Abstract
Several recent publications have focused on the newly described interactions between natural-killer (NK) cells and dendritic cells (DCs). Activated NK cells induce DC maturation either directly or in synergy with suboptimal levels of microbial signals. Immature DCs appear susceptible to autologous NK-cell-mediated cytolysis while mature DCs are protected. NK-cell-induced DC activation is dependent on both tumor necrosis factor-α (TNF-α)/interferon-γ (IFN-γ) secretion and a cell-cell contact involving NKp30. In vitro, interleukin-12 (IL-12)/IL-18, IL-15, and IFN-α/β production by activated DCs enhance, in turn, NK-cell IFN-γ production, proliferation, and cytotoxic potential, respectively. In vivo, NK-cell/DC interactions may occur in lymphoid organs as well as in nonlymphoid tissues, and their consequences are multiple. By inducing DC activation, NK-cell activation induced by tumor cells can indirectly promote antitumoral T-cell responses. Reciprocally, DCs activated through Toll-like receptors (TLRs) induce potent NK-cell activation in antiviral responses. Thus, DCs and NK cells are equipped with complementary sets of receptors that allow the recognition of various pathogenic agents, emphasizing the role of NK-cell/DC crosstalk in the coordination of innate and adaptive immune responses.
Introduction
Natural-killer (NK) cells are lymphocytes of the innate immune system that are involved in the early defense against foreign cells and autologous cells undergoing various forms of stress, such as microbial infection or tumor transformation. Upon stimulation, NK cells secrete large amounts of cytokines including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF), and chemokines such as CC chemokine ligand 3 (CCL3; macrophage inflammatory protein 1-α [MIP1-α], CCL4 (MIP1-β) and CCL5 (RANTES [regulated on activation, normal T expressed and secreted]). NK cells are also cytotoxic, inducing apoptosis of cells recognized as targets. NK cells identify their targets through a set of activating or inhibitory receptors, that recognize pathogen-encoded molecules (“nonself recognition”), self proteins whose expression is up-regulated in transformed or infected cells (“stress-induced self recognition”), or self proteins that are expressed by normal cells but down-regulated by infected or transformed cells (“missing-self recognition”).1-3 NK-cell activation is controlled by the dynamic balance between these activating and inhibitory signals.
Dendritic cells (DCs) are classically defined as the sentinels of the immune system. At an “immature” stage of development, DCs continuously uptake antigens in peripheral tissues and migrate at a slow rate to lymph nodes (LNs). Under these steady-state conditions, DCs express low levels of major histocompatibility complex (MHC) and costimulatory molecules and it has been suggested that their interaction with naive T cells is involved in peripheral T-cell tolerance.4 By contrast, encounter with microbial products or tissue damage in the periphery initiates DC maturation and their rapid migration to LNs. This activation program is due in part to the engagement of a complex set of innate sensors, such as the Toll-like receptors (TLR), which are able to recognize molecules and molecular patterns shared by various classes of microbes. Mature DCs express high levels of MHC and costimulatory molecules, which license them to activate naive T cells.
In 1999, it was discovered that NK-cell effector functions are stimulated through direct contact with activated DCs.5 These findings led to the novel concept of dendritic-cell-activated killers (DAKs).5 Subsequently, it was found that DC/NK-cell interaction was bi-directional and complex, as it could result not only in NK-cell activation but also in DC maturation or apoptosis, depending on the activation status of both players. The NK-cell-activating receptor NKp30 appears to play a central role in DC maturation or apoptosis induced by NK cells.6 Here, we provide an overview of how and where NK cells and DCs interact and what are the consequences of their interactions on the outcome of the immune response.
How do NK cells and DCs interact?
NK-cell activation by DCs
Original studies by Fernandez et al revealed that the adoptive transfer of DC- or fms-like tyrosine kinase 3-ligand (Flt3-L) expansion of DCs in mice bearing MHC class I-negative tumors promote NK-cell-dependent antitumor effects.5 In vitro studies demonstrated that the coculture of DCs and resting NK cells results in a substantial increase in both NK-cell cytolytic activity and IFN-γ production. Subsequent in vitro investigations identified that NK-cell activation induced by DCs requires both cytokine signals and a contact between NK cells and DCs.
Role of cytokines. Under conditions of stimulation by various pathogens or by TLR ligands, DCs constitute a source of several cytokines known to trigger NK-cell functions.
These include interleukin-12 (IL-12), IL-18, IL-15, and type I interferons (reviewed in Iwasaki and Medzhitov7 ).
In vitro studies have demonstrated a central role for DC-derived IL-12 in the induction of IFN-γ production by NK cells in different systems. These include lipopolysaccharide (LPS)-stimulated CD34+-derived DCs,8,9 actinobacillus-stimulated monocyte-derived DCs,10 or polyriboinosinic-polyribocytidilic acid (poly[I: C])-stimulated myeloid DCs11 in humans, and virus-activated plasmacytoid DCs (pDCs) in mice.12 IL-18 is known to synergize with IL-12 to induce IFN-γ secretion by NK cells. In the case of the NK/DC crosstalk, IL-12/IL-18 secretion by CD34+-derived DCs has been shown to enhance NK-cell cytotoxicity.13 Importantly, NK cells can also be activated independently of IL-12 or IL-18. Indeed, Granucci et al found that following stimulation with TLR4 ligands such as LPS or Escherichia coli bacteria, IL-12- and IL-18-deficient mouse bone marrow-derived DCs (BMDCs) were as efficient as wild-type BMDCs in inducing IFN-γ production by NK cells.14 In this system, bacterially activated DCs need to produce IL-2 but also need to establish cell-to-cell contact with NK cells for IFN-γ release by NK cells to occur.14
Another cytokine, IL-15, is secreted by mouse GM-CSF + IL-4-derived BMDCs and enhances in vitro NK-cell function. This effect requires the expression of the IL-15Rα on DCs,15 suggesting that murine DCs use IL-15Rα to present IL-15 in trans to NK cells during NK-cell priming. IL-15 production by DCs could be particularly important to trigger NK-cell proliferation, as demonstrated in another system by Ferlazzo et al, who reported that IL-15 production by human monocyte-derived DCs or spleen DCs induces NK cells to proliferate.16
Lastly, in vitro studies have also demonstrated a critical role for type I interferons in the induction of NK-cell cytotoxicity by various DC types, such as mouse GM-CSF-derived BMDCs stimulated with TLR4 ligands,14 and mouse12 or human11 pDCs activated by viruses or by TLR7/8/9 ligands. IFN-α/β can be produced by all DC subsets. However, pDCs appear specialized in high-level secretion of these cytokines in response to viral particles, viral single-stranded RNA (ssRNA), or viral and bacterial unmethylated cytosine-phosphoguanine (CpG) sequences (reviewed in Colonna et al17 ).
Role of cell-cell contacts. Optimal NK-cell activation by DCs also requires direct cell-to-cell contacts, as established in the previously mentioned study by Fernandez et al.5 In another system, MHC class I-related (MIC) A and B expression by IFN-α-stimulated DCs were found to be critical for NK-cell activation induced by DCs.18 Recently, Borg et al found that NK cells and DCs form stimulatory synapses involving cytoskeleton rearrangement and lipid raft mobilization in DCs.9 Synapse formation allows the polarized secretion of preassembled stores of IL-12 by DCs toward the NK-cell.
This IL-12 secretion leads to IFN-γ production. Similar findings have been reported for IL-18.19
Collectively, these studies indicate that in vitro NK-cell activation induced by DCs requires the synergistic action of several cytokines and a direct contact between DCs and NK cells (Figure 1Ai). The requirement for cell-cell contact is likely to reflect (1) the implication of membrane-bound receptor/ligand pairs; and/or (2) the necessity for local delivery of cytokines at high concentration at the interface between DCs and NK cells.
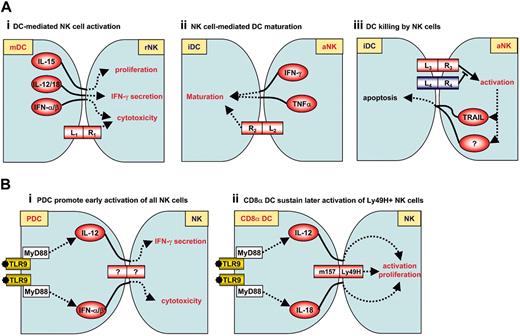
In vitro and in vivo NK-cell-DC interactions. (A) In vitro NK cell-DC interactions. Three types of NK-cell-DC interactions have been described. They are all dependent on cell-cell contact and cytokine signals.1 Activated DCs mediate NK-cell activation. In particular, DC-derived IL-15, IL-12/IL-18, and IFN-α/β could specifically enhance NK-cell proliferation, IFN-γ secretion, and cytotoxic function, respectively.2 Activated NK cells promote DC maturation. In human, NKp30 appears to play a central role in this interaction.3 Immature DCs, but not mature DCs, are killed by NK cells. This phenomenon appears to be dependent on NKp30 as well. iDC indicates immature DCs; mDC, mature DCs; rNK, resting NK cells; aNK, activated NK cells. (Ai-Aiii) Examples of L (ligand)-R (receptor) interactions. In panel Ai, L1 indicates MICA/B; R1, NKG2D. In panel Aii, R2 indicates TREM2 (mouse) or NKp30 (human). In panel Aiii, R3 indicates NKp30; and L4, HLA-E. (B) In vivo NK-cell-DC interactions during MCMV infection. (Bi) pDCs promote early activation of NK cells through IL-12 and IFN-α/β production following activation by viral CpG motifs. (Bii) CD8α DCs sustain later activation of Ly49H+ NK cells. Although Ly49H is known to bind to m157, it should be stressed that m157-Ly49H interaction between NK cells and DCs has not been formally demonstrated.
In vitro and in vivo NK-cell-DC interactions. (A) In vitro NK cell-DC interactions. Three types of NK-cell-DC interactions have been described. They are all dependent on cell-cell contact and cytokine signals.1 Activated DCs mediate NK-cell activation. In particular, DC-derived IL-15, IL-12/IL-18, and IFN-α/β could specifically enhance NK-cell proliferation, IFN-γ secretion, and cytotoxic function, respectively.2 Activated NK cells promote DC maturation. In human, NKp30 appears to play a central role in this interaction.3 Immature DCs, but not mature DCs, are killed by NK cells. This phenomenon appears to be dependent on NKp30 as well. iDC indicates immature DCs; mDC, mature DCs; rNK, resting NK cells; aNK, activated NK cells. (Ai-Aiii) Examples of L (ligand)-R (receptor) interactions. In panel Ai, L1 indicates MICA/B; R1, NKG2D. In panel Aii, R2 indicates TREM2 (mouse) or NKp30 (human). In panel Aiii, R3 indicates NKp30; and L4, HLA-E. (B) In vivo NK-cell-DC interactions during MCMV infection. (Bi) pDCs promote early activation of NK cells through IL-12 and IFN-α/β production following activation by viral CpG motifs. (Bii) CD8α DCs sustain later activation of Ly49H+ NK cells. Although Ly49H is known to bind to m157, it should be stressed that m157-Ly49H interaction between NK cells and DCs has not been formally demonstrated.
DC maturation induced by NK cells
Piccioli et al found that culture of activated human NK cells with immature monocyte-derived DCs, at low NK/DC ratios (1:5), led to an increase in DC cytokine production.20 In this context, soluble factors such as TNF-α and IFN-γ, as well as cell-to-cell contacts, are required for NK-cell-mediated DC activation (Figure 1Aii). Similarly, IL-2-activated NK cells can induce maturation of blood pDCs and myeloid DCs, and synergize with suboptimal concentrations of CpG oligonucleotides to promote strong IFN-α and TNF production by blood pDCs. Here again, both effects are dependent on cell-cell contacts.11 Vitale et al6 recently shed light on the cell-cell contact dependency of DC maturation induced by NK cells. Indeed, they reported that NK-cell-mediated monocyte-derived DC maturation depends on the triggering of NKp30 on NK cells and is counterregulated by killer cell inhibitory receptors (KIRs) and NKG2A inhibitory receptors.6 Another mechanism has been demonstrated in the mouse whereby IL-2 activated NK cells can trigger TREM2 (triggering receptor expressed on myeloid cells 2) signaling in GM-CSF/IL-4-derived BMDCs, thereby promoting up-regulation of CD86 molecules.21
Activated NK cells can therefore induce DC maturation either directly or in synergy with suboptimal levels of microbial signals. NK-cell-induced DC activation is dependent on both TNF-α/IFN-γ secretion and a cell-cell contact. It follows that NK-cell help might be critical for optimal DC activation and subsequent induction of T-cell responses in conditions where inflammation is poor but where NK-cell activation could occur through direct recognition of target cells. In particular, this may be relevant to defense against cancer, as discussed in “Antitumor responses.”
DC killing by NK cells
Whereas low NK-cell-immature DC ratios (1:5) lead to DC maturation, higher NK-cell-immature DC ratios (5:1) result in the killing of immature DCs by the autologous NK cells.20,22-25 Importantly, immature DCs are susceptible to autologous NK-cell-mediated cytolysis while mature DCs are protected. In vitro, signals delivered by NKp30 are critical for lysis of immature DCs25 while resistance to NK-cell lysis is mediated by up-regulation of MHC class I molecules during DC maturation,26 in particular histocompatibility leukocyte antigen-E (HLA-E) (Figure 1Aiii).27 Other signals may be involved in this phenomenon, as Carbone et al23 reported that immature DC lysis by NK cells in vitro was partly dependent on CD40 expressed by DCs. In a second article, they also found that expression of CD1 by DCs can inhibit NK-cell-mediated lysis.28
In vivo, NK-cell-mediated DC elimination has also been observed in different mouse models. Hayakawa et al reported that immature DCs are rapidly eliminated by NK cells via a pathway dependent on the TNF-related apoptosis-inducing ligand (TRAIL).29 Similarly, transplantation of alloreactive NK cells has been shown to suppress T-cell-mediated graft-versus-host disease by eliminating host DCs.30
By killing immature DCs, NK cells might thus regulate DC homeostasis and thereby the balance between tolerance and immunity. However, it remains to be determined whether DC killing by NK cells occurs in vivo under normal physiologic conditions.
Where do NK-cell/DC interactions occur?
DCs are distributed across all lymphoid and nonlymphoid tissues and include at least 2 major types: myeloid and pDCs that differ in terms of function and phenotype.31 Mature NK cells are found in the circulation and in several tissues, including the bone marrow, spleen, lymph nodes, liver, lung, omentum, intestine, and placenta. In human, 2 subsets of NK cells have been identified. Most CD56dim NK cells express high levels of FcγRIII (CD16) and perforin, whereas CD56bright NK cells are CD16-/low and perforin-/low. CD56dim NK cells harbor immediate cytotoxic activity while CD56bright become cytolytic upon culture with IL-2.32 CD56dim and CD56bright have different tissue distributions. CD56bright NK cells express L-selectin and CCR7 and are preferentially localized in lymph nodes. By contrast, CD56dim NK cells are poorly represented in lymph nodes but comprise 95% of blood NK cells and 85% of spleen NK cells. CD56dim NK cells also express receptors for inflammatory chemokines (Table 1).
Chemokine receptors expressed by NK cells and DCs
. | CCR1 . | CCR2 . | CCR3 . | CCR4 . | CCR5 . | CCR6 . | CCR7 . | CCR8 . | CCR9 . | CXCR1 . | CXCR2 . | CXCR3 . | CXCR4 . | CXCR5 . | CX3CR1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human NK cells | |||||||||||||||
| Resting CD56dim NK cells | – | – | – | + | – | – | – | – | – | +++ | ND | ++ | +++ | – | +++ |
| Resting CD56bright NK cells | – | – | – | – | ++ | +/– | ++ | – | – | + | ND | +++ | +++ | – | +/– |
| Activated NK cells | – | +/– | – | ++ | – | + | +/– | – | ND | – | ND | +++ | + | – | + |
| Mouse NK cells | |||||||||||||||
| Resting | (–) | (++) | (–) | (–) | +++ | – | (–) | (–) | (–) | ND | ND | +++ | +++ | – | (+) |
| Human DCs* | |||||||||||||||
| Immature | + | +/– | ND | + | + | + | +/– | ND | ND | ND | ND | ND | + | – | ND |
| Mature | – | – | ND | + | – | – | ++ | ND | ND | ND | ND | ND | ++ | – | ND |
| Mouse DCs† | |||||||||||||||
| Immature | +++ | + | – | +/– | + | ND | – | ND | ND | ND | – | ND | + | – | ND |
| Mature | + | – | – | – | – | ND | ++ | ND | ND | ND | – | ND | + | – | ND |
. | CCR1 . | CCR2 . | CCR3 . | CCR4 . | CCR5 . | CCR6 . | CCR7 . | CCR8 . | CCR9 . | CXCR1 . | CXCR2 . | CXCR3 . | CXCR4 . | CXCR5 . | CX3CR1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human NK cells | |||||||||||||||
| Resting CD56dim NK cells | – | – | – | + | – | – | – | – | – | +++ | ND | ++ | +++ | – | +++ |
| Resting CD56bright NK cells | – | – | – | – | ++ | +/– | ++ | – | – | + | ND | +++ | +++ | – | +/– |
| Activated NK cells | – | +/– | – | ++ | – | + | +/– | – | ND | – | ND | +++ | + | – | + |
| Mouse NK cells | |||||||||||||||
| Resting | (–) | (++) | (–) | (–) | +++ | – | (–) | (–) | (–) | ND | ND | +++ | +++ | – | (+) |
| Human DCs* | |||||||||||||||
| Immature | + | +/– | ND | + | + | + | +/– | ND | ND | ND | ND | ND | + | – | ND |
| Mature | – | – | ND | + | – | – | ++ | ND | ND | ND | ND | ND | ++ | – | ND |
| Mouse DCs† | |||||||||||||||
| Immature | +++ | + | – | +/– | + | ND | – | ND | ND | ND | – | ND | + | – | ND |
| Mature | + | – | – | – | – | ND | ++ | ND | ND | ND | – | ND | + | – | ND |
Symbols in parentheses indicate a demonstrated activity of the corresponding receptor using in vitro chemotaxis assays. Based on Sozzani et al,35 Campbell et al,81 Hanna et al,82 Inngjerdingen et al,83 and Vecchi et al.84
ND indicates not determined; +, detectable; ++, intermediate expression; +++, high expression; +/–, low-to-undetectable expression; and –, undetectable expression.
Monocyte-derived DCs
Bone marrow–derived DCs
In secondary lymphoid organs
In the mouse, few NK cells are found in LNs but they can be recruited in response to inflammation induced by adjuvant,33 DC injection,33 or Leishmania major inoculation (M. Bajenoff, Breart B, and Glaichenhaus N, unpublished results, June 2004). Under steady-state conditions, NK cells have been reported to reside in the LN's outer paracortex in close vicinity to DCs near high endothelial venules (HEV). In contrast to T cells, which move rapidly, the majority of NK cells appear to be slow motile cells (M. Bajenoff et al, unpublished results).
In humans, NK cells and DC colocalize in T-cell areas of LNs.16 Two studies have compared the ability of CD56bright and CD56dim NK cells to interact with DCs. Vitale et al found that both NK subsets purified from peripheral blood up-regulate CD69 expression when cultured with mature DCs.34 However, only CD56bright NK cells secrete IFN-γ and proliferate in these cocultures. Ferlazzo et al reported a similar phenomenon using lymph node or splenic human NK cells.16
In nonlymphoid peripheral tissues
Both immature DCs and NK cells express receptors for inflammatory chemokines (Table 1). In in vitro assays, both subsets strongly migrate in response to CXC chemokine receptor 3 (CXCR3), CXCR4, and CCR5 ligands.35-37 In vivo, it has been demonstrated that hepatic production of CCL3 (MIP1-α, a CCR5 ligand) is required for mouse NK cells homing to the liver and effective control of murine cytomegalovirus (MCMV) infection.38 Additionally, NK cells migrate to inflamed LNs and lungs in a CXCR3-dependent fashion.33,39 DCs have been shown to dramatically accumulate in inflamed tissues.40 As both NK cells and DCs have the potential to attract each other through their production of various chemokines (Table 2), it therefore follows that DCs and NK cells are likely to colocalize in inflamed tissues.
Chemokines secreted by actived NK cells and DCs
. | CCL2 . | CCL3 . | CCL4 . | CCL5 . | CCL17 . | CCL22 . | XCL1 . | CXCL8 . | CXCL10 . | CX3CL1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Other name | MCP-1 | MIP1-α | MIP1-β | RANTES | Tarc | MDC | Lymphotactin | IL-8 | IP-10 | Fractalkine |
| Receptor | CCR2 | CCR1/5 | CCR5 | CCR1/3/5 | CCR4 | CCR4 | XCR1 | CXCR1/2 | CXCR3 | CX3CR1 |
| Human NK cells | ND | ++ | +++ | ++ | ND | ND | ND | +++ | ND | ND |
| Mouse NK cells | ND | +++ | +++ | ++ | ND | ND | +++ | NA | (+) | ND |
| Human/mouse DCs* | +++ | +++ | ND | +++ | +++ | +++ | ND | +++ | ND | +++ |
. | CCL2 . | CCL3 . | CCL4 . | CCL5 . | CCL17 . | CCL22 . | XCL1 . | CXCL8 . | CXCL10 . | CX3CL1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Other name | MCP-1 | MIP1-α | MIP1-β | RANTES | Tarc | MDC | Lymphotactin | IL-8 | IP-10 | Fractalkine |
| Receptor | CCR2 | CCR1/5 | CCR5 | CCR1/3/5 | CCR4 | CCR4 | XCR1 | CXCR1/2 | CXCR3 | CX3CR1 |
| Human NK cells | ND | ++ | +++ | ++ | ND | ND | ND | +++ | ND | ND |
| Mouse NK cells | ND | +++ | +++ | ++ | ND | ND | +++ | NA | (+) | ND |
| Human/mouse DCs* | +++ | +++ | ND | +++ | +++ | +++ | ND | +++ | ND | +++ |
Direct contact between DCs and NK cells in vivo have been observed in multiple settings. DC-SIGN+ (dendritic-cell-specific intercellular adhesion molecule [ICAM] 3-grabbing nonintegrin) decidual antigen-presenting cells (APCs) were observed in situ to have intimate contact with CD56+/CD16-/ICAM-3+ decidual NK cells, during human pregnancy.41 NK-cell/DC interactions were also found in Malassezia-induced atopic skin lesions, and in imatinib mesylate-induced lichenoid dermatitis.42 PEN5+ NK cells can also be found in tissues infiltrated with malignant Langherans histiocytosis (L.Z., unpublished data, January 2005), suggesting that a dysregulation of the Langerhans cell (LC)/NK-cell cross talk could participate in this chronic inflammation. Although NK cells and DCs have been shown to infiltrate into tumors,43,44 further investigations are required to demonstrate and define their interactions within tumors.
NK-cell-DC interactions in immune responses
LN NK cells may have an important role in the initiation of T-cell responses by contributing to DC maturation. Indeed, under in vitro conditions where DCs are suboptimally activated with type-I IFN, NK cells license DCs to prime T-cell responses.45 LN NK cells also contribute to skew T-cell responses. Indeed, CD56bright NK cells secrete high levels of IFN-γ, TNF-β, IL-10, IL-13, and GM-CSF in response to IL-12/IL-1, IL12/IL-15, or IL-12/IL-18 stimulation,46 which gives them the potential to modulate the type of T-cell responses initiated in the LN. This point has been demonstrated in the mouse by Martin-Fontecha et al,33 who reported that NK-cell recruitment in LN provides the early IFN-γ production necessary for T helper 1 (Th1) polarization in a model of CD4+ T-cell response to ovalbumin (OVA). NK-cell recruitment into the LN may be induced by footpad injection of LPS-activated DCs or adjuvants such as R848 (TLR7/8 ligand) or RC-529 (TLR4 ligand).33 NK-cell depletion leads to defective Th1 polarization of CD4+ T cells activated by LPS-matured, OVA-pulsed DCs. However, it is not clear which cells—naive T cells or DCs presenting the MHC class II/OVA complexes—are the target of IFN-γ produced by NK cells.33 In vitro, Mailliard et al47 reported that NK cells activated by both IFN-α and K562 target cells may in turn induce high IL-12 production by DCs. In addition, they demonstrate that such “DC1s” are primed to induce Th1 CD4+ T-cell responses in vitro.47
Antitumor responses
It is assumed that tumor cells that develop into cancer are poorly immunogenic and not recognized by the immune system because “danger signals” are missing in vivo. NK-cell activation by tumor cells (MHC class I low, transporter associated with antigen processing [TAP]-deficient or overexpressing CD27, glycoprotein [gp] 96, or NKG2D ligands) has been shown to promote the elicitation of cognate and protective T-cell responses against the tumor (reviewed in Raulet2 ). In these examples, tumor recognition by NK cells involves receptors that allow them to detect “missing self” and/or “induced-self.” In some cases, T-cell-mediated tumor rejection was shown to be dependent on DC activation by NK cells.48 IFN-γ secreted during NK-cell-mediated tumor rejection is critical for cytotoxic T lymphocyte (CTL) generation, particularly when tumors express CD70 or CD80 and CD86.49 Furthermore, Adam et al reported that NK-cell-DC crosstalk may bypass the T helper arm in CTL induction against tumors expressing NKG2D ligands.50 They found that efficient CTL priming and long term CD8+ T-cell memory against A20 lymphoma may be achieved by injecting mature CD40-/- BMDCs, unpulsed with tumor antigens but capable of NK-cell triggering. Their data clearly show that IFN-γ produced by NK cells was necessary for activation of endogenous DCs and IL-12 production leading to CTL induction.50
Besides this NK-cell → DC → T-cell pathway, a DC → T-cell → NK-cell pathway has also been identified in vivo. Indeed, Van Den Broeke et al51 reported that unpulsed mature BMDC injection protects BALB/C mice against syngeneic CT26 colon carcinoma or LL/2 lung carcinoma inoculation. They found that protection against tumor was long lasting and NK-cell mediated. Activation of NK cells was CD4+ T-cell-dependent and strongly relied on the expression of costimulatory molecules on DCs, but did not require expression of IL-12 or IL-15 by the DCs. These findings led to the hypothesis that mature DCs stimulate CD4+ T cells that could in turn directly activate NK cells through IL-2 secretion.51
Lastly, activation of NK cells by DCs is potentially important for the promotion of tumor regression. For example, Turner at al showed that anti-CD40 therapy induces substantial NK-cell-mediated antitumor and antimetastatic effects.52 As CD40 is not expressed by NK cells or by the tumor cells used in their study, it was suggested that NK-cell activation is mediated by increased cytokine production upon CD40 ligation on DCs. Similarly, modulation of the c-kit signaling pathway in DCs leads to marked NK-cell activation in various strains of mice.42 Indeed, a 10- to 21-day oral therapy with the c-kit inhibitor imatinib mesylate promotes the selective expansion of CD69+ NK cells and NK-cell-dependent antitumor effects in tumor models resistant to imatinib mesylate in vitro. Nanomolar concentrations of imatinib mesylate are sufficient to endow DCs with NK-cell stimulatory capacities in vitro without promoting DC maturation. The effect of imatinib mesylate does not depend on IL-12 secretion by DCs but does require NK-cell-DC contacts. The capacity of imatinib mesylate to endow DCs with NK-cell stimulatory capacities has also been shown in patients with gastrointestinal stromal tumor (GIST). GISTs are tumors displaying the typical features of NK-cell sensitivity (TAP1 deficiency, overexpression of MIC and UL16-binding protein [ULBP] molecules) and are lysed as efficiently as K562 cells by NK cells.42 Up to 50% of patients bearing a GIST and treated with imatinib mesylate acquire enhanced NK-cell effector functions. The relevance of NK-cell activation for the clinical outcome was suggested by a correlation between the objective responses at 1 year and early NK-cell triggering (at 2 months) on a cohort of 42 patients.42
Antimicrobial responses
A role for NK cells in the control of hepatitis,53 Ebola,54 and HIV55,56 infections in human has been proposed. In the mouse, a critical role for NK cells, DC and their crosstalk in the defense against murine cytomegalovirus (MCMV) infection has been demonstrated.3 NK-cell cytotoxicity and IFN-γ expression, proliferation, and accumulation are rapidly induced after MCMV infection. IL-12/STAT4 is critical for NK-cell IFN-γ expression, whereas IFN-α/β/ signal transducer and activator of transcription 1 (STAT1) is required for induction of cytotoxicity.57 The accumulation/survival of proliferating NK cells is STAT4 independent but requires IFN-α/β/STAT1 induction of IL-15.57
pDCs recognize viral CpG DNA sequences through the TLR9-myeloid differentiation factor 88 (MyD88) pathway and are the main source of IFN-α/β and IL-12 during MCMV infection (Figure 1Bi).12,57-60 However, while depletion of pDCs leads to a drastic reduction in IFN-α/β production, other cell types compensate for IL-12 production, ensuring normal IFN-γ and NK-cell responses to MCMV.59,60 Thus, the TLR9/MyD88 pathway contributes to coordinate antiviral cytokine responses by pDCs, DCs, and other cell types to promote effective NK-cell function and MCMV clearance.
In mice, resistance to MCMV is associated with a single dominant locus, Cmv1, which controls viral replication in the spleen. Cmv1 encodes the NK-cell-activating receptor Ly49H that binds a virus-encoded molecule with a predicted MHC class I structure m157 (Figure 1Aii).61-65 Transgenesis of Ly49H is necessary and sufficient to confer to susceptible mouse strains increased resistance to MCMV infection.66 During MCMV infection, a specific expansion of the Ly49H+ NK-cell subset occurs before antiviral CD8 T-cell responses become readily detectable in the spleen.67 This expansion depends on the presence of CD8α+ DCs and also on IL-12 and IL-18 (Figure 1Bii).68 CD8α+ DCs are a major early target for MCMV infection in vivo and infected CD8α+ DCs produce very high levels of IL-12.12 Thus, the amplification of Ly49H+ NK cells may be driven by infected CD8α+ DCs. In this setting, m157 expression by DCs may allow the establishment of a synapse and the local delivery of IL-12 and IL-18 toward NK cells. Interestingly, the presence of the Ly49H+ subset of NK cells results in the maintenance of spleen CD8α+ DCs during acute infection by MCMV.68,69 This may be due at least in part to the induction of the expression of viral resistance genes in DCs in response to IFN-γ production.
Concluding remarks
DCs are classically defined as sentinels of the immune system, capable of recognizing “danger” and specialized in the initiation of the adaptive response against danger-causing agents.70 In addition, DC-dependent activation of CD8+ T cells requires CD4+ T-cell help.71,72 A growing body of evidence now shows that (1) not all receptors sensing “danger” or pathogenic agents are expressed by DCs; (2) other cells than CD4 T cells may provide help to DC maturation; and (3) DCs have the potential to influence both innate and adaptive immunity. In particular, NK cells make functional interactions with DCs that can clearly influence induction/regulation of innate and adaptative immune responses. NK activation through NK-cell receptors can promote T-cell responses against tumors by inducing DC activation. Reciprocally, DCs have been found to induce potent NK-cell activation in antiviral responses; IL-12, IL-15, IL-18, and IFN-α/β production by DCs as well as cell-cell contact are critical for the enhancement of NK-cell effector functions.
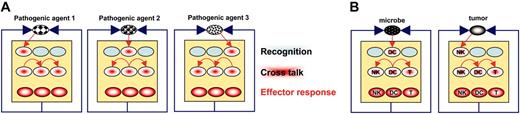
NK-cell/DC interactions are critical in situations where receptors allowing the recognition of the pathogenic agent are only expressed by one of the 2 subsets. By extrapolation, one may hypothesize that the expression of receptors allowing early detection of all pathogenic agents is compartmentalized in (innate) immune cell types. In this model, activation of the whole immune system is thus dependent on cell-cell crosstalk that spreads activation among cells that compose it (Figure 2A). At one extreme, DCs would be the first cells to be activated by microbes, thanks to their expression of relevant innate sensors (TLRs, nucleotide-binding oligomerization domain [NOD]) and turn on the system. At another extreme, in the case of a tumor that does not cause overt inflammation but does express ligands for activating NK-cell receptors, NK cells would be the first cells to be activated and turn on the system (Figure 2B).
It is intriguing to note the close similarities between NK cells and DCs. Indeed, the development of NK cells and some DC subtypes is selectively dependent on Flt3L.73 Some NK-cell markers are expressed on DC (ie, NKRP1) and vice versa (CD11c).74,75 Leukemic cells expressing both DCs and NK-cell markers have also been described,76 and in the course of lymphocytic choriomeningitis virus (LCMV) infection bitypic cells expressing both CD11c and DX5 have been identified and shown to protect against autoimmune diabetes.77 DCs may have cytotoxic properties74,78 or acquire them in response to stimulation with type I IFN,79 whereas activated NK cells have recently been identified as potent antigen-presenting cells.80
The central role of cell-cell crosstalk in the immune function. (A) In this model, each immune cell type specifically expresses a receptor for 1 pathogenic agent. The elimination of this agent, however, needs the coordinated effort of several immune cell types. This may only be achieved through cell-cell crosstalk, resulting in activation spreading. (B) As a prototypical example, in the case of a tumor or a microbe, initial recognition may involve NK-cells and DCs, respectively.
The central role of cell-cell crosstalk in the immune function. (A) In this model, each immune cell type specifically expresses a receptor for 1 pathogenic agent. The elimination of this agent, however, needs the coordinated effort of several immune cell types. This may only be achieved through cell-cell crosstalk, resulting in activation spreading. (B) As a prototypical example, in the case of a tumor or a microbe, initial recognition may involve NK-cells and DCs, respectively.
As most studies on NK-cell-DC interactions have focused on conditions of microbial and tumoral challenge, the role of NK/DC crosstalk under other conditions, such as autoimmune diseases, remains to be determined.
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2005-03-1154.
Supported in part by specific grants from the European Union (“ALLOSTEM”), Ligue Nationale contre le Cancer (“Equipes labellisées La Ligue”) and institutional grants from INSERM, CNRS, and Ministère de l'Enseignement Supérieur et de la Recherche (E.V., L.Z.), and by an Action Thématique et Inicitative sur Programme (ATIPE) grant from the CNRS and by a grant from the Agence pour la Recherche contre le Cancer (ARC) (M.D).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal