Abstract
Virus-specific CD8+ T cells emerge after infection with herpesviruses and maintain latency to these persistent pathogens. It has been demonstrated that murine memory CD8+ T-cell precursors specific for acute lymphocytic choriomeningitis virus express interleukin-7 receptor α (IL-7Rα), and IL-7 is involved in maintaining memory populations after the clearance of antigen. To investigate whether human CD8+ T cells reactive toward persistent viruses are maintained similarly, we analyzed IL-7Rα expression and function on these virus-specific cells. During primary infection, all cytomegalovirus (CMV)-specific CD8+ T cells and most Epstein-Barr virus (EBV)-specific CD8+ T cells lacked IL-7Rα expression. Only some virus-specific T cells expressed IL-7Rα late after viral replication became undetectable. CD8+ T cells specific for cleared viruses, influenza (FLU), and respiratory syncytial virus (RSV) all expressed IL-7Rα. Remarkably, the percentage of IL-7Rα- CMV-specific T cells correlated with the height of viral replication in the acute phase. Virus-specific IL-7Rα+ cells proliferated vigorously in response to IL-7, IL-15, or peptide, whereas IL-7Rα- cells required both peptide and helper-cell activation or IL-2 or IL-15 for optimal expansion. Our data suggest that although IL-7 is essential for the maintenance of memory cells in the absence of antigen, CD8+ T cells specific for latent viruses need T-cell receptor activation plus helper factors to persist. (Blood. 2005;106:2091-2098)
Introduction
Antiviral CD8+ T-cell responses can be divided into 3 distinct phases. In the first phase, CD8+ T cells clonally expand and differentiate into effector T cells that eliminate virus-producing cells. During the contraction phase that follows, most CD8+ T cells die by apoptosis. Finally, in the third phase, the establishment of a CD8+ T-cell memory pool takes place (for a review, see Kaech et al1 ). The generation of a stable memory T-cell pool is a central feature of the adaptive immune system. On a second encounter with a virus, the immune system will be able to respond faster and more efficiently, thereby limiting cytopathic viral effects. This is achieved because the frequency of antigen-specific cells is greatly enhanced after the primary antigenic challenge, leading to higher numbers of responding T cells.2 Moreover, on a per-cell basis, memory T cells are better equipped than naive T cells to respond to antigenic challenge because they can respond rapidly by producing in larger quantities important mediators such as interferon γ (IFNγ); regulated on activation, normal T-cell expressed and secreted (RANTES); and cytotoxic molecules perforin and granzyme.3-6 Several studies have suggested that the expansion and differentiation program is imprinted shortly after antigenic stimulation.7-9 Moreover, CD4+ T-cell help, either during priming or during the memory phase, is required for CD8+ T cells to be able to mount a proper secondary response.10-13
The mechanism behind the development of long-lived memory cells is incompletely understood but depends on the survival of a few antigen-specific cells in the contraction phase. Two recent studies in mice infected with lymphocytic choriomeningitis virus (LCMV) or Listeria monocytogenes showed that during acute infection only a small population (5%-15%) of the effector cells expressed interleukin-7 receptor α (IL-7Rα), whereas in the memory phase all specific CD8+ T cells were IL-7Rα+.14,15 The IL-7Rα+ effector cells expressed higher levels of Bcl-2 than their IL-7Rα- counterparts. Consistent with their antiapoptotic profile, IL-7Rα+ cells had a superior recall response and showed enhanced proliferation in response to homeostatic signals compared with IL-7Rα- cells. This indicated that IL-7Rα+ effector T cells are the cells that survive and develop into long-lived memory CD8+ T cells, which would make IL-7Rα a useful marker for cells destined to become memory cells. In addition, Madakamutil et al16 have shown that the homotypic form of CD8, CD8αα, is selectively expressed by CD8+ memory precursors and is required for CD8+ memory T-cell generation and survival. It is noteworthy that one of the consequences of CD8αα expression in memory precursors is the up-regulation of IL-7Rα, which links the 2 findings together. The long-term maintenance of memory CD8+ T cells at relatively constant numbers in mice has been described as independent of antigen17 and of major histocompatibility complex (MHC) class I molecules.18 The cytokines IL-7 and IL-15 are responsible for the homeostatic proliferation of memory CD8+ T cells.19-22 It may be that IL-15 and IL-7 act cooperatively in maintaining the CD8+ T-cell memory pool such that IL-15 regulates T-cell division and IL-7 mediates T-cell survival.23
Human viruses belonging to the herpesvirus family are among the viruses that are not completely cleared by the immune system after primary infection but that persist as latent pathogens. In recent years, CD8+ T cells specific for these viruses have been extensively characterized using HLA-peptide tetramer technology. In healthy people, these T cells are resting cells that may vary in function, depending on their specificity. For example, Epstein-Barr virus (EBV)-specific T cells are mostly noncytotoxic, whereas many cytomegalovirus (CMV)-specific T cells display a constitutive cytolytic function.24,25 Irrespective of their functional properties, T cells contribute to the maintenance of viral latency because depletion or inhibition of T cells leads to viral reactivation.26,27 It is unknown, however, how CD8+ T cells specific for persistent viruses are maintained in stable pools for years. Therefore, we sought to determine whether human CMV- and EBV-specific T cells could be maintained by the homeostatic cytokines IL-7 and IL-15 in a manner similar to that of murine memory cells after acute infection.
Materials and methods
Subjects
Healthy volunteers and recipients of kidney transplants were included in this study. Recipients were treated with basic immunosuppression therapy consisting of prednisolone (10 mg daily), mycophenolate mofetil (1000 mg twice daily), and cyclosporine (at dosages guided by trough levels aimed at 100 ng/mL). In addition to recipients who were not studied until more than 1 year after transplantation, we longitudinally studied 6 recipients with primary CMV infection, 2 with CMV reactivation, and one with primary EBV infection. All subjects gave written informed consent, and the local medical ethics committee approved the study.
PBMCs
Heparinized peripheral blood samples were collected, and peripheral blood mononuclear cells (PBMCs) were isolated using standard density-gradient centrifugation techniques. Subsequently these cells were cryopreserved until the day of analysis.
CMV-PCR, EBV-PCR, anti-CMV IgG, and anti-EBV IgG
Quantitative polymerase chain reaction (PCR) for CMV was performed in EDTA (ethylenediaminetetraacetic acid) whole blood samples, as described.28 To determine CMV serostatus, anti-CMV immunoglobulin G (IgG) was measured in serum using the AxSYM microparticle enzyme immunoassay (Abbott Laboratories, Abbott Park, IL) according to the manufacturer's instructions. Measurements were calibrated relative to a standard serum. Quantitative PCR for EBV was performed in EDTA plasma. EBV serostatus was investigated by determining IgG specific for Epstein-Barr viral capsid antigen (EB-VCA) and Epstein-Barr nuclear antigen (EBNA) by enzyme-linked immunosorbent assay (ELISA; Biotest, Dreieich, Germany).
Tetrameric complexes
The following HLA-peptide tetrameric complexes were kindly provided by Kiki Tesselaar and Debbie van Baarle (both from Sanquin, Amsterdam, The Netherlands): HLA-A2 tetramer loaded with the CMV pp65-derived NLVPTMVATV peptide, HLA-B7 tetramer loaded with the CMV pp65-derived TPRVTGGGAM peptide, HLA-A2 tetramer loaded with EBV BMLF1-derived GLCTLVAML peptide, HLA-B7 tetramer loaded with EBV EBNA3A-derived RPPIFIRRL peptide, HLA-B8 tetramer loaded with EBV EBNA3A-derived FLRGRAYGL peptide, HLA-B8 tetramer loaded with EBV BZLF1-derived RAKFKQLL peptide, HLA-A2 tetramer loaded with influenza (FLU) M1-derived GILGFVFTL peptide, HLA-A1 tetramer loaded with respiratory syncytial virus (RSV) M-derived YLE-KESIYY peptide, and HLA-B7 tetramer loaded with RSV NL-derived peptide NPKASLLSL. HLA-A1 tetramer loaded with FLU NP-derived CTELKLSDY peptide was obtained from Proimmune (Oxford, United Kingdom). All used tetramers were allophycocyanin conjugated. Hereafter the different tetramers will be named after the virus, the HLA type, and the first 3 amino acids of the peptide sequence (eg, CMV A2 NLV tetramer).
Quantification of IL-7 in serum
Serum was separated from peripheral blood when PBMCs were obtained and stored at -20°C until analysis. IL-7 levels were measured using a commercially available human IL-7 ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Immunofluorescence staining and flow cytometry
PBMCs were washed in phosphate-buffered saline (PBS) containing 0.01% (wt/vol) NaN3 and 0.5% (wt/vol) bovine serum albumin. Five hundred thousand PBMCs were incubated with an appropriate concentration of tetrameric complexes in a small volume for 30 minutes at 4°C, protected from light. Fluorescence-labeled monoclonal antibodies (mAbs) were then added and incubated for 30 minutes at 4°C, protected from light, at concentrations according to the manufacturer's instructions. For surface marker expression analysis, the following antibodies were used in different combinations: CD127 (IL-7Rα)-phycoerythrin (PE) (Immunotech, Marseille, France), CD27-fluorescein isothiocyanate (FITC) (homemade clone 3A12), CD38-PE, CD45RA-FITC, CD57-FITC, CD62L-FITC, anti-HLA-DR-FITC (all BD Biosciences, San Jose, CA), and CD28-FITC (Sanquin, Amsterdam, The Netherlands). Cells were washed and analyzed using a FACScalibur flow cytometer and CellQuest software (BD Biosciences).
Intracellular perforin and Bcl-2 staining
For intracellular staining, cells were fixed with 50 μL buffered formaldehyde acetone solution and subsequently permeabilized by washing with 0.1% saponin in 50 mM d-glucose. Cells were then incubated with anti-perforin-FITC (BD Biosciences) or anti-Bcl-2-FITC (DAKO Cytomation, Carpinteria, CA) antibodies according to the manufacturers' instructions, followed by flow cytometric analysis.
CFSE labeling
PBMCs were resuspended in PBS at a final concentration of 5 to 10 × 106 cells/mL. PBMCs were labeled with 0.5 μM (final concentration) of 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in PBS for 8 to 10 minutes at 37°C under constant agitation. Cells were washed and subsequently resuspended in Iscove modified Dulbecco medium (IMDM) supplemented with 10% human pool serum (BioWhittaker, Verviers, Belgium), antibiotics, and 3.57 × 10-4% (vol/vol) β-mercaptoethanol (Merck, West Point, PA) (cIMDM).
Proliferation assays
Either total PBMCs or sorted cells from healthy donors were labeled with CFSE and cultured in cIMDM for 3 to 6 days. HLA-A2 CMV NLVPTM-VATV peptide, HLA-B7 CMV TPRVTGGGAM peptide, and HLA-A2 FLU GILGFVFTL peptide were obtained from IHB-LUMC peptide synthesis library facility (Leiden, The Netherlands), dissolved in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany), and used in a concentration of 1.25 ng/mL for stimulations. CMV antigen and FLU antigen (Microbix Biosystems, Toronto, Canada) were used in a final concentration of 10 μL/mL for stimulation of CD4+ T cells. Cells were stimulated with the specific CMV or FLU peptide alone or in combination with CMV antigen or FLU antigen, respectively, or with either IL-2 (50 U/mL; Biotest), IL-7 (10 ng/mL; Strathmann Biotec, Hamburg, Germany), or IL-15 (10 ng/mL; R&D Systems). Cells were sorted into a CD8+ CMV tetramer+ IL-7Rα+ population and a CD8+ CMV tetramer+ IL-7Rα+ population using a FACSAria (BD Biosciences). After CFSE labeling, these cells were cocultured with irradiated autologous PBMCs. Before irradiation, these autologous cells were cultured for 5 hours with the specific CMV peptide, CMV antigen, or both. Irradiated autologous cells were not added when cells were stimulated with IL-7 or IL-15. The precursor frequency (percentage of cells in the initial population that underwent one or more divisions after culture) was calculated as follows: [(Σn≥1(Pn/2n))/(Σn≥0(Pn/2n))], where n is the division number that cells have gone through and Pn is the number of cells in division n.29 The mean number of divisions of the divided cells was calculated as follows: [(Σn≥1(nPn/2n))/(Σn≥1(Pn/2n))].
Statistical analysis
The 2-tailed Mann-Whitney U test was used for analysis of differences between groups. For correlations, the Spearman nonparametric correlation test was used. P less than .05 was considered statistically significant.
Results
During primary CMV infection all CMV-specific and most circulating CD8+ T cells do not express IL-7Rα
Insight into primary virus infections in humans is difficult to attain. In the past we have been able to gather valuable information by carefully monitoring immune responses in CMV-seronegative recipients of kidney transplants who received organs from seropositive donors.30,31 We first wanted to assess whether, after primary infection with CMV, the same IL-7Rα+ memory cell precursors described in mice could be found. During primary CMV infection, none of the circulating CMV-specific CD8+ T cells, visualized by tetramer staining, expressed IL-7Rα (Figure 1A). Several weeks after the viral load became undetectable, a fraction of the CMV-specific cells expressed IL-7Rα. CD27 followed a completely different temporal expression pattern than did IL-7Rα, with considerable numbers of CD27- CMV-specific cells appearing only at later time points (Figure 1A). Data obtained from 6 primary CMV infections are summarized in Table 1.
Summary of data obtained from donors with primary CMV infections
. | Donor 1 . | . | Donor 2 . | . | Donor 3 . | . | Donor 4 . | . | Donor 5 . | . | Donor 6 . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | ||||||
| During positive PCR, % | 1.8 | 0.4 | 4.9 | 0.4 | 0.7 | 1.1 | 0.6 | 2.2 | 6.4 | 1.5 | 1.0 | 1.1 | ||||||
| 1 y after transplantation, % | 0.3 | 7.8 | 2.3 | 3.9 | 0.5 | 2.2 | 0.5 | 4.9 | 4.0 | 7.5 | 0.5 | 3.9 | ||||||
| More than 2 y after transplantation, % | 0.9 | 17.2 | NA | NA | NA | NA | NA | NA | 2.9 | 10.1 | 2.0 | 4.9 | ||||||
. | Donor 1 . | . | Donor 2 . | . | Donor 3 . | . | Donor 4 . | . | Donor 5 . | . | Donor 6 . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | Tetr+ . | IL-7Rα+ . | ||||||
| During positive PCR, % | 1.8 | 0.4 | 4.9 | 0.4 | 0.7 | 1.1 | 0.6 | 2.2 | 6.4 | 1.5 | 1.0 | 1.1 | ||||||
| 1 y after transplantation, % | 0.3 | 7.8 | 2.3 | 3.9 | 0.5 | 2.2 | 0.5 | 4.9 | 4.0 | 7.5 | 0.5 | 3.9 | ||||||
| More than 2 y after transplantation, % | 0.9 | 17.2 | NA | NA | NA | NA | NA | NA | 2.9 | 10.1 | 2.0 | 4.9 | ||||||
Tetr+ indicates percentages of CMV-specific cells within CD8+ T cells; IL-7Rα+, percentages of CMV-specific cells expressing IL-7Rα; positive PCR, time point during positive CMV PCR; NA, not available.
Donor 1 is the donor shown in Figure 1A.
Primary infection with EBV, another persistent herpesvirus, was studied by means of 2 tetramers, one specific for the lytic peptide RAKFKQLL (RAK) and the other specific for the latent peptide FLRGRAYGL (FLR). The expression of IL-7Rα by RAK-specific cells started off at 8% at week 10 (data not shown) and then steadily increased to 69% in the latency stage. The percentage of IL-7Rα+ FLR-specific cells followed the same kinetics but at a higher level, increasing from 37% to 81% late after primary infection (Figure 1B). As for CMV-specific cells, a subpopulation of the EBV-specific cells still did not express IL-7Rα. In contrast to CMV-specific T cells, the phenotype of the EBV-specific cells remained mostly CD27+ for RAK- and FLR-specific cells.
Some patients who are seropositive for CMV before transplantation experience a reactivation of CMV after transplantation because of the start of immunosuppression therapy.32 During CMV reactivation, the percentage of IL-7Rα+ CMV-specific cells diminished from 22% to 10% at week 7 (Figure 1C) and was slightly lower (7%) at week 13, even though the viral load was undetectable at that moment. The percentage of IL-7Rα+ CMV-specific cells partially recovered to 12% (Figure 1C, upper panels) only late after the viral load became undetectable. Changes in IL-7Rα expression coincided with the activation of CMV-specific cells, as shown by HLA-DR and CD38 expression (Figure 1C, lower panels).
Expression of IL-7Rα on virus-specific cells is low during primary infection. (A) (top row) CMV-specific CD8+ T cells during primary CMV infection. (bottom row) Phenotypes of total CD8+ T cells (red) and CMV-specific T cells (black). (B) Same as panel A but showing primary EBV infection measured by CD8+ T cells specific for the lytic epitope RAK (first 2 rows) and the latent epitope FLR (last 2 rows). (C) Changes in phenotype of CD8+ T cells and CMV-specific CD8+ T cells (measured with CMV A2 tetramer) during CMV reactivation. wk indicates the number of weeks after transplantation; + and - indicate the relative heights of the viral load as measured by PCR. Percentages are the percentages of virus-specific cells within CD8+ T cells. Dot plots show the phenotypes of CD8+ T cells (red) and of virus-specific CD8+ T cells (black). Numbers indicate percentages within virus-specific cells in the corresponding quadrants. For clarity, only 10% of the dots are shown from dot plots showing RAK-specific cells during primary EBV infection; all other plots show 100% of measured events. Representative results of flow cytometric analysis are shown for 1 of 6 patients with primary CMV infection and 1 of 2 patients with CMV reactivation.
Expression of IL-7Rα on virus-specific cells is low during primary infection. (A) (top row) CMV-specific CD8+ T cells during primary CMV infection. (bottom row) Phenotypes of total CD8+ T cells (red) and CMV-specific T cells (black). (B) Same as panel A but showing primary EBV infection measured by CD8+ T cells specific for the lytic epitope RAK (first 2 rows) and the latent epitope FLR (last 2 rows). (C) Changes in phenotype of CD8+ T cells and CMV-specific CD8+ T cells (measured with CMV A2 tetramer) during CMV reactivation. wk indicates the number of weeks after transplantation; + and - indicate the relative heights of the viral load as measured by PCR. Percentages are the percentages of virus-specific cells within CD8+ T cells. Dot plots show the phenotypes of CD8+ T cells (red) and of virus-specific CD8+ T cells (black). Numbers indicate percentages within virus-specific cells in the corresponding quadrants. For clarity, only 10% of the dots are shown from dot plots showing RAK-specific cells during primary EBV infection; all other plots show 100% of measured events. Representative results of flow cytometric analysis are shown for 1 of 6 patients with primary CMV infection and 1 of 2 patients with CMV reactivation.
Many T cells specific for persistent viruses do not express IL-7Rα
The data described here were obtained using cells from recipients of kidney transplants who received standard immunosuppression therapy that could possibly have influenced the expression of IL-7Rα. Therefore, we extended our findings and analyzed a group of healthy people likely infected with CMV or EBV for years. When the percentage of virus-specific cells expressing IL-7Rα was plotted against the different T-cell specificities, an apparent relationship was observed between the type of virus and the percentage of IL-7Rα+ virus-specific cells (Figure 2A). CD8+ T cells specific for CMV showed a variation in levels of IL-7Rα expression between 12% and 74%, with a median of 46% for CMV A2 NLV-specific cells and 39% for CMV B7 TPR-specific cells (Figure 2B). These numbers were higher for cells directed against the different EBV peptides measured, of which 30% to 79% were IL-7Rα+ (median, 60%). Almost all CD8+ T cells specific for FLU or RSV were IL-7Rα+, making the level of expression of this cytokine receptor a distinguishing marker between T-cell populations specific for persistent viruses and viruses that are cleared from the host (Figure 2B).
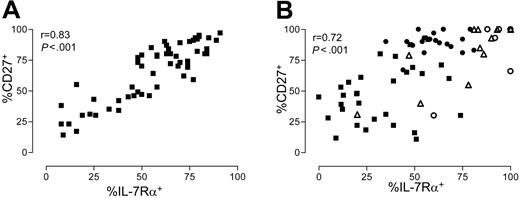
Expression of IL-7Rα correlates with the expression of CD27 on CD8+ T cells
From the dot plots shown in Figure 2A, it seemed that many CD8+ T cells that lacked IL-7Rα also lacked CD27, and vice versa. CD27 is expressed on all naive cells, absent on effector-type cells, and differentially expressed on memory-type cells.33 Although the regulation during primary immune responses is different (Figure 1), a strong correlation was found between the percentage of CD27+ and IL-7Rα+ cells in the total CD8+ T-cell population (Figure 3A).
The correlation between IL-7Rα and CD27 was also observed in virus-specific cells, as shown in Figure 3B, for all different specificities measured. CMV-specific cells were mostly IL-7Rα- and CD27-, in contrast to EBV-specific cells, which were all CD27+ and had a variable expression of IL-7Rα. FLU- and RSV-specific cells expressed both IL-7Rα and CD27 (Figure 3B). The relation between these 2 surface markers was less strict within virus-specific cells than within the total CD8+ T-cell pool, possibly reflecting clonal-specific differences in activation and differentiation status that remained unobserved in the total CD8+ T-cell population.
Consistent with the difference in CD27 expression, phenotypic and functional properties of IL-7Rα+ and IL-7Rα- cells were clearly distinct. As shown in Figure 4A, IL-7Rα- cells were enriched in the CD28- and the CD62L- populations, often expressed CD45RA, and, in contrast to IL-7Rα+ cells, partially expressed CD57. Furthermore, IL-7Rα- cells expressed higher levels of the cytotoxic molecule perforin, both in the total CD8+ T-cell population and within CMV-specific cells (Figure 4B). Not only was the mean fluorescence intensity of perforin higher, but perforinhi cells were also only present in the IL-7Rα- cells. Expression of the antiapoptotic molecule Bcl-2 was higher in IL-7Rα+ cells (Figure 4B).
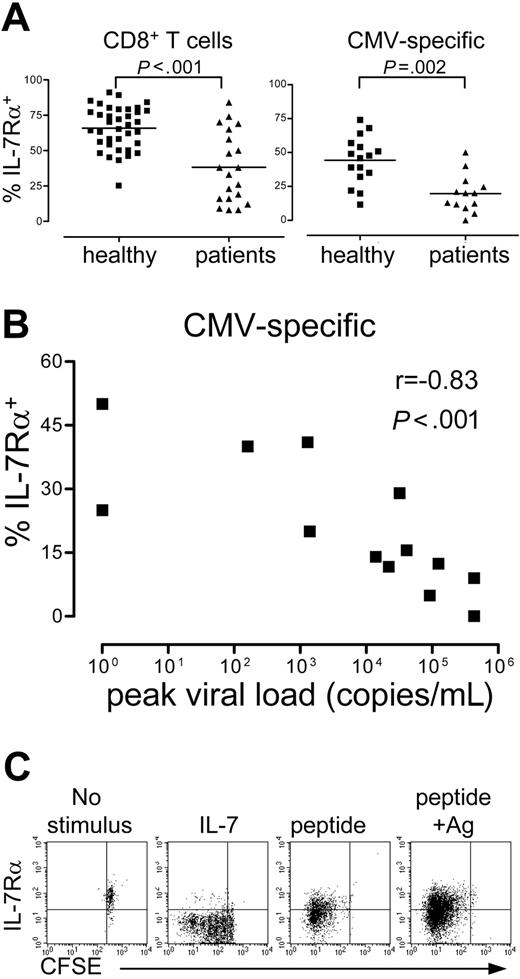
Virus-specific T cells expressing IL-7Rα are related to viral load during the acute phase
It has been shown that recipients of kidney transplants have higher amounts of effector-type (CD27-) cells than healthy persons.25 Regarding the expression of IL-7Rα in total CD8+ and CMV-specific CD8+ T cells, the frequency of IL-7Rα+ cells is significantly lower in patients (Figure 5A). Interestingly, within the patient group, a strong inverse correlation was seen between the percentage of IL-7Rα+ CMV-specific cells at late time points and the peak viral load, defined as the highest viral load measured during the posttransplantation period (Figure 5B). Patients with high viral loads (more than 10 000 copies/mL) experienced either primary CMV infection or strong CMV reactivation, whereas patients with low viral loads were all CMV seropositive before transplantation and had only mild or no detectable reactivation.
Expression of IL-7Rα differs between T cells specific for various viruses. (A) Dot plots of 2 representative healthy persons in whom T cells with different specificities could be visualized. CD8+ T cells (red); virus-specific CD8+ T cells (black). Numbers indicate percentages within virus-specific cells in the corresponding quadrants. (B) Percentages of IL-7Rα+ cells within virus-specific cells measured in healthy persons with different tetramers. Horizontal lines indicate median values.
Expression of IL-7Rα differs between T cells specific for various viruses. (A) Dot plots of 2 representative healthy persons in whom T cells with different specificities could be visualized. CD8+ T cells (red); virus-specific CD8+ T cells (black). Numbers indicate percentages within virus-specific cells in the corresponding quadrants. (B) Percentages of IL-7Rα+ cells within virus-specific cells measured in healthy persons with different tetramers. Horizontal lines indicate median values.
A possible explanation for the variation in the percentage of IL-7Rα+ cells could be differences in IL-7 levels in the circulation because IL-7Rα is down-regulated after stimulation with IL-7.34 Indeed, with the addition of IL-7 in experiments using isolated IL-7Rα+ CMV-specific cells, all IL-7Rα+ cells—including nondividing cells—lost IL-7Rα expression. The receptor was also down-regulated in the cells that divided on antigen-specific stimulation (Figure 5C).
However, when serum IL-7 levels of a group of patients with high percentages of IL-7Rα+ CD8+ T cells (n = 5; median, 69%; range, 58%-84%) were compared with serum IL-7 levels of patients with low frequencies of IL-7Rα+ CD8+ T cells (n = 5; median, 9%; range, 8%-16%), no significant differences were found. Excluding a role for IL-7 in the variation of IL-7Rα expression, the median serum IL-7 level in each group was 16 pg/mL (ranges, 6-27 pg/mL for the group with high percentages and 7-24 pg/mL for the group with low percentages).
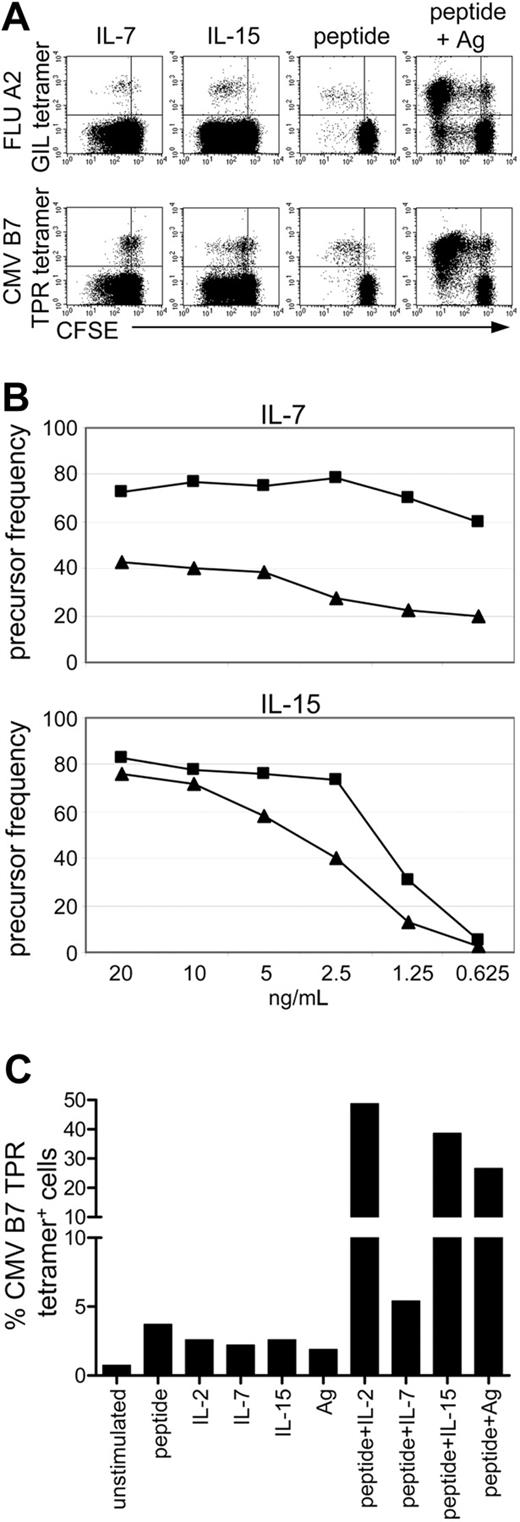
Memory cells specific for cleared viruses proliferate better than memory cells specific for persistent viruses
IL-7 was of major importance for the survival of memory cells after acute infection, but memory cells specific for persistent viruses only expressed IL-7Rα at low frequencies. Therefore, we tested the responsiveness of memory cells to cytokines and antigen-specific stimulation in humans by comparing the proliferation of FLU-specific (acute) and CMV-specific (persistent) CD8+ T cells derived from the same healthy donor. Figure 6A shows that although both populations proliferated, FLU-specific cells showed enhanced proliferation compared with CMV-specific cells when stimulated with IL-7. This is consistent with the expression of IL-7Rα on all FLU-specific cells, whereas only 27% of the CMV-specific cells expressed IL-7Rα in this donor (Figure 6A). In addition, FLU-specific cells proliferated better on stimulation with the other homeostatic cytokine, IL-15. The differences in the proliferative capacity of FLU- and CMV-specific cells to IL-7 and IL-15 were even more apparent when dose titrations of the cytokines were performed (Figure 6B). The precursor frequency of FLU-specific cells was also higher than that of CMV-specific cells, and maximum proliferation of FLU-specific cells was achieved at lower concentrations. Cell culture in the presence of the specific (FLU or CMV) peptide resulted in similar proliferation of FLU- and CMV-specific cells; this did not change when lower concentrations of the peptides were used. Combined stimulation with peptide and either FLU or CMV antigen, which stimulates CD4+ T cells, resulted in increased proliferation of FLU- and CMV-specific cells (Figure 6A). To study the relative expansion of virus-specific cells, we cultured PBMCs from healthy donors with different stimuli, as indicated in Figure 6C. Although all conditions induced proliferation (Figure 6A and data not shown), a considerable increase in the percentage of CMV-tetramer+ cells was only seen when cells were stimulated with the specific CMV peptide in combination with IL-2, IL-15, or antigen (Figure 6C). Stimulation with peptide plus IL-7 caused little more expansion than with peptide alone (5.4% vs 3.7%), consistent with the low percentage of IL-7Rα+ CMV-specific cells (27% in this donor). Indeed, RSV- and FLU-specific cells, all of which express IL-7Rα, do show enhanced proliferation when peptide is combined with IL-7 (data not shown and de Bree et al35 ).
Expression of IL-7Rα correlates with expression of CD27. (A) Relation between IL-7Rα expression and CD27 in the total CD8+ T-cell pool (n = 57, healthy persons and patients). (B) Relation between IL-7Rα expression and CD27 within virus-specific cells. (▪) CMV-specific cells. (•) EBV-specific cells. (▵) FLU-specific cells. (○) RSV-specific cells. For each virus, measurements with the different available tetramers are shown together. Measurements are obtained from cells of 40 healthy donors and patients, all staining with one or more tetramers.
Expression of IL-7Rα correlates with expression of CD27. (A) Relation between IL-7Rα expression and CD27 in the total CD8+ T-cell pool (n = 57, healthy persons and patients). (B) Relation between IL-7Rα expression and CD27 within virus-specific cells. (▪) CMV-specific cells. (•) EBV-specific cells. (▵) FLU-specific cells. (○) RSV-specific cells. For each virus, measurements with the different available tetramers are shown together. Measurements are obtained from cells of 40 healthy donors and patients, all staining with one or more tetramers.
Phenotypic differences between IL-7Rα+and IL-7Rα-cells. (A) Dot plots gated on total CD8+ T cells in which IL-7Rα is plotted against known markers for T-cell subset differentiation, CD28, CD62L, CD57, and CD45RA. Numbers indicate percentages within CD8+ T cells in the corresponding quadrants. (B) Overlay histograms of IL-7Rα- cells (shaded histogram) and IL-7Rα+ cells (black line) within total CD8+ T cells (left) or CMV-specific CD8+ T cells (right). Numbers indicate mean fluorescence intensity of perforin (top panels) and Bcl-2 (bottom panels). Representative results of flow cytometric analysis are shown from 4 independent measurements.
Phenotypic differences between IL-7Rα+and IL-7Rα-cells. (A) Dot plots gated on total CD8+ T cells in which IL-7Rα is plotted against known markers for T-cell subset differentiation, CD28, CD62L, CD57, and CD45RA. Numbers indicate percentages within CD8+ T cells in the corresponding quadrants. (B) Overlay histograms of IL-7Rα- cells (shaded histogram) and IL-7Rα+ cells (black line) within total CD8+ T cells (left) or CMV-specific CD8+ T cells (right). Numbers indicate mean fluorescence intensity of perforin (top panels) and Bcl-2 (bottom panels). Representative results of flow cytometric analysis are shown from 4 independent measurements.
Recipients of kidney transplants have lower frequencies of IL-7Rα+cells. (A) Two graphs in which the percentages of IL-7Rα+ cells are plotted within total CD8+ T cells (left graph) or within CMV-specific CD8+ T cells (right graph) in healthy persons and in recipients of kidney transplants. Horizontal lines indicate the median values. (B) Relation between IL-7Rα expression on CMV-specific CD8+ T cells in recipients of kidney transplants (measured more than 1 year after transplantation) and the peak viral load measured since transplantation in copies per milliliter whole blood. (C) Dot plots of CFSE compared with IL-7Rα expression from isolated IL-7Rα+ CMV-specific CD8+ T cells, cultured for 6 days.
Recipients of kidney transplants have lower frequencies of IL-7Rα+cells. (A) Two graphs in which the percentages of IL-7Rα+ cells are plotted within total CD8+ T cells (left graph) or within CMV-specific CD8+ T cells (right graph) in healthy persons and in recipients of kidney transplants. Horizontal lines indicate the median values. (B) Relation between IL-7Rα expression on CMV-specific CD8+ T cells in recipients of kidney transplants (measured more than 1 year after transplantation) and the peak viral load measured since transplantation in copies per milliliter whole blood. (C) Dot plots of CFSE compared with IL-7Rα expression from isolated IL-7Rα+ CMV-specific CD8+ T cells, cultured for 6 days.
Proliferation and survival differ between IL-7Rα+ and IL-7Rα CMV-specific T cells
The last question we addressed was whether the difference in proliferation between FLU- and CMV-specific cells could be attributed to the type of memory cells (specific for acute or persistent viruses) or whether it reflects a difference in IL-7Rα expression. To examine this, CMV-specific CD8+ T cells from healthy donors were sorted into IL-7Rα+ and IL-7Rα- T cells. IL-7 induced the proliferation of IL-7Rα+ CMV-specific cells but not of IL-7Rα- CMV-specific cells on day 6 (Figure 7A). Stimulation of the sorted cell populations with IL-15 resulted in higher numbers of dividing cells in the IL-7Rα+ population with a more than 2-fold elevated precursor frequency on day 3. In addition, stimulation with the specific CMV peptide, alone or in combination with CMV antigen, led to more proliferating cells in the population expressing IL-7Rα. Again, the difference was larger on day 3 than on day 6, suggesting that the IL-7Rα- cells caught up with the IL-7Rα+ cells, though a disparity remained (Figure 7A). The dividing cells in the IL-7Rα+ population also underwent more cell divisions than IL-7Rα cells. Although we found a good correlation between the expression of IL-7Rα and that of CD27 (Figure 3), the higher proliferative capacity of IL-7Rα+ CMV-specific cells could not be attributed to the expression of CD27 because we found similar data in a donor whose CMV-specific cells all lacked CD27 expression (data not shown). It also should be noted that although experiments were started with the same number of cells from both populations, more living cells were always recovered from the IL-7Rα+ population (Figure 7A). This points to a survival advantage of IL-7Rα+ cells consistent with their higher Bcl-2 content (Figure 4B). Figure 7B shows that the difference in proliferation after stimulation with IL-7 or peptide alone was statistically significant over several experiments.
FLU-specific cells proliferate better than CMV-specific cells. (A) Dot plots gated on CD8+ lymphocytes in which FLU or CMV tetramer staining is plotted against CFSE to visualize cell divisions. Total PBMCs were cultured for 6 days in the presence of IL-7, IL-15, FLU peptide, CMV peptide, or the specific peptide in combination with FLU or CMV antigen, respectively. (top panels) Staining with the FLU tetramer. (bottom panels) Staining with the CMV tetramer. For clarity, only 50% of the measured events is shown in the CMV tetramer staining. Representative data are shown from 1 of 3 independent experiments with healthy donors. (B) Precursor frequencies of FLU-specific (▪) and CMV-specific cells (▴) from one donor on dose titrations of IL-7 and IL-15 in 6-day total PBMC cultures. (C) Percentage of CMV B7 tetramer+ cells within CD8+ T cells before culture (unstimulated) and after 6 days' stimulation with the CMV B7 peptide, cytokines, or CMV antigen, alone or in combination. Representative data are shown from 2 independent experiments with healthy donors.
FLU-specific cells proliferate better than CMV-specific cells. (A) Dot plots gated on CD8+ lymphocytes in which FLU or CMV tetramer staining is plotted against CFSE to visualize cell divisions. Total PBMCs were cultured for 6 days in the presence of IL-7, IL-15, FLU peptide, CMV peptide, or the specific peptide in combination with FLU or CMV antigen, respectively. (top panels) Staining with the FLU tetramer. (bottom panels) Staining with the CMV tetramer. For clarity, only 50% of the measured events is shown in the CMV tetramer staining. Representative data are shown from 1 of 3 independent experiments with healthy donors. (B) Precursor frequencies of FLU-specific (▪) and CMV-specific cells (▴) from one donor on dose titrations of IL-7 and IL-15 in 6-day total PBMC cultures. (C) Percentage of CMV B7 tetramer+ cells within CD8+ T cells before culture (unstimulated) and after 6 days' stimulation with the CMV B7 peptide, cytokines, or CMV antigen, alone or in combination. Representative data are shown from 2 independent experiments with healthy donors.
Discussion
In this study we show that CD8+ T cells specific for the persistent human viruses CMV and EBV expressed IL-7Rα only at low frequencies. This was in contrast to observations in memory cells specific for acute viruses (FLU and RSV), which were all IL-7Rα+, as they are in mice after acute LCMV infection.14,15 Comparing human and murine studies was difficult because of the limitation imposed by examining only peripheral blood in humans, whereas in mice spleen and lymph nodes were analyzed. It may be that CD8+ T cells specific for persistent viruses have a different appearance in other compartments, but, because FLU- and RSV-specific cells do express IL-7Rα, we think the difference is between T cells specific for persistent and acute viruses. This finding suggests distinct requirements for IL-7 in maintaining the survival of memory cells specific for persistent viruses compared with memory cells specific for acutely cleared viruses.19,36,37
How are IL-7Rα- CD8+ T cells, specific for persistent viruses, maintained, considering that IL-7 appears not to be involved and that their response to IL-15 is minimal? Fairly high numbers of IL-7Rα- CD8+ T cells circulate in humans, suggesting potent mechanisms for generation, maintenance, or both. One possibility might be that IL-7Rα- cells continuously differentiate from the pool of IL-7Rα+ cells on activation. However, in healthy persons, almost no recently activated cells can be found, as evidenced by the absence of the activation molecules CD38 and HLA-DR. This makes it unlikely that IL-7Rα- cells are constantly formed on antigenic stimulation. Still, it cannot be ignored that help from activated CD4+ T cells has been described as playing an important role in the maintenance of CD8+ T-cell memory.10-13 In addition, helper-derived factors have been shown to greatly enhance the expansion of peptide-stimulated CMV-specific CD8+ T cells, which largely do not express IL-7Rα.38,39 Another possibility for the maintenance of IL-7Rα- cells is that the population of IL-7Rα- cells is stable and, once formed, persists in peripheral blood. This is supported by the finding by Wallace et al40 that human primed CD8+CD45RA+ T cells, which largely overlap with IL-7Rα- CD8+ T cells, show a low rate of cell death in vivo. Thus, the persistence of these cells might be attributed to long half-life in vivo rather than to proliferation.
In agreement with other studies,34,41 we found that the low frequency of IL-7Rα+ CD8+ T cells specific for latent viruses might be related to the down-regulation of the receptor after stimulation with IL-7 or T-cell receptor (TCR) stimulation. It is unlikely that circulating IL-7 plays a role here because in the same donor CD8+ T cells with different specificities varied in the expression of IL-7Rα (Figure 1). In addition, no differences were observed in serum IL-7 levels between patients with high or low frequencies of IL-7Rα+ CD8+ T cells. However, we showed several lines of evidence that indicate the importance of TCR stimulation in the regulation of IL-7Rα expression. First, we observed that CD8+ T cells specific for cleared viruses were all IL-7Rα+. Second, we showed that long after primary CMV infection, when viral load was no longer detectable, CMV-specific cells started to express IL-7Rα. Third, during reactivation, the frequency of IL-7Rα+ virus-specific cells decreased. Finally, in vitro antigen-specific stimulation also induced the down-regulation of IL-7Rα on IL-7Rα+ cells (Figure 5C). Therefore, it is possible that as long as CD8+ T cells frequently encounter their specific epitope and are thus triggered through TCR, the cells will remain IL-7Rα-. This could explain the higher frequencies of IL-7Rα- cells in recipients of kidney transplants. It can be assumed that because of immunosuppression therapy, patients will experience viral infections and reactivation episodes more often than healthy persons. This corresponds to earlier studies showing high numbers of effector-type cells in immunosuppressed persons.25,42,43
High CMV load during the initial period after transplantation apparently determines the IL-7Rα phenotype on CMV-specific cells at later time points (Figure 5B). Several factors may account for this finding. The first is that high viral loads just after transplantation may result in more antigen during the latency phase, either through higher expression of viral epitopes or more frequent reactivations, consequently inducing IL-7Rα- cells. The large variation in percentages of IL-7Rα- cells between persons could be explained by differences in susceptibility to CMV or virulence of the CMV strain. A second explanation could be related to imprinting; the amount of antigen present at early time points after activation might force the differentiation of CD8+ T cells in a particular direction. Accordingly, high viral loads may induce the formation of a memory population that is more IL-7Rα-. Finally, high viral loads may lead to widespread infection and eventually result in high numbers of latently infected cells, among which are monocytes. On CMV reactivation, these monocytes may produce IL-15, which has several effects on CD8+ T cells including the down-regulation of IL-7Rα34 (E.M.M.V.L. and N.L. Alves, unpublished data, January 2005), thus giving rise to high frequencies of IL-7Rα- CMV-specific cells.
Sorted CMV-specific IL-7Rα+cells proliferate better than IL-7Rα-cells. From PBMCs, IL-7Rα+ and IL-7Rα CMV-specific (tetramer+) CD8+ T cells were sorted by fluorescence-activated cell sorter (FACS) analysis. (A) CFSE profiles of sorted IL-7Rα- or IL-7Rα+ CMV-specific CD8+ T cells on stimulation with IL-7, IL-15, specific CMV peptide, or CMV peptide in combination with CMV antigen on day 3 (top 2 rows) and day 6 (bottom 2 rows). Numbers indicate the calculated precursor frequency that indicates the percentage of cells from the original population that proliferated. Italic numbers indicate the calculated mean number of divisions of the divided cells and the amount of divisions that the proliferating cells underwent. Representative data are shown from 4 independent experiments with cells from 3 different donors. (B) Means of the precursor frequencies after 6 days of culture, calculated from 4 independent experiments (2 experiments for IL-15). *Significant difference (P = .029). ▦ indicates IL-7Rα-; ▪, IL-7Rα+.
Sorted CMV-specific IL-7Rα+cells proliferate better than IL-7Rα-cells. From PBMCs, IL-7Rα+ and IL-7Rα CMV-specific (tetramer+) CD8+ T cells were sorted by fluorescence-activated cell sorter (FACS) analysis. (A) CFSE profiles of sorted IL-7Rα- or IL-7Rα+ CMV-specific CD8+ T cells on stimulation with IL-7, IL-15, specific CMV peptide, or CMV peptide in combination with CMV antigen on day 3 (top 2 rows) and day 6 (bottom 2 rows). Numbers indicate the calculated precursor frequency that indicates the percentage of cells from the original population that proliferated. Italic numbers indicate the calculated mean number of divisions of the divided cells and the amount of divisions that the proliferating cells underwent. Representative data are shown from 4 independent experiments with cells from 3 different donors. (B) Means of the precursor frequencies after 6 days of culture, calculated from 4 independent experiments (2 experiments for IL-15). *Significant difference (P = .029). ▦ indicates IL-7Rα-; ▪, IL-7Rα+.
Regarding the differentiation of CD8+ T cells, the correlation between IL-7Rα and CD27 expression is striking. Loss of CD27 expression is a relatively late and irreversible event during the differentiation of CD8+ T cells,31 whereas IL-7Rα is quickly down-regulated on TCR triggering or on stimulation with IL-7 and can be re-expressed34 (E.M.M.V.L. and N.L. Alves, unpublished data, January 2005). Although the kinetics are different, we found, in agreement with Gamadia et al,32 that IL-7Rα and CD27 expression are regulated by the presence of antigen, which could explain the correlation.
We found that IL-7Rα expression is different on cells specific for persistent viruses (CMV and EBV) than it is on cells of viruses cleared from the host (FLU and RSV). These cell types were investigated in mice by Wherry et al,44 who compared T cells in acute and chronic LCMV infection and who found that IL-7Rα expression was reduced on memory cells in chronically infected mice that responded poorly to IL-7 and IL-15. Our findings were consistent with this. CD8+ T cells specific for chronic LCMV also showed diminished proliferation on in vitro peptide stimulation. However, we showed that the expansion of CD8+ T cells specific for persistent viruses is better when CD4+ T-cell help or cytokines are provided (Figure 6), as described previously for CD8+CD45RA+CD27- T cells.38,39
It is debatable whether memory CD8+ T cells specific for persistent viruses can actually be named memory cells and, as such, can be compared with memory cells specific for viruses that are cleared. The viruses that these cells recognize are not cleared but are only kept under control of the immune system in a latency stage. Frequencies of CD8+ T cells specific for latent viruses are much higher than they are for acute viruses. Moreover, the phenotype of cells specific for persistent viruses is also different from that of memory cells specific for acute viruses. For example, CMV-specific cells often resemble effector-type cells (CCR7-CD28-CD27)- and contain high levels of granzyme B and perforin.24,25 Wherry et al44 suggest that the continuous presence of antigen turns these cells into antigen-addicted memory cells lacking the features of memory cells after acute infection. We propose to classify CD8+ T cells specific for persistent viruses not in the same category as cells specific for acute viruses but as vigilant, resting effector cells that adequately control latent virus and prevent frequent reactivation.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-02-0449.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients and the healthy volunteers for their blood donations, Berend Hooibrink (Department of Cell Biology and Histology) for sorting the different cell populations, technicians from the Department of Clinical Virology for performing CMV and EBV PCR and serology, and Jessica van der Sluijs-Videler for performing IL-7 ELISA. In addition, we thank Drs E. John Wherry, Robert M. Hoek, Kris A. Reedquist, Amber van Stijn, and Hans L. Zaaijer for critical reading of the manuscript and for useful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal