Abstract
The role of the proteolytic enzyme elastase in motility and proliferation of leukemic human acute myeloblastic leukemia (AML) cells is currently unknown. We report a correlation between abnormally high levels of elastase in the blood of AML patients and the number of leukemic blast cells in the circulation. In AML cells, we observed expression of cell-surface elastase, which was regulated by the chemokine stromal cell-derived factor-1 (SDF-1). In vitro inhibition of elastase prevented SDF-1-induced cell polarization, podia formation, and reduced migration of human AML cells as well as their adhesion. Elastase inhibition also significantly impaired in vivo homing of most human AML cells to the bone marrow (BM) of nonobese diabetic-severe combined immunodeficient (NOD/SCID)/beta-2 microglobulin knock-out (B2mnull) mice that underwent transplantation. Moreover, in vitro proliferation of AML cells was elastase dependent. In contrast, treatment with elastase inhibitor enhanced the proliferation rate of human cord blood CD34+ cells, including primitive CD34+/CD38- cells, and their in vivo homing. Finally, NOD/SCID mice previously engrafted with human AML cells and treated with elastase inhibitor had significantly reduced egress of leukemic cells into the circulation. Taken together, our data demonstrate that human AML cells constitutively secrete and express SDF-1-dependent cell-surface elastase, which regulates their migration and proliferation. (Blood. 2005;106:2120-2127)
Introduction
Acute myeloblastic leukemia (AML) is characterized by uncontrolled proliferation within the bone marrow (BM) of malignant myeloid progenitors arrested in their maturation process and the egress of these abnormal cells into the circulation. Many observations suggest that similar genes and pathways regulate cancer and normal stem cells. We have previously shown that homing and engraftment of normal stem and progenitor cells are tightly regulated by the chemokine stromal cell-derived factor-1 (SDF-1) expressed in the BM and by its receptor CXCR4 expressed on the cell surface of progenitors.1,2 The functional, preclinical immunedeficient nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice model for human hematopoietic cells allows engraftment of both normal (SCID-repopulating cell [SRC]) and AML (SCID leukemia-initiating cell [SLIC]) stem cells and mimics many aspects of human AML.1,3-5 Recently, we have demonstrated that neutralization of SDF-1/CXCR4 interactions inhibits the homing and engraftment of human AML cells in NOD/SCID mice.6 In support of this data, Rombouts et al have found that in AML patients the levels of CXCR4 is a negative predictor of overall survival and relapse-free survival.7
Throughout adult life, the hematopoietic system is maintained by constant production of maturing lymphoid, myeloid, and erythroid cells, which are released from the BM to the periphery and to secondary organs. Of interest, hematopoietic stem cells, which mainly reside within the BM, are also found circulating in the blood at very low levels. This egress of maturing and immature cells must be tightly regulated, but the molecular mechanisms controlling migration, development, and cell egress are largely uncharacterized.
Studies aimed at deciphering the mechanism of stem and progenitor cell egress from the BM have focused on leukocytosis induced by various stress-inducing agents such as chemotherapy drugs, lipopolysaccharide (LPS), and repeated daily stimulations with chemokines or cytokines, including granulocyte colony-stimulating factor (G-CSF), which is widely used to mobilize and harvest stem cells for clinical transplantation.8,9 In recent years, the mechanism governing G-CSF-induced mobilization has began to emerge: it was found that G-CSF induces expansion of myeloid cells in the BM and release of large amounts of neutrophil proteases, such as elastase and cathepsin G, that degrade stromal vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), the P-selectin receptor P-selectin glycoprotein ligand-1 (PSGL-1), as well as the chemokine SDF-1 and the cytokine kit ligand.10 Moreover, it was shown that elastase can cleave CXCR4.11 VCAM-1/very late antigen 4 (VLA-4), c-kit/kit ligand, and SDF-1/CXCR4 interactions are believed to be crucial regulators of hematopoietic cell anchorage and retention within the BM. In addition, G-CSF enhances the release of metalloproteinases such as matrix metalloproteinase-9 (MMP-9), which also participates in the egress of cells to the circulation.12 Of interest, heterozygous germ-line mutations in the ELA2 gene encoding human leukocyte elastase have been associated with several inherited neutropenic syndromes such as cyclic neutropenia and Kostmann disease, which are characterized by a severe impairment in neutrophil release into the circulation.13 These observations suggest that elastase may have a key role in regulating egress of human neutrophils from the BM.
Leukocyte or neutrophil elastase (NE) is a serine protease stored in azurophilic granules of myeloid cells and is released upon activation and degranulation. Elastase is a broad-range proteolytic enzyme, whose substrates include various extracellular matrix (ECM) proteins, such as elastin, fibronectin, and collagen as well as adhesive molecules such as ICAM-1 and junctional cadherins, suggesting a role for elastase in facilitating cell motility and transendothelial migration.14 Moreover, elastase degrades numerous soluble proteins, namely coagulation factors, immunoglobulins, complements, protease inhibitors, cytokines, and chemokines and their receptors.15,16 Previous studies reported high levels of intracellular elastase activity and secretion of elastase protein by AML cells.17-19 Evidence recently emerged that elastase has a major role in the development of myeloid leukemias. Elastase produced by chronic myelogenous leukemia (CML) neutrophils preferentially suppresses normal hematopoiesis.20 Elastase can cleave G-CSF and its receptor, abolishing its proliferative effect on normal human CD34+ progenitor cells.21,22 Moreover, proteolytic processing by elastase was recently suggested to play an important role in the development of leukemogenic processes. Lane and Ley showed that the fusion protein promyelocytic leukemia-retinoic acid receptor (PML-RAR) associated with acute promyelocytic leukemia (APL) is cleaved by neutrophil elastase and that NE-deficient mice are partially protected from development of APL.23
In the present study, we studied the interactions between elastase and the key molecule SDF-1, and we explored the involvement of elastase in migration and development of human AML cells, using the preclinical NOD/SCID-AML model.
Material and methods
Cell lines
Human myeloid U937, HL-60, ML-2, and ML-1 (kindly provided by Dr A. Peled, Hadassah University Hospital, Jerusalem, Israel) were grown in RPMI with 10% fetal calf serum (FCS).
Human cells
Normal human cord blood (CB) cells from full-term deliveries and malignant leukemic peripheral blood (PB) and/or BM cells, were obtained from 23 newly diagnosed AML patients and one resistant AML patient after chemotherapy treatment (no. 22) after patients provided informed consent. The diagnosis of leukemia was based on routine morphologic evaluation, immunophenotyping, and cytochemical smears using the French-American-British (FAB) classification. Plasma was collected after centrifugation and stored at -70°C. Cell samples were diluted 1:1 in phosphate-buffered saline (PBS). Low-density mononuclear cells (MNCs) were collected after standard separation on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) and washed in PBS. In some experiments, AML and CB CD34+ cells were enriched using the magnetic activated cell sorting (MACS) cell isolation kit and AutoMacs magnetic cell sorter (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions, obtaining purity of more than 95%. Cells were used fresh or frozen in FCS plus 10% dimethyl sulfoxide (DMSO) for storage in liquid nitrogen. Human samples were used in accordance with approved procedures by the human experimentation and ethics committee of the Weizmann Institute and the Sourasky Medical Center. Informed consent was provided according to the Declaration of Helsinki.
Migration assay
A total of 600 μL RPMI supplemented with 10% FCS in the presence or absence of 125 ng/mL recombinant human SDF-1 (rhSDF-1; Peprotech, Rocky Hill, NJ) was added to the lower chamber of a Costar 24-well transwell plate with 5-μm pore filters (Corning Costar, Cambridge, MA). One hundred thousand primary MNC leukemic cells or AML cell lines with or without pretreatment with elastase inhibitor (EI) MeOSuc-AAPV-CMK (10 μg/mL; Calbiochem, La Jolla, CA), α1-antitrypsin (300 μg/mL; Sigma Chemicals, St Louis, MO), or monoclonal mouse anti-human neutrophil elastase antibody (Ab, 30 μg/mL; Dako, Glostrup, Denmark) for 30 minutes were added to the upper chamber and were allowed to migrate for 4 hours at 37°C. Migrating cells were collected from the lower chamber and counted using a fluorescence-activated cell sorter (FACS) Calibur (Becton Dickinson [BD], San Jose, CA).
Elastase enzyme-linked immunoabsorbent assay (ELISA)
Levels of soluble elastase in BM and PB plasma samples were quantified by ELISA according to the manufacturer's instructions (Bender MedSystems, San Bruno, CA).
Elastase immunofluorescence
Cell-surface elastase on primary AML cells and AML cell lines was analyzed by flow cytometry. Cells, after fixation with 3.7% paraformaldehyde for 20 minutes and permeabilization with 0.5% triton for 10 minutes for intracellular detection, were incubated with polyclonal rabbit antihuman elastase (Biodesign, Kennebunkport, ME) for 30 minutes, washed, and then stained with secondary donkey anti-rabbit fluorescein isothiocyanate (FITC) Ab (Jackson ImmunoResearch, West Grove, Pennsylvania). In some experiments, cells were pretreated for 3 hours with SDF-1 (200 ng/mL) or anti-CXCR4 Ab (10 μg/mL, 12G5; R&D Systems, Minneapolis, MN) before elastase immunolabeling. After staining, cells were analyzed on a FACS Calibur using Cell Quest software (Becton Dickinson, San Jose, CA).
Mice
NOD-SCID mice (NOD/LtSz PrKdcscid/PrKdcscid) and NOD/SCID β2 microglobulin knock-out mice (NOD/SCID/B2mnull) were bred and maintained under defined flora conditions in individually ventilated (highefficiency particulate air [HEPA]-filtered air) sterile microisolator cages (Techniplast, Buguggiate, Italy) at the Weizmann Institute. All experiments were approved by the animal care committee of the Weizmann Institute. Mice, 8 to 10 weeks old, were irradiated with a sublethal dose of 375 cGy at 67cG/min from a cobalt source 5 to 24 hours prior to transplantation.
Homing assay
Human primary AML MNCs, enriched CD34+ AML cells, or CB cells, at the indicated cell doses, were suspended in 500 μL RPMI with 10% FCS and incubated for 30 minutes in 37°C either with or without EI (10 μg/mL) before injection via the dorsal tail vein. NOD/SCID/B2mnull mice were killed 16 hours after transplantation. BM cells flushed from both femur and tibia bones were harvested and resuspended into single-cell suspension. The percentage of human cells was determined by flow cytometry immunostaining with anti-human CD45-FITC mAB (Immuno Quality Products, Groningen, the Netherlands). Human Fc receptors were blocked with human plasma (1%) and murine Fc receptors by mouse immunoglobulin G (IgG; Pharmingen, San Diego, CA). Isotype control antibodies were used in order to exclude false-positive cells (BD). After staining, cells were analyzed on a FACS Calibur using Cell Quest software.
Engraftment and egress assays
Human primary AML MNCs (10-30 × 106) or HL-60 AML cell line (20 × 106) was injected intravenously into sublethally irradiated NOD/SCID mice. Two to 4 weeks later, EI (1 mg) was injected once a day for 4 consecutive days. Mice were asphyxiated with dry ice, PB was collected by cardiac aspiration in heparinized tubes, and BM was collected as described in “Homing assay.” Percentage of human cells was determined by immunofluorescence for human CD45 Abs as described in “Homing assay.”
Immunocytochemical procedures
Primary PB AML and HL-60 cells, either pretreated for 30 minutes with 10 μg/mL elastase inhibitor or left untreated, were allowed to adhere for 30 minutes to Poly-l-Lysine (Sigma Chemicals)-coated glass coverslips, in the presence or absence of SDF-1 (200 ng/mL), fixed in 3% paraformaldehyde (Merck, Darmstadt, Germany) in PBS, and indirectly immunolabeled with anti-human elastase (Biodesign) and anti-human CXCR4 (12G5; R&D Systems) followed by goat anti-rabbit Alexa488 (Molecular Probes, Eugene, OR) and goat anti-mouse cyanin 3 (Cy3; Jackson ImmunoResearch), respectively. Samples were mounted in Mowiol-488 (Hoechst, Frankfurt, Germany) and observed using a confocal microscope (Bio-Rad Laboratories, Hercules, CA).
Adhesion assay
Human AML cell lines were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) (according to the manufacturer's instructions) incubated for 30 minutes at 37°C with or without EI (10 μg/mL for 30 minutes), and allowed to adhere to confluent BM endothelial cell line (BMEC) cells (kindly provided by Dr S. Rafii, Cornell University, NY) or 25 μg/cm2 fibronectin-coated 96-well plate for 45 minutes at 37°C in serum-free RPMI plus 0.2% bovine serum albumin (BSA). Nonadherent cells were washed twice in FACS buffer (PBS + 1% FCS + 0.02% NaNO3). Adherent cells were collected in 150 μL FACS buffer plus 5 mM ethylenediaminetetraacetic acid (EDTA). The number of CFSE+ cells was determined by FACS analysis (FACSCalibur; BD).
Proliferation assay
Primary human AML cells (1 × 106/mL), AML cell lines (1 × 104/mL), or CD34+ enriched human CB cells (1 × 105/mL) were grown for 3 to 7 days in RPMI supplemented with 10% FCS with or without EI (10 μg/mL). Cultures of CB CD34+ cells were supplemented with stem cell factor (SCF) 50 ng/mL, FLT-3 ligand (FLT-3L) 50 ng/mL, and interleukin-6 (IL-6) 50 ng/mL. The number of viable cells was determined by trypan blue exclusion.
Results
Primary human AML cells secrete abnormally high amounts of elastase that correlate with the blast levels in patients' circulation
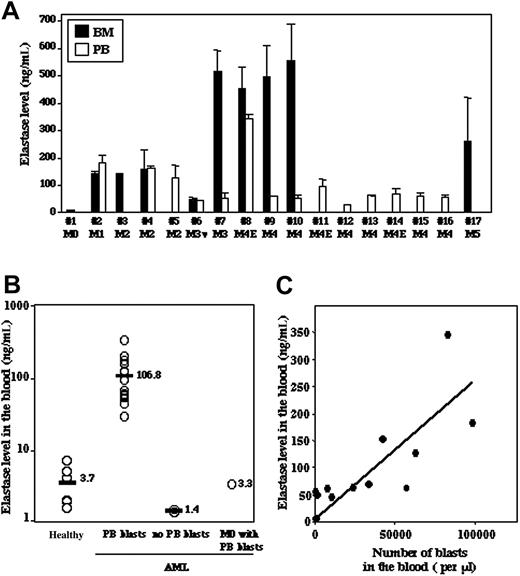
We analyzed by ELISA the plasma levels of elastase in the BM and PB of 17 newly diagnosed AML patients with different FAB subtypes and found variable amounts of elastase (Figure 1A). Of interest, despite the relatively low number of AML samples examined, a correlation between the level of elastase in the BM and the subtypes of AML could be observed. The poorly differentiated AML type (M0) exhibited the lowest levels of elastase, while more differentiated APL (M3) and myelomonocytic leukemia (M4) cells had the highest levels of the enzyme secreted in the BM (Figure 1A). Compared with PB plasma of healthy individuals, a 30-times higher concentration of elastase was observed in most AML patients (Figure 1B). Of note, in 2 AML patients (no. 3-M2 and no. 17-M5) who had almost no blast cells in the circulation, normal levels of elastase were detected (Figure 1B, no PB blasts), suggesting that the concentration of PB elastase may correlate with the number of circulating leukemic blasts. Indeed, a significant correlation (r = 0.6, P ≤ .01) between the amount of secreted elastase protein in the plasma and the number of AML blast cells in the peripheral blood was found (Figure 1C). These results demonstrate that the levels of secreted elastase protein in the plasma are mostly due to AML blast cells present in the blood. It is noteworthy that elastase concentration was very low in the PB plasma from a M0 type patient (no. 1) despite a high number of blasts (Figure 1B, M0 with PB blast).
Plasma levels of elastase in BM and PB of AML patients. (A) The levels of elastase in BM and PB plasma of AML patients were determined by ELISA. (B) The levels of elastase in PB plasma of 6 healthy donors were compared with those of AML patients that showed the presence (PB blasts) or absence (no PB blasts, patients no. 3 and no. 17) of leukemic blasts in the PB and with one M0 patient with no blast present in the circulation (patient no. 1). (C) Correlation between levels of elastase and number of leukemic blasts in the PB (r = 0.6, P ≤ .01). Results indicate the mean ± SD of 2 to 3 independent experiments.
Plasma levels of elastase in BM and PB of AML patients. (A) The levels of elastase in BM and PB plasma of AML patients were determined by ELISA. (B) The levels of elastase in PB plasma of 6 healthy donors were compared with those of AML patients that showed the presence (PB blasts) or absence (no PB blasts, patients no. 3 and no. 17) of leukemic blasts in the PB and with one M0 patient with no blast present in the circulation (patient no. 1). (C) Correlation between levels of elastase and number of leukemic blasts in the PB (r = 0.6, P ≤ .01). Results indicate the mean ± SD of 2 to 3 independent experiments.
AML cells constitutively express cell-surface elastase, which is regulated by the SDF-1/CXCR4 axis
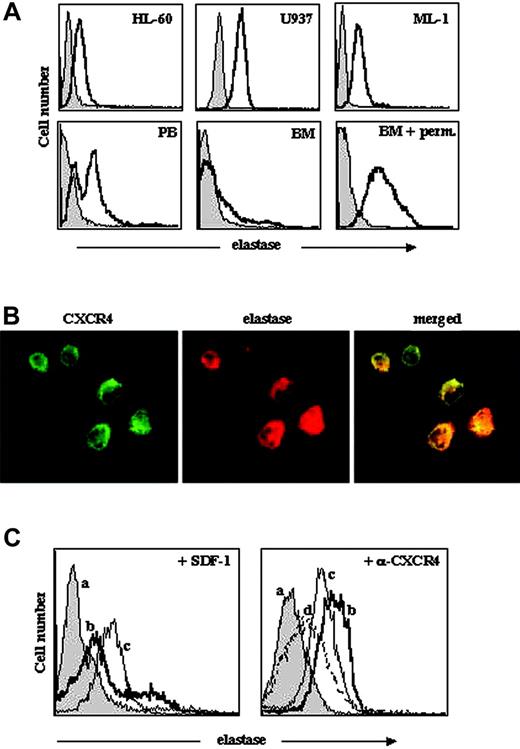
In neutrophils, which are short lived, elastase is secreted after cell activation or during degranulation when the cells die and is normally found in condition medium/sera. Activated neutrophils also express membrane-bound elastase, which is relocalized on the leading edge of migrating cells.24,25 Since most leukemic myeloid cells display deregulated activation and dissemination patterns, we evaluated the expression of elastase in primary human AML cells as well as in 3 AML cell lines. Of interest, elastase was detected by flow cytometry on the cell surface of all PB primary AML cells and AML cell lines tested (Figure 2A). We found that BM-derived AML cells had low levels of surface elastase; however, staining after permeabilization revealed high levels of intracellular elastase expression (Figure 2A).
Previously, others and we demonstrated that human AML stem cells express CXCR4 and migrate toward a gradient of SDF-1, and that CXCR4 neutralization had a remarkable effect on engraftment and survival of AML cells in a NOD/SCID transplant model.6,7,26 Moreover, the levels of CXCR4 are a negative predictor of overall survival and relapse-free survival of AML patients.7 Next, we tested whether the CXCR4/SDF-1 axis is involved in the regulation of cell-surface elastase expression in human AML cells. Immunofluorescence staining revealed localization of both elastase and CXCR4 on the cell surface of AML cells (Figure 2B). Of interest, we observed a remarkable increase in the amount of cell-surface elastase on primary BM AML cells, which express low levels of surface elastase, following exposure to 200 ng/mL SDF-1 for 3 hours (Figure 2C, left panel). In line with this observation, incubation of highly expressing elastase AML PB cells with neutralizing anti-CXCR4 antibodies decreased the expression of surface elastase compared with control cells (Figure 2C, right panel). Longer incubation (16 hours) led to further down-regulation of cell-surface elastase (Figure 2C).
Cell-surface localization of elastase in AML cells. (A) Representative FACS analysis, determined using rabbit antielastase Abs and secondary anti-rabbit FITC Abs, of elastase expression on AML cell lines (U937, HL-60, and ML-1) and primary AML PB and BM cells. Staining with only secondary Abs is shown in grey. Elastase internal staining of BM AML cells was performed after cell permeabilization as described in “Materials and methods.” Perm. indicates permeabilization. (B) Immunofluorescence staining for membranal elastase and CXCR4 in primary PB AML cells. Original magnification, × 100. (C) Cell-surface expression of elastase on AML cells after incubation with SDF-1 or anti-CXCR4 Abs. BM-derived cells were treated with 200 ng/mL SDF-1 (left panel) and PB-derived cells with 10 μg/mL anti-CXCR4 Abs (right panel) for 3 hours and stained for elastase cell-surface expression. Control cells stained with only secondary anti-rabbit FITC Abs are shown with grey plain line (a); control cells stained with antielastase Abs, with bold black line (b); cells treated with SDF-1 or anti-CXCR4 for 3 hours, with thin black line (c); and cells treated with anti-CXCR4 for 16 hours, with dotted line (d).
Cell-surface localization of elastase in AML cells. (A) Representative FACS analysis, determined using rabbit antielastase Abs and secondary anti-rabbit FITC Abs, of elastase expression on AML cell lines (U937, HL-60, and ML-1) and primary AML PB and BM cells. Staining with only secondary Abs is shown in grey. Elastase internal staining of BM AML cells was performed after cell permeabilization as described in “Materials and methods.” Perm. indicates permeabilization. (B) Immunofluorescence staining for membranal elastase and CXCR4 in primary PB AML cells. Original magnification, × 100. (C) Cell-surface expression of elastase on AML cells after incubation with SDF-1 or anti-CXCR4 Abs. BM-derived cells were treated with 200 ng/mL SDF-1 (left panel) and PB-derived cells with 10 μg/mL anti-CXCR4 Abs (right panel) for 3 hours and stained for elastase cell-surface expression. Control cells stained with only secondary anti-rabbit FITC Abs are shown with grey plain line (a); control cells stained with antielastase Abs, with bold black line (b); cells treated with SDF-1 or anti-CXCR4 for 3 hours, with thin black line (c); and cells treated with anti-CXCR4 for 16 hours, with dotted line (d).
By immunofluorescence microscopy, we examined the cellular localization of elastase in AML cells after incubation with 200 ng/mL SDF-1 and did not observe any relocalization of elastase to specific structures (data not shown). Taken together, these results suggest that AML cells not only secrete elastase, but also constitutively express elastase on the cell surface, which is regulated by the CXCR4/SDF-1 axis.
Elastase inhibitor decreases SDF-1-induced migration of AML cells
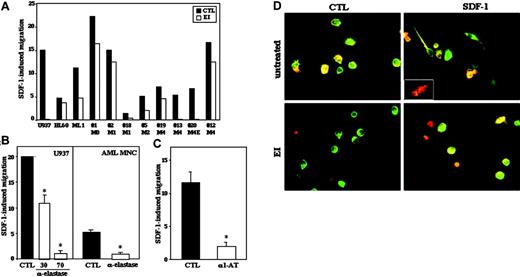
We next examined the effect of EI on SDF-1-induced migration of human AML cells in transwell assay. Preincubation of primary AML cells and cell lines for 30 minutes with 5 to 10 μg/mL EI significantly reduced their SDF-1-dependent migration in all AML cells tested (Figure 3A). Spontaneous migration in the absence of exogenous SDF-1 was also reduced (data not shown). In order to confirm these results, we used specific neutralizing antielastase Abs as well as the physiologic inhibitor of elastase, α1-antitrypsin. We found similar, significant inhibition of migration toward SDF-1 after pretreatment of primary AML cells or cell lines with neutralizing antielastase Abs (Figure 3B) or with α1-antitrypsin (Figure 3C).
These observations demonstrate that elastase is important for the migration of human AML cells, both spontaneous and SDF-1 induced. Of interest, EI did not change the levels of cell-surface CXCR4 expression as examined by FACS (data not shown). Cell migration requires cytoskeletal rearrangements and SDF-1 was shown to induce formation of protrusions and cell polarization in AML cells.27 When leukemic cells were pretreated with EI, the formation of SDF-1-induced protrusions was prevented (Figure 3D). Identical results were observed after preincubation of AML cells with α1-antitrypsin (data not shown). Of note, basal cell polarization in the absence of exogenous SDF-1 was also abolished by EI (Figure 3D) or α1-antitrypsin (data not shown). These results suggest that elastase participates in leukemic cell motility through direct regulation of cytoskeletal rearrangements and cell polarization in response to CXCR4 signaling.
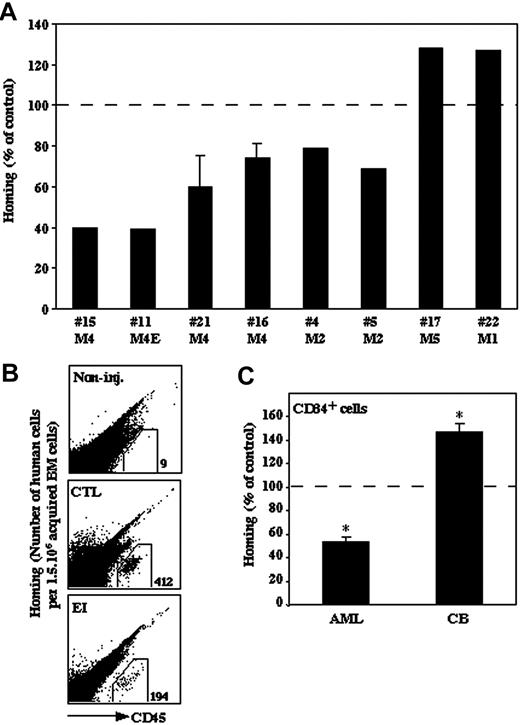
Homing of human AML cells to the BM of NOD/SCID mice is impaired by pretreatment with EI in contrast to enhanced homing of normal CB CD34+ cells
SDF-1/CXCR4 interactions are crucial for homing and repopulation of normal human stem cells transplanted into immunedeficient NOD/SCID mice.1,2 We recently showed that malignant human AML and pre-B-acute lymphoblastic leukemia (ALL) cell homing to the BM and spleen of NOD/SCID/B2mnull mice is CXCR4 dependent.6,28 In order to assess the effect of elastase inhibition on the in vivo migration and homing of human AML cells to the BM, primary AML MNCs (5 × 106) were injected into sublethally irradiated NOD/SCID/B2mnull mice, either untreated or after 30 minutes of incubation with EI (10 μg/mL). Homing of primary M2 and M4 AML cells from 6 patients into the BM was significantly decreased after treatment with EI compared with the untreated control (Figure 4A-B). However, homing of AML cells from one primary M1 (no. 22) and one M5 (no. 17) patient was not inhibited but rather increased (of note, patient no. 22 was on relapse at time of collection) (Figure 4A). We next examined the effect of elastase inhibition on the homing of normal human enriched CD34+ CB cells. In order to analyze similar primitive stem cell populations, CB CD34+ cells were compared with primary enriched CD34+ AML cells from 2 other AML patients (1.5-3 × 106) and were injected into sublethally irradiated NOD/SCID/B2mnull mice with or without preincubation with EI. Similar to the result observed with MNCs, this pretreatment decreased the homing of AML-enriched human CD34+ cells to the murine BM (Figure 4C). In contrast, homing of normal CB CD34+ cells was increased (Figure 4C). Taken together, these results indicate an opposite effect of elastase inhibition on the in vivo migration of normal and most myeloid leukemic cells including immature CD34+ cells, suggesting a specific role for elastase in migration of most AML cells.
Elastase expression by AML cells affects their SDF-1-induced transwell migration. (A) In vitro transwell migration assay of AML cells either untreated (▪) or treated for 30 minutes with EI (10 μg/mL; □). The results show the percentage of migrated cells toward 125 ng/mL SDF-1 after 4 hours. The percent of migrating cells in the absence of SDF-1 (spontaneous migration) was deduced from the percent of migrating cells in the presence of SDF-1. Statistical significance of the inhibition from all samples taken together was P = .039. (B-C) U937 and primary AML cells were assayed for SDF-1-induced migration after pretreatment with neutralizing antielastase Abs (30 and 70 μg/mL) for 30 minutes (B) or α1-antitrypsin (100 μg/mL, α1-AT) (C). Results represent mean ± SE of 3 experiments with U937 cells and 2 patient samples done in duplicate; *P ≤ .03. (D) Immunocytochemical analysis of membranal elastase and CXCR4 localization in primary PB AML cells either untreated or pretreated with EI in the presence or absence of 200 ng/mL SDF-1. Merged images are shown. Original magnification, × 40. Insert shows a cell that acquired highly polarized morphology in the presence of SDF-1.
Elastase expression by AML cells affects their SDF-1-induced transwell migration. (A) In vitro transwell migration assay of AML cells either untreated (▪) or treated for 30 minutes with EI (10 μg/mL; □). The results show the percentage of migrated cells toward 125 ng/mL SDF-1 after 4 hours. The percent of migrating cells in the absence of SDF-1 (spontaneous migration) was deduced from the percent of migrating cells in the presence of SDF-1. Statistical significance of the inhibition from all samples taken together was P = .039. (B-C) U937 and primary AML cells were assayed for SDF-1-induced migration after pretreatment with neutralizing antielastase Abs (30 and 70 μg/mL) for 30 minutes (B) or α1-antitrypsin (100 μg/mL, α1-AT) (C). Results represent mean ± SE of 3 experiments with U937 cells and 2 patient samples done in duplicate; *P ≤ .03. (D) Immunocytochemical analysis of membranal elastase and CXCR4 localization in primary PB AML cells either untreated or pretreated with EI in the presence or absence of 200 ng/mL SDF-1. Merged images are shown. Original magnification, × 40. Insert shows a cell that acquired highly polarized morphology in the presence of SDF-1.
In vivo elastase inhibition decreases AML-cell egress into the circulation
Our present results demonstrate that elastase is involved in regulation of the motility of human AML cells. Therefore, we hypothesized that elastase may play a role in the abnormal AML-cell egress from the BM into the circulation. Primary human AML cells (20-40 × 106) were injected into sublethally irradiated NOD/SCID mice to establish human AML chimerism, and 2 to 4 weeks later EI (1 mg) was administrated daily for 4 consecutive days. Following EI treatment, AML-cell egress to the PB (calculated as the ratio of the percentage of CD45+ cells in the PB/BM) was significantly decreased by 73% ± 17.5% (P ≤ .001) when compared with untreated mice (Table 1). Similar results were observed with the human HL-60 cell line (Table 1). These results suggest that elastase participates in the regulation of human AML-cell emigration from the BM into the circulation and that elastase inhibition can efficiently prevent AML-cell egress in chimeric NOD/SCID mice.
Effect of elastase inhibition on AML-cell egress in NOD/SCID mice that underwent transplantation
. | CTLs . | . | . | EI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | BM,% . | PB,% . | PB/BM . | BM,% . | PB,% . | PB/BM . | ||||
| No. 8 M4E | 78.5 | 40.6 | 0.7 | 79 | 3.3 | 0.07 | ||||
| No. 20 M4E | 35 | 5 | 0.14 | 50 | 2.5 | 0.05 | ||||
| No. 23 M4 | 79 | 14 | 0.177 | 12.7 | 0.5 | 0.039 | ||||
| No. 24 M4 | 7.4 | 0.18 | 0.024 | 16.9 | 0.25 | 0.014 | ||||
| HL-60 | 3.1* | 13.5 | 4.8 | 4 | 2.4 | 0.6 | ||||
. | CTLs . | . | . | EI . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | BM,% . | PB,% . | PB/BM . | BM,% . | PB,% . | PB/BM . | ||||
| No. 8 M4E | 78.5 | 40.6 | 0.7 | 79 | 3.3 | 0.07 | ||||
| No. 20 M4E | 35 | 5 | 0.14 | 50 | 2.5 | 0.05 | ||||
| No. 23 M4 | 79 | 14 | 0.177 | 12.7 | 0.5 | 0.039 | ||||
| No. 24 M4 | 7.4 | 0.18 | 0.024 | 16.9 | 0.25 | 0.014 | ||||
| HL-60 | 3.1* | 13.5 | 4.8 | 4 | 2.4 | 0.6 | ||||
Value is mean ± 0.85.
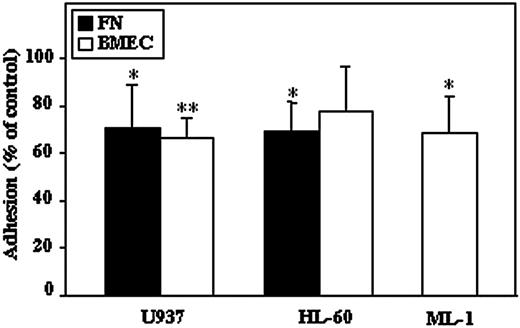
Inhibition of elastase decreases adhesion of AML cells to fibronectin and BMEC
Adhesion of hematopoietic cells to ECM or to other types of cells such as stromal or endothelial cells is a crucial process for cell retention, development, and motility within the BM. We assessed the effect of elastase inhibition on adhesive properties of AML cell lines to fibronectin and BMEC. We found that pretreatment of U937, HL-60, and ML-1 cell lines with EI decreased their SDF-1-induced adhesion on fibronectin as well as on BMEC (Figure 5). These results suggest the involvement of elastase in adhesion of AML cells to endothelial cells and to ECM.
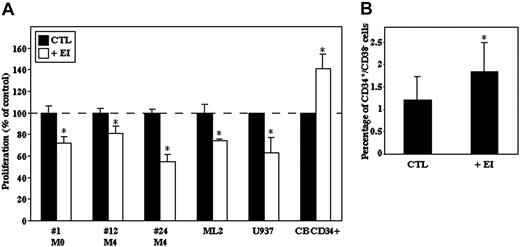
Inhibition of elastase decreases AML-cell proliferation but increases the number of primitive normal CB CD34+/CD38- cells
The effect of EI on the proliferative rates of primary AML MNCs and AML cell lines (ML-2, U937) was evaluated after 3 and 7 days, respectively, in culture. The number of primary cells was reduced after 3 days in culture, and addition of EI significantly decreased the number of viable AML cells (Figure 6A). In contrast to primary AML cells, after 7 days in culture the number of ML-2 and U937 cells was increased; however, their proliferation was also inhibited in the presence of EI (Figure 6A). In contrast, when normal CB CD34+ cells were cultured for 3 to 4 days with EI, elastase inhibition enhanced their proliferation rate (Figure 6A). Moreover, we found that the percentage of primitive CD34+/CD38- progenitor cells was significantly increased after 3 days in culture in the presence of cytokines and EI (Figure 6B), suggesting a role for elastase in differentiation of normal human progenitor cells.
Discussion
The increased motility and egress into the circulation of immature malignant myeloid cells is one of the hallmarks of AML. Recently, others and we began to identify the factors and mechanisms that control the emigration of normal cells from the BM into the circulation. The finding that elastase, among other proteolytic enzymes such as cathepsin G and MMPs, participates in the process of G-CSF-induced mobilization of normal stem cells, progenitors, and maturing cells29,30 led us to hypothesize that the abnormally high levels of elastase in AML patients' plasma17,19 may facilitate the cells' egress into the circulation.
Effect of elastase inhibitor on the homing of AML and normal progenitor cells. Primary human AML MNCs from 8 patients, either untreated or after 30 minutes of incubation with EI (10 μg/mL), were injected into sublethally irradiated NOD/SCID/B2mnull mice. (A) Data show the percentage of human CD45+ cells present in the murine BM compared with control (100%). Statistical significance of inhibition from the 8 samples taken together was P = .05. Experiment with cells from patients no. 16 and no. 21 was done in duplicate, and results show mean ± SE. (B) Representative FACS plot. A mouse that was not injected is shown as control (non-inj.). (C) Comparison of the effect of EI (10 μg/mL) on the homing of CD34+ cells derived from normal CB and primary AML (M4E, no. 8 and M4, no. 16) cells. Data show the percentage of human CD45+ cells present in the murine BM compared with control (100%). Results are average ± SE of 3 independent experiments; *P ≤ .05.
Effect of elastase inhibitor on the homing of AML and normal progenitor cells. Primary human AML MNCs from 8 patients, either untreated or after 30 minutes of incubation with EI (10 μg/mL), were injected into sublethally irradiated NOD/SCID/B2mnull mice. (A) Data show the percentage of human CD45+ cells present in the murine BM compared with control (100%). Statistical significance of inhibition from the 8 samples taken together was P = .05. Experiment with cells from patients no. 16 and no. 21 was done in duplicate, and results show mean ± SE. (B) Representative FACS plot. A mouse that was not injected is shown as control (non-inj.). (C) Comparison of the effect of EI (10 μg/mL) on the homing of CD34+ cells derived from normal CB and primary AML (M4E, no. 8 and M4, no. 16) cells. Data show the percentage of human CD45+ cells present in the murine BM compared with control (100%). Results are average ± SE of 3 independent experiments; *P ≤ .05.
Elastase, along with other azurophil (primary) granule proteins, is synthesized early in myelopoiesis, during the myeloblast-to-promyelocyte transition. In this study, we showed that the amount of elastase in the BM plasma of AML patients was dependent on the maturation stage of the leukemic clone. Although tested in a small number of patients, we observed the highest concentrations of BM elastase in differentiated M3 and M4 AML subtypes and very low levels in undifferentiated M0 AML cells. As previously reported,17 we confirmed that AML patients have higher levels of elastase in the blood when compared with healthy individuals. Moreover, we found a significant positive correlation between the level of blood plasma elastase and the number of blast cells present in the circulation. These observations suggest that AML cells secrete elastase both in the BM and in the blood after their egress. Of interest, AML patients with no dissemination of blasts in the circulation had normal levels of elastase in the PB. In addition, the myelomonocytic leukemia (M4), which usually disseminates more extensively to extramedullary sites in patients as well as in the functional preclinical SCID-leukemia model,3-5 was found to express the highest levels of elastase in the BM.
AML-cell adhesion to fibronectin and BMEC is decreased after elastase inhibition. AML cell lines (U937, HL-60, and ML-1) either untreated or treated for 30 minutes with EI (10 μg/mL) were placed on fibronectin (FN; ▪) or BMEC-coated (□) plates with SDF-1 (100 ng/mL) for 2 hours, and adhesion was measured as described in “Materials and methods.” Results are mean ± SE of 3 to 5 independent experiments; *P ≤ .05; **P ≤ .001.
AML-cell adhesion to fibronectin and BMEC is decreased after elastase inhibition. AML cell lines (U937, HL-60, and ML-1) either untreated or treated for 30 minutes with EI (10 μg/mL) were placed on fibronectin (FN; ▪) or BMEC-coated (□) plates with SDF-1 (100 ng/mL) for 2 hours, and adhesion was measured as described in “Materials and methods.” Results are mean ± SE of 3 to 5 independent experiments; *P ≤ .05; **P ≤ .001.
Of interest, in cyclic neutropenia, a disease in which heterozygous mutations in the leukocyte elastase gene were found and that is characterized by impaired egress of maturing BM hematopoietic cells,13 a patient's serum has approximately half of the level of elastase present in healthy individuals.31
During inflammation, activated neutrophils invade tissues as part of host defense response. This process is facilitated by a shift to functional expression of membrane-bound elastase, which is localized in the migrating leading edge and has catalytic activity.25,32,33 Unstimulated resting neutrophils have only minimal cell-surface elastase expression; however, it is up-regulated upon activation with cytokines such as platelet-activating factor, tumor necrosis factor α (TNF-α), or IL-8.33 We found that PBAML cells constitutively express cell-surface-bound elastase, independently of external stimulus in vitro. Constitutive elastase expression might confer AML cells selective advantage for egress from the BM and for their dissemination pattern. Up-regulation of cell-surface elastase and/or secretion of elastase might therefore be a prerequisite for the egress of most leukemic cells from the BM. The essential role of elastase in AML-cell emigration out from the BM was tested by injecting elastase inhibitor in NOD/SCID mice previously receiving a transplant of AML cells. Egress was severely impaired, supporting the notion that elastase critically regulates human AML-cell emigration from the BM. Cell adhesion to BM endothelium is required for the initial step in transendothelial migration, which takes place during the processes of entering and exiting the BM. In the present study, we found that inhibition of elastase decreased AML-cell adhesion to BMEC. These results suggest a role for elastase in promoting AML-cell adhesion to BM endothelial cells. Studies previously showed that cancer cell adhesion to endothelial cells is also elastase dependent. Elastase enhanced both E-selectin and ICAM-1 expression on endothelial cells, thereby facilitating cancer cell motility and dissemination.34,35 Therefore, high levels of elastase expressed by AML cells may promote their transendothelial migration and egress by increasing their interactions with BM endothelial cells. Evidence suggests that elastase disrupts cell retention by modulating cell-ECM interactions, thereby facilitating cell dissemination. However, our data demonstrate that adhesion of AML cells to fibronectin was impaired after elastase inhibition, implying that elastase can also promote adhesion to fibronectin. Taken together these data challenge the well-known role of elastase in degrading ECM and suggest additional properties.
To evaluate whether elastase also regulates directly the cell motility machinery, we evaluated the effect of elastase inhibition in transwell migration assay in vitro. Since elastase was shown to cleave and inactivate SDF-1 and CXCR4,11 one might expect increased SDF-1-induced migration after EI treatment. Surprisingly, inhibition of elastase by either inhibitors or neutralizing Abs decreased migration of AML cells, both spontaneous and SDF-1 induced. As transwell assays were performed with bare filters, the observed effect is not exerted by degradation of ECM macromolecules but rather a direct effect on the cells. Indeed, we demonstrated that elastase inhibition prevented cell polarization and protrusion formation on AML cells, implying regulation of cytoskeletal rearrangements by elastase in addition to its role in ECM degradation. In vivo homing of most MNCs or enriched CD34+ AML cells was significantly inhibited after pretreatment of cells with EI, whereas homing of normal CB progenitor cells was enhanced. It therefore appears that unlike normal immature cells, elastase activity is beneficial for the motility of most leukemic AML cells and that repression of elastase activity can partially prevent their migration. Similar to in vitro migration, we observed the strongest effect of elastase inhibition on homing of AML cells from M4 subtype, which was shown to express high levels of elastase.
Of interest, we found that addition of exogenous SDF-1 up-regulates the expression of membranal elastase on AML cells in vitro. Moreover, our observation that neutralization of the receptor CXCR4 down-regulates cell-surface elastase suggests that constitutive secretion of SDF-1 by AML cells is an important regulator of elastase expression in AML. Indeed, SDF-1 has been found to have numerous biologic roles. In addition to controlling cell motility and mediating retention of normal and leukemic cells within the BM, SDF-1 can regulate cell proliferation and also acts as a survival factor for human AML progenitor cells.6 Our present results suggest that effects of SDF-1 on AML cells could be mediated through activation of cell-surface elastase. We recently showed that inhibition of CXCR4/SDF-1 axis in AML cells in culture led to reduced proliferation/survival.6 In light of the data presented here, it may be possible that SDF-1 promotes AML-cell proliferation also by regulating the expression of elastase, which we found to be involved in regulation of AML-cell proliferation.
Inhibition of elastase reduces AML-cell proliferation while maintaining normal undifferentiated CD34+cells. (A) AML cell lines (U937 and ML-2), primary AML cells, and CB CD34+ cells were cultured for 3 to 7 days with or without EI (10 μg/mL), and the number of viable cells was determined using trypan blue exclusion. Results are shown as percentage of viable cells compared with CTL-untreated cells plotted as 100%; *P ≤ .005. (B) CB CD34+ cells were cultured for 3 days with or without EI (10 μg/mL). The percentage of CD34+/CD38- cells was determined by flow cytometry. Results are mean ± SE of 4 independent experiments; *P ≤ .05.
Inhibition of elastase reduces AML-cell proliferation while maintaining normal undifferentiated CD34+cells. (A) AML cell lines (U937 and ML-2), primary AML cells, and CB CD34+ cells were cultured for 3 to 7 days with or without EI (10 μg/mL), and the number of viable cells was determined using trypan blue exclusion. Results are shown as percentage of viable cells compared with CTL-untreated cells plotted as 100%; *P ≤ .005. (B) CB CD34+ cells were cultured for 3 days with or without EI (10 μg/mL). The percentage of CD34+/CD38- cells was determined by flow cytometry. Results are mean ± SE of 4 independent experiments; *P ≤ .05.
The role of the myeloid serine protease family in cell growth regulation was first revealed when membrane extracts from AML cells (called common antigen of myelogenous leukemia, CAMAL) that contains elastase, cathepsin G, azurocidin, and protease 3 were shown to inhibit normal hematopoiesis of humans and mice.36,37 Moreover, El-Ouriaghli et al20 recently demonstrated that elastase could negatively affect normal hematopoietic regulation by G-CSF inactivation and provide a selective advantage to the CML leukemic clone, which survives and proliferates without exogenous growth factors via autocrine cytokine secretion.38 We addressed the involvement of elastase in the regulation of AML-cell proliferation and normal CD34+ progenitor cells and found that elastase inhibition decreased the proliferation rates of AML cells, demonstrating that elastase directly promotes AML-cell growth. On the contrary, elastase inhibition not only increased the proliferation of immature normal CD34+ cells but also maintained increased levels of undifferentiated primitive CD34+/CD38- stem cells. However, since it was suggested that the CD34+/CD38- cell phenotype does not strictly correlate with SRC function, and eventual reduction of membrane CD38 may be interpreted as CD38-cell expansion,39,40 one must be careful in interpreting these results. Future studies that include functional assays are required to elucidate the role of elastase in the differentiation of normal stem cells.
In conclusion, our data indicate that elastase participates in the migration, development, and egress into the circulation of human AML cells in most patient samples studied (combining in vitro assay and the functional, preclinical NOD/SCID mouse model) by regulating their motility machinery and cell survival. A better understanding of the role of elastase in AML-cell biology will possibly lead to new therapeutic approaches for AML based on specific inhibitory molecules of elastase.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-12-4969.
Supported in part by grants from the Gabriella Rich Center for Transplantation Biology, Israel Science Foundation (ISF) grant 796/04, and the Ares-Serono group.
S.T. and I.P. contributed equally to the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr O. Kollet for critically reviewing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal