion due to CR | |
| 3; M; 54; lymphomatoid papulosis | + | ++ | Topical steroids, PUVA | 100 TCID50/right upper arm/day 4, cycle 1; 100 TCID50/right upper arm/day 17, cycle 1 | Partial regression of the treated plaque (2.5×2.5×0.3 cm) surrounded by papules (flattening) | Partial regression of distant lesion and new plaque | 16/16 = 1.0 (SD) | None | Discontinued treatment for personal reasons | |
| 4; M; 64; CD30+ pleomorphic large-cell CTCL | ++ | ++ | Tumor excision, radiotherapy, IFN-α, topical steroids, BCNU, Acitretin | 500 TCID50/right plantar/day 4, cycle 1; 500 TCID50/right plantar/day 17, cycle 1; 1000 TCID50/right plantar/day 4, cycle 2 = day 32; 1000 TCID50/right plantar/day 17, cycle 2 = day 45 | Minor response (flattening) of a tumor (1.5×2×0.8 cm) | No response, additional lesions | 24/16 = 1.5 (PD) | Burning sensation, slight erythema, and itching at injection site, arthralgias (day 30/31, eg, after IFN before full dose MV injection) | None | |
| 5; F; 54; MF stage IIb | + | ++ | Radiotherapy, BCNU, Neotigason, PUVA, IFN-α, topical steroids | 500 TCID50/inguinal left/day 4, cycle 1; 500 TCID50/inguinal left/day 17, cycle 1 | Partial regression (flattening) of the treated tumor (3×2×1 cm) | No response, additional lesions | 54/40 = 1.35 (PD) | Itching | Overall progression of disease required treatment with cortico-steroids | |
Patient no.; sex; age, y; diagnosis . | IHC for MV receptors . | . | . | Dose/injection sites/time of injection . | . | Response of distant lesions* . | Overall response, TBI ratio . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|
| . | CD 46 . | CD 150 . | Previous treatment . | . | Local response* . | . | . | Adverse reactions . | Comments . | |
| 1; M; 65; MF with mucinosis follicularis stage IIb | + | ++ | Tumor excisions, IFN-α, radiotherapy, pegylated interferon, PUVA, topical steroids | 100 TCID50/cervical left/day 4, cycle 1; 100 TCID50/cervical left/day 17, cycle 1; 500 TCID50/cervical left/day 4, cycle 2 = day 32; 500 TCID50/cervical left/day 17, cycle 2 = day 45 | Partial regression of the treated plaque (4×4×0.3 cm) (Figure 3A-B) | Partial regression of distant lesions (Figure 3C-D) | 6.5/21 = 0.3 (PR) | Slight erythema at injection site, itching, stabbing sensation in left shoulder after the first MV injection | none | |
| 2; M; 54; MF stage IIb | ++ | ++ | UV therapy, radiotherapy, PUVA, IFN-α, methotrexate, radiotherapy, topical steroids | 100 TCID50/right thigh/day 4, cycle 1; 100 TCID50/right thigh/day 17, cycle 1; 500 TCID50/right plantar/day 4, cycle 2 = day 32; 500 TCID50/right plantar/day 17, cycle 2 = day 45 | Complete regression of the treated plaque (3×3×0.3 cm) (Figure 3E-F) | Distant tumor (1×1×0.5 cm) became injected lesion and also responded (Figure 3G-H) | 17/18 = 0.94 (SD) | Slight erythema at injection site, dizziness on the day after the first MV injection | Change of injected lesion due to CR | |
| 3; M; 54; lymphomatoid papulosis | + | ++ | Topical steroids, PUVA | 100 TCID50/right upper arm/day 4, cycle 1; 100 TCID50/right upper arm/day 17, cycle 1 | Partial regression of the treated plaque (2.5×2.5×0.3 cm) surrounded by papules (flattening) | Partial regression of distant lesion and new plaque | 16/16 = 1.0 (SD) | None | Discontinued treatment for personal reasons | |
| 4; M; 64; CD30+ pleomorphic large-cell CTCL | ++ | ++ | Tumor excision, radiotherapy, IFN-α, topical steroids, BCNU, Acitretin | 500 TCID50/right plantar/day 4, cycle 1; 500 TCID50/right plantar/day 17, cycle 1; 1000 TCID50/right plantar/day 4, cycle 2 = day 32; 1000 TCID50/right plantar/day 17, cycle 2 = day 45 | Minor response (flattening) of a tumor (1.5×2×0.8 cm) | No response, additional lesions | 24/16 = 1.5 (PD) | Burning sensation, slight erythema, and itching at injection site, arthralgias (day 30/31, eg, after IFN before full dose MV injection) | None | |
| 5; F; 54; MF stage IIb | + | ++ | Radiotherapy, BCNU, Neotigason, PUVA, IFN-α, topical steroids | 500 TCID50/inguinal left/day 4, cycle 1; 500 TCID50/inguinal left/day 17, cycle 1 | Partial regression (flattening) of the treated tumor (3×2×1 cm) | No response, additional lesions | 54/40 = 1.35 (PD) | Itching | Overall progression of disease required treatment with cortico-steroids | |
Characteristics of the 5 patients with CTCL treated within the clinical trial (M indicates male; F, female), pretreatment regimens (PUVA, psoralen + UV-A therapy), expression of surface molecules CD46 and CD150 in immunohistochemistry (IHC: +, positive; ++, strong positive) that can act as receptors for measles virus, doses received during the course of the study, individual responses (SD, stable disease; PD, progressive disease) and adverse reactions.
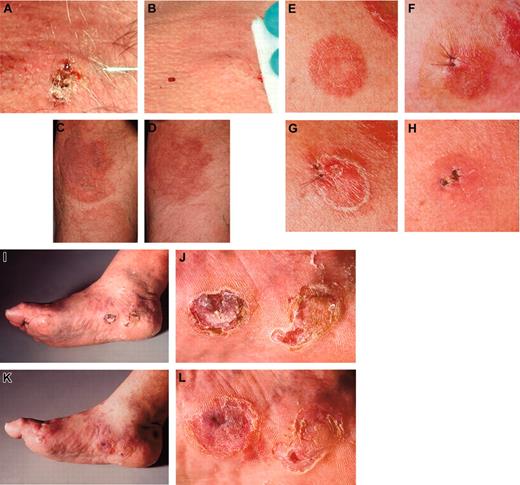
Five of 6 MV-treated lesions (in patient 2, 2 lesions were injected) showed clear regressions (examples are shown in Figure 3).
All tumor responses occurred within 28 days after initiation of treatment. One of the treated lesions completely disappeared. Distant noninjected lesions improved in 2 patients, but remained unchanged in the other 3 of 5 (see Table 2; Figure 3); one had PD.
Course of lymphocyte counts during treatment. Absolute cell counts of CD3+, CD4+, and CD8+ cells as well as CD4/CD8 ratio for the individual study patients. Arrows indicate time of measles-virus injections.
Course of lymphocyte counts during treatment. Absolute cell counts of CD3+, CD4+, and CD8+ cells as well as CD4/CD8 ratio for the individual study patients. Arrows indicate time of measles-virus injections.
Measles vaccine virus enters lymphoma cells in vivo in humans and leads to formation of syncytia
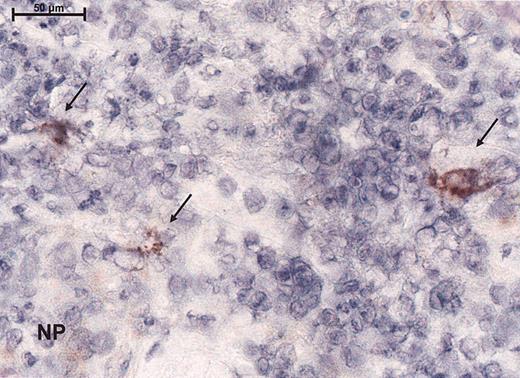
To assess the effect of the measles virus on lymphoma from study patients, before and after injection of measles vaccine virus, biopsies were analyzed histologically and by immunohistochemical staining (for the expression of MV proteins, CD4, CD8, and Mx protein). Within the lymphocytic bands syncytia formation could be seen upon histologic examination. No measles nucleoprotein was detected before injection or in the negative controls, whereas 4 of 4 biopsies tested after measles-virus injection showed large cells with immunoreactivity for the measles NP (Figure 4). Within the injected lesions, there was a clear decrease of CD4 mRNA (from a mean of 70% to a mean of 54%) and an increase of CD8 mRNA consistent with the CD8 infiltrates observed in the tumor (Table 3). Mx protein expression showed a slight up-regulation in the dermis (Table 3) in 1 patient. One had down-regulation. The other biopsies demonstrated little or no Mx immunoreactivity compatible with interferon resistance.
Summary of results of CD4 and CD8 staining and expression of Mx protein before and after therapy in CTCL biopsy samples
. | . | . | . | . | Mx† . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | CD4, % positive* . | . | CD8, % positive* . | . | Before therapy . | . | After therapy . | . | ||||
| . | Before therapy . | After therapy . | Before therapy . | After therapy . | Epidermis . | Dermis . | Epidermis . | Dermis . | ||||
| 1 | 90 | 90 | 10 | 10 | 2 | 1 | 4 | 3 | ||||
| 2 | 90 | 60 | 10 | 40 | 3 | 1 | NA | 1 | ||||
| 3 | 90 | 70 | 10 | 30 | 0 | 0 | 0 | 0 | ||||
| 4 | 0‡ | 0‡ | 5 | 20 | 3 | 1 | 0 | 0 | ||||
| 5 | 80 | 50 | 20 | 50 | 4 | 1 | 3 | 1 | ||||
| Mean | 70 | 54 | 11 | 30 | 2.4 | 0.8 | 1.75 | 1 | ||||
. | . | . | . | . | Mx† . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | CD4, % positive* . | . | CD8, % positive* . | . | Before therapy . | . | After therapy . | . | ||||
| . | Before therapy . | After therapy . | Before therapy . | After therapy . | Epidermis . | Dermis . | Epidermis . | Dermis . | ||||
| 1 | 90 | 90 | 10 | 10 | 2 | 1 | 4 | 3 | ||||
| 2 | 90 | 60 | 10 | 40 | 3 | 1 | NA | 1 | ||||
| 3 | 90 | 70 | 10 | 30 | 0 | 0 | 0 | 0 | ||||
| 4 | 0‡ | 0‡ | 5 | 20 | 3 | 1 | 0 | 0 | ||||
| 5 | 80 | 50 | 20 | 50 | 4 | 1 | 3 | 1 | ||||
| Mean | 70 | 54 | 11 | 30 | 2.4 | 0.8 | 1.75 | 1 | ||||
NA indicates not applicable.
Immunohistochemistry results of CD4 and CD8 is stained percentage of mononuclear cells
Mx staining intensity is as follows: 4 is less than 75% of all cells, 3 is greater than 50% but less than 75%, 2 is greater than 25% but less than 50%, and 1 is less than 25%
Tumor-cell population is assumed to have an aberrant T-cell phenotype with loss of CD4
Measles vaccine virus treatment leads to an increase in IFN-γ and CD8+ cells at the tumor site and a reduction in CD4+ cells
To investigate immunologic changes induced by the treatment 8 tumor biopsy specimens were collected before therapy (baseline) and 6 after 2 treatment cycles (after treatment) and were analyzed by real-time quantitative PCR for mRNA expression levels of CD4, CD8, and IFN-γ.
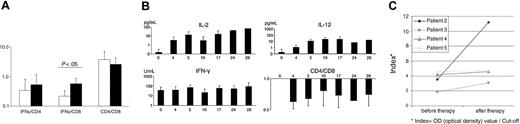
Following the treatment with MV-EZ, a mean increase of the IFN-γ/CD4 mRNA ratio of 1.6-fold and a 2.6-fold increase of the IFN-γ/CD8 mRNA ratio (P = .55 and .02, respectively) were observed (Figure 5A). CD4/CD8 ratio was reduced by a factor of 0.7 in posttreatment samples (P = .045; Figure 5A), consistent with the infiltration of CD8+ cells and the immune response via IFN-γ (Table 3).
Measles vaccine virus induces increases of inflammatory cytokines in peripheral blood and antimeasles antibody titers
To determine the systemic immune reaction to the intratumoral injections of the measles vaccine serum levels of cytokines and IgG antibodies against measles virus were monitored. Treatment was associated with increased serum cytokine levels. Serum IL-2 levels increased following the first treatment with a decline after day 5 and a boost upon the second injection (Figure 5B). During the second treatment cycle IL-2 levels remained elevated. Serum levels of IL-12 increased considerably after the first treatment and remained elevated throughout the treatment course. IFN-γ demonstrated constant high serum levels during the treatment (Figure 5B). Comparison of antimeasles antibodies before and after 2 treatment cycles showed a clear increase in all 4 of the tested patients (mean increase 2-fold; Figure 5C).
Discussion
T-cell lines established from patients with CTCL5 and purified clonal T cells from the blood of patients with Sézary syndrome6,27 are deficient in interferon signaling, including pathways necessary to induce antiviral molecules such as the Mx protein. This inhibits an adequate antiviral response and renders them susceptible to viral oncolysis.27 We successfully used VSV to target this Achilles heel. However, the use of replicating VSV in humans would require intensive precautions, especially because VSV is a neurotropic virus. Therefore, the attenuated MV vaccine is a safe alternative, particularly because it has been used in millions of children over decades with an excellent safety profile. Several reports have shown the potential and efficacy of recombinant MV or MV vaccine against cancers, B-cell lymphoma,13 myeloma,14,27 and gliomas28 in murine models. This is the first clinical study to test safety and oncolytic potential of attenuated measles virus in patients with cancer. It shows the successful use of the measles vaccine strain Edmonston Zagreb in human cancer, that is, in patients with CTCL, and thus confirms the potential of MV for treatment of neoplasias in humans.
Clinical presentations. The injected lesion (A) and a representative noninjected plaque (C) of patient 1 before (A,C) and after (B,D) treatment with intratumoral injection of measles vaccine virus preceded by subcutaneous IFN-α application. (E) The first injected plaque lesion of patient 2 before therapy, (F) after 6 days presenting edematous swelling and inflammation, (G) after 12 days and (H) clearing after 28 days. Thereafter, a tumor lesion was treated (I, overview) and (J, detail), again resulting in regression (K, overview; L, detail). The tumor lesions showed reduction in size and thickness after therapy. Images were acquired with a Leica RG camera (Leica, Jena, Germany).
Clinical presentations. The injected lesion (A) and a representative noninjected plaque (C) of patient 1 before (A,C) and after (B,D) treatment with intratumoral injection of measles vaccine virus preceded by subcutaneous IFN-α application. (E) The first injected plaque lesion of patient 2 before therapy, (F) after 6 days presenting edematous swelling and inflammation, (G) after 12 days and (H) clearing after 28 days. Thereafter, a tumor lesion was treated (I, overview) and (J, detail), again resulting in regression (K, overview; L, detail). The tumor lesions showed reduction in size and thickness after therapy. Images were acquired with a Leica RG camera (Leica, Jena, Germany).
Immunoreactivity for the virus as demonstrated by peroxidase staining for the measles nucleoprotein immunohistochemically. Magnification, × 100 (scale bar indicates 100 μm). Arrows indicate immunoreactivity for nucleoprotein (NP).
Immunoreactivity for the virus as demonstrated by peroxidase staining for the measles nucleoprotein immunohistochemically. Magnification, × 100 (scale bar indicates 100 μm). Arrows indicate immunoreactivity for nucleoprotein (NP).
In the biopsies from patients with CTCL expression of measles-virus receptors CD46 and CD150 by the tumor cells was shown by immunohistochemistry, thus rendering these tumors susceptible for infection and lysis by MV. In contrast to wild-type MV, certain members of the Edmonston vaccine strains (MV-Ed) are oncolytic against a broad spectrum of lymphoid and nonlymphoid human malignancies.13,15,16,28,29 It is well established that the broadened
host range of attenuated MV vaccine is a consequence of mutations in the viral genome, including the attachment glycoprotein that enhances its ability to interact with the CD46 receptor, a regulator of complement inactivation that is expressed at high levels on the surface of most human tumors.30-33 In summary, apart from the broadened receptor specificity, tissue culture-adapted strains of MV-Ed have attenuated pathogenicity for normal human tissues and are coincidentally more potent and more oncolytic against tumor cells.
Despite the excellent safety profile of the MV vaccine, the risk of uncontrolled virus spread in the patients who are immunocompromised by the lymphoma had to be considered. Therefore, the dose escalation protocol allowed only patients in the study with detectable antimeasles antibody in the serum. In addition, IFN-α was applied to protect normal tissues. Indeed, all patients experienced only minor side effects such as local injection site reactions (all below grade 1 WHO). The application of the virus was well tolerated at all dose levels. There was no sign of infectious diseases, rashes, or febrile episodes. The selective regression of MV-injected lesions during systemic interferon therapy suggests that the antilymphoma activity is at least in part mediated by the virus.
Immunohistochemical monitoring and quantitative RT-PCR both document an enhanced immune response with an influx of cytotoxic T lymphocytes. The increase of IFN-γ transcription might help to correct the T helper 1/T helper 2 imbalance in CTCL lesions. As both the IFN-γ/CD4 and the IFN-γ/CD8 ratios rise, IFN-γ seems to be produced from both CD8+ as well as from CD4+ cells. Because of the combined application of IFN-α and MV, it is hard to determine the contribution of each player in this context. However, the appearance of large syncytial cells expressing the measles nucleoprotein NP proves the local activity of the MV vaccine. In line with these findings local clinical responses were observed.
Immunomonitoring shows local and syngeneic changes during therapy. (A) Levels of IFN-γ, CD4, and CD8 mRNA as detected by RT-PCR in biopsies at baseline in comparison with posttreatment biopsies. (B) Kinetics of IL-2, IFN-γ, and IL-12 serum levels and CD4/CD8 ratio. (C) Antibody titers of individual anti-measles IgG antibodies before and after therapy as measured by ELISA. Values presented as indexes (Index = OD optical density value/cut-off). Error bars indicate standard deviation.
Immunomonitoring shows local and syngeneic changes during therapy. (A) Levels of IFN-γ, CD4, and CD8 mRNA as detected by RT-PCR in biopsies at baseline in comparison with posttreatment biopsies. (B) Kinetics of IL-2, IFN-γ, and IL-12 serum levels and CD4/CD8 ratio. (C) Antibody titers of individual anti-measles IgG antibodies before and after therapy as measured by ELISA. Values presented as indexes (Index = OD optical density value/cut-off). Error bars indicate standard deviation.
The local immune intervention also induced systemic immune activation. An increase in serum IL-2 levels was detected in most patients. Despite the disease-associated immunosuppression, all patients investigated demonstrated increased antimeasles antibody titers after the therapy. This study demonstrates that treatment of patients with CTCL with measles vaccine strain preceded by treatment with IFN-α is feasible, safe, and induces local and systemic immune responses. Tumor-cell killing is most likely a consequence of cell-to-cell transmission of MV34 and subsequently causing syncytial apoptosis.35 The in vivo results also clearly demonstrate that MV can infect cells despite preexisting immunity and IFN-α activity locally after intralesional injection. This is in accordance with findings using oncolytic viruses in various animal models.36-38 Together with earlier reports by groups from the Mayo clinic, Rochester, MN, this work shows the potential of MV vaccine for clinical application.
Oncolytic viruses have the potential to become multimodal therapeutics in the future because they can be genetically engineered to (1) exhibit tumor specificity, that is, if tumor-specific surface proteins are known; (2) induce immune responses against tumor cells by introducing new epitopes or transferring cytokine genes into tumor cells; and (3) transfer genes exhibiting tumor-cell-killing properties directly into the target cells.
A lot of work has been done to develop viruses targeted to tumor cells with known tumor-specific surface proteins.39-43 The idea of arming oncolytic viruses with “payloads” to enhance their antitumor activity is one fundamental technique in viro-therapy.37 This could be achieved by using oncolytic vectors coding for IFN-α as an additional transcription unit because it has recently been shown for a related virus.44 MV provides all characteristics required for developing a future gene-delivery system, ensuring efficient targeted gene transfer, sustained expression of therapeutic transgenes, and applicability of the virus to humans.
Prepublished online as Blood First Edition Paper, June 16, 2005; DOI 10.1182/blood-2004-11-4558.
Supported by the Swiss Cancer League (project SKL 01217-022002).
Two of the authors (V.K. and H.N.) are employed by Berna Biotech AG, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This project received the project award 2003 by the Swiss Cancer League. We thank Prof Dr Jürgen Schneider-Schaulies and Dr Fabian Wild for generously providing antibodies. We also thank Dr Walter Bossart, Department of Medical Virology, for measuring titers of measles IgG antibodies; Christa Dudli who has continuously supported the immunohistological work; and Dr Tanja Maier for support with the preparation of the protocol, the clinical trial, and the statistical analysis. We thank Ian Metcalfe for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal