Abstract
Natural killer (NK) cell-mediated cytotoxicity is triggered by multiple activating receptors associated with the signaling adaptor protein DNAX activation protein 12/killer cell-activating receptor-associated protein (DAP12/KARAP). Here, we show that one of these receptors, NKp44, is present on a subset of natural interferon-producing cells (IPCs) in tonsils. NKp44 expression can also be induced on blood IPCs after in vitro culture with interleukin 3 (IL-3). Crosslinking of NKp44 does not trigger IPC-mediated cytotoxicity but, paradoxically, inhibits interferon α (IFN-α) production by IPCs in response to cytosine-phosphate-guanosine (CpG) oligonucleotides. We find that IPCs in tonsils are in close contact with CD8+ T cells and demonstrate that a subset of memory CD8+ T cells produces IL-3. Therefore, IL-3-mediated induction of NKp44 on IPCs may be an important component of the ongoing crosstalk between the innate and adaptive immune response that allows memory CD8+ T cells to control the IPC response to virus. (Blood. 2005;106: 2076-2082)
Introduction
Natural interferon-producing cells (IPCs), also termed plasmacytoid dendritic cells (pDCs), play an important role in the first-line defense against viral infections.1-3 IPCs recognize viral components, such as cytosine-phosphate-guanosine (CpG)-rich DNA and single-stranded RNA, through the Toll-like receptors TLR9 and TLR7, respectively.4 Detection of viral infection triggers IPC secretion of type I interferons (IFNs), that is, IFN-α and IFN-β, which inhibit viral replication in infected cells and activate host immune responses.5-7 In particular, type I IFNs enhance the cytotoxicity of natural killer (NK) cells and CD8 T cells,5,8 induce survival of CD8 T cells,9 activate dendritic cells (DCs),10 promote differentiation of helper CD4 T cells into T helper 1 cells5 and the differentiation of memory B cells into antibody-secreting plasma cells.11,12 IPCs further activate adaptive immune responses by secreting interleukin 12 (IL-12)13 and proinflammatory chemokines,14 and by presenting antigens to T cells.15-17
IPCs are generated in the bone marrow2 and circulate in peripheral blood in very low numbers. Upon viral infection, IPCs presumably leave the blood stream and migrate into the lymph nodes draining the site of infection through the high endothelial venules (HEVs).18-20 IPCs localize predominantly in the T-cell zone of the lymph node, where they secrete type I IFNs and activate innate and adaptive immune responses. IPCs also migrate into inflamed peripheral tissues, particularly skin of patients affected by autoimmune disorders like systemic lupus erythematosus.21,22 Moreover, IPCs are recruited into tumors, such as ovarian carcinomas and melanomas, but, in these sites, they appear to be functionally paralyzed by tumor microenvironment and incapable of secreting type I IFNs and activating immune responses.23,24
Human IPCs are identified by the exclusive expression of a cell-surface molecule termed BDCA-2 (blood dendritic cell antigen 2).25 BDCA-2 is a C-type lectin receptor that acts as an antigen-capturing molecule. Moreover, engagement of BDCA-2 by a specific antibody inhibits IFN-α production by IPCs in response to CpG. Activation of IPCs in vitro with TLR ligands causes a rapid down-regulation of BDCA-2 from the cell surface, together with increased expression of major histocompatability complex (MHC) and costimulatory molecules, such as CD80, CD86, and CD40. IPCs also express the α chain of the IL-3 receptor (CD123) and depend on IL-3 for their survival in vitro.26,27 Resting and activated IPCs express additional cell-surface markers of the myeloid lineage (CD31, CD36, CD68) and the lymphoid lineage (CD45RA),28 as well as T-cell- and B-cell-specific transcripts, like pre-T-cell receptor α and Spi-B.2 Although a rare leukemia with the surface characteristics of IPCs expresses the NK-cell marker CD56,29 no other NK-cell-surface molecules have been identified on normal IPCs to date.
NKp44 is an activating receptor expressed on human NK cells that belongs to the immunoglobulin (Ig) superfamily.30,31 It consists of a single extracellular V-type Ig domain, a transmembrane region, and a short cytoplasmic domain. Through its transmembrane region, NKp44 associates with the adaptor molecule DNAX activation protein 12 (DAP12) (killer cell-activating receptor-associated protein; KARAP), which contains an immunoreceptor tyrosine-based activation motif (ITAM). DAP12 is required for NKp44 cell-surface expression and intracellular signaling. The cytoplasmic domain of NKp44 contains a tyrosine-based motif with unknown function.32 NKp44 mediates cytotoxicity against various tumor cells, such as neuroblastoma and choriocarcinoma.30,31,33,34 The NKp44 ligand on these tumors has not yet been identified. There may also be a role for NKp44 recognition of virally infected cells.35 NKp44 is not expressed on the surface of resting NK cells. Expression is induced upon in vitro activation of NK cells with IL-2.30 Recently, NKp44 was also found on a subset of NK cells in tonsils, possibly representing in vivo-activated NK cells.36,37 Here, we report that tonsil IPCs and blood IPCs cultured in vitro with IL-3 express NKp44. We demonstrate that crosslinking of NKp44 leads to inhibition of IFN-α production by IPCs in response to CpG. Moreover, we show that IPCs in tonsils are in close contact with CD8 T cells and that a subset of memory CD8 T cells produces IL-3, indicating a potential crosstalk between IPCs and CD8 T cells through IL-3-mediated induction of NKp44.
Materials and methods
Cell preparations and cultures
Buffy coats from healthy volunteers were obtained from the American Red Cross (St Louis, MO). Peripheral blood from patients with Nasu-Hakola disease was obtained from the Nagasaki Medical Center of Neurology, Nagasaki, Japan.38 Peripheral blood mononuclear cells (PBMCs) were prepared from buffy coats by standard Ficoll density gradient centrifugation (Ficoll-Paque; Amersham Biosciences, Piscataway, NJ). IPCs were isolated from PBMCs using the BDCA-4 isolation kit (Miltenyi Biotec, Auburn, CA). Tonsils were obtained from pediatric patients undergoing elective tonsillectomy (Children's Hospital, Washington University School of Medicine, St Louis, MO). Approval was obtained from the Washington University School of Medicine and the Nagasaki Medical Center of Neurology institutional review boards for these studies. Informed consent was provided in accordance with the Declaration of Helsinki. Lymphocyte suspensions were prepared from tonsils by dissociation of the tissue, followed by Ficoll density gradient centrifugation. NK92 cells were kindly provided by M.L. Botet (University Pompeu-Fabre, Barcelona, Spain). Peripheral blood NK cells were obtained from CD56+CD3- PBMCs as described.39 Peripheral blood CD8 T cells were isolated using CD8 MicroBeads (Miltenyi Biotec) and then sorted into CD45RA+2B4- naive cells, CD45RA-2B4- central memory cells, CD45RA+2B4+ effector cells, and CD45RA-2B4+ effector memory cells on a MoFlo cytometer (Cytomation, Fort Collins, CO). Sorted cells were cultured for 48 hours in IL-2-containing medium before stimulation with 10-7 M PMA (phorbol 12-myristate-13-acetate) and 0.5 μg/mL ionomycin (both from Sigma Aldrich, St Louis, MO). Following 2 hours of stimulation, 2 μM monensin was added to the cultures, and incubation was continued for 4 hours. Cells were then fixed for detection of intracellular cytokines.
PBMCs and purified IPCs were cultured in RPMI 1640 (Gibco BRL, Rockville, MD), supplemented with 10% fetal calf serum (HyClone, Logan, UT), GlutaMAX, kanamycin, and Na-pyruvate (all from Gibco BRL). IL-3 (R&D Systems, Minneapolis, MN) was added to a final concentration of 20 ng/mL, where indicated. For IPC stimulation, 5 μg/mL CpG 2216 (Invitrogen, Carlsbad, CA) or influenza virus WSN strain (a kind gift from Andrew Pekosz, Department of Molecular Microbiology, Washington University, St Louis, MO) was added to cultures. For receptor crosslinking experiments, cells were stimulated in enzyme-linked immunosorbent assay (ELISA) plate wells coated with F(ab′)2 goat anti-mouse IgG antibody (10 μg/mL; Southern Biotechnology Associates, Birmingham, AL) and hybridoma supernatants containing the indicated mouse monoclonal antibody (mAb). Alternatively, wells were coated with purified mouse mAbs (10 μg/mL) or their F(ab′)2 fragments (50 μg/mL). F(ab′)2 fragments were prepared from the anti-NKp44 mAb (clone 3.43) and anti-CD155 mAb (clone SKII.4, mouse IgG1, as control antibody) using the ImmunoPure F(ab′)2 Preparation Kit from Pierce (Rockford, IL).
Cytotoxicity assays
The target cell lines P815 (murine mastocytoma) and K562 (human erythroleukemia) were labeled with 0.1 mCi (3.7 MBq) chromium-51 (Amersham) for 1 hour, washed 3 times, and seeded at 5000 cells/well in a 96-well plate. The indicated effector cells (NK92, activated NK cells, or IPCs cultured overnight in IL-3) were added in serial dilutions, with a maximum effector-target ratio of 10:1. Cytotoxicity was measured as chromium-51 release following 4 hours or 18 hours of culture at 37°C. Specific lysis was calculated from the following formula: % lysis = 100 × (test cpm - spontaneous cpm)/(total cpm - spontaneous cpm).
Flow cytometric analysis and immunofluorescence
Anti-NKp44 mAbs (clones 2.29 and 3.43, both mouse IgG1) were generated in our laboratory by immunizing mice with a NKp44-human crystallizable fragment (Fc) fusion protein. Anti-NKp44 (clone 2.29) was conjugated to allophycocyanin (Cyanotech, Kailua-Kona, HI) according to standard protocols. Biotinylated anti-TREM-1 (triggering receptor expressed on myeloid cells 1) antibody was generated in our laboratory40 and used in combination with Streptavidin-allophycocyanin (APC; Molecular Probes, Eugene, OR). Unconjugated anti-BDCA-2 antibody (clones 13A11 and AC144) and fluorescein isothiocyanate (FITC)-conjugated anti-BDCA-2 (AC144) were a kind gift of Miltenyi Biotec (Bergisch Gladbach, Germany). Fluorochrome-labeled antibodies against CD45RA, CD123, Granzyme B, IL-2, IL-3, tumor necrosis factor α (TNF-α), and IFN-γ were purchased from BD Biosciences (San Jose, CA). Fluorochrome-labeled antibodies against CD8, CD19, CD20, CD56, CD14, CD16, CD83, CD86, CD40, and CD244 (2B4) were from Beckman Coulter (Miami, FL). Samples were analyzed on a FACSCalibur (BD Biosciences) using the CellQuest software.
Cryosections of tonsil tissues were stained with anti-BDCA-2 (AC144, mouse IgG1) and anti-CD8 (OKT8, mouse IgG2a; from American Type Culture Collection [ATCC], Manassas, VA), followed by biotin-labeled anti-mouse IgG2a (Southern Biotechnology Associates). Antibody binding was detected using a Texas Red-conjugated streptavidin and Alexa 488-conjugated anti-mouse IgG1 antibody (Molecular Probes). Slides were mounted with VectaShield (Vector Laboratories, Burlingame, CA) containing DAPI (4′, 6-diamino-2-phenylindole) stain and visualized with an Olympus BX51 microscope (Olympus, Melville, NY) coupled to a Spot RT Slider camera (Diagnostic Instruments, Sterling Heights, MI). Images were acquired with the Spot Advanced software (Diagnostic Instruments) and further processed using Adobe Photoshop (Adobe, San Jose, CA).
Analysis of cytokine and chemokine secretion
IFN-α levels in culture supernatants were measured by evaluating the inhibition of Daudi-cell proliferation with reference to a standard IFN-α curve.41 Concentrations of the chemokines monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein 1-α (MIP-1α), MIP-1β, RANTES (regulated on activation normal T cell expressed and secreted), and IL-8 were assessed using the Chemokine II Cytometric Bead Array (BD Biosciences).
Results
A subset of tonsil IPCs expresses NKp44
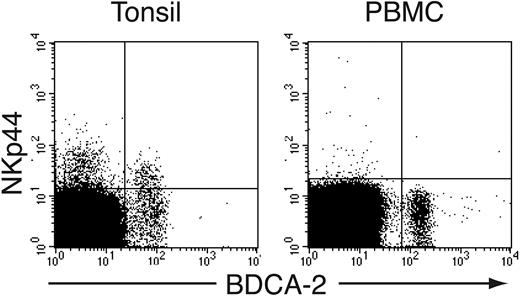
NKp44 is induced by in vitro activation of NK cells and TCR γδ cell clones, whereas no cell types express NKp44 within PBMCs.30,31 In addition, NKp44 is present on a subset of tonsil NK cells, presumably corresponding to NK cells activated in vivo.36,37 We studied in depth the phenotype of NKp44+ cells in human tonsils and observed that NKp44 is expressed not only on NK cells but also on a small cell population that lacks NK-cell markers. This cell subset expressed the IL-3 receptor α chain (CD123) (not shown) and BDCA-2, a specific marker of IPCs25 (Figure 1). NKp44+ IPCs represented approximately 10% to 50% of tonsil IPCs and expressed NKp44 at lower levels than tonsil NK cells (Figure 1). In contrast, peripheral blood IPCs did not express NKp44 (Figure 1). We conclude that primary IPCs express NKp44 in tonsils but not in blood.
IL-3 induces NKp44 on blood IPCs
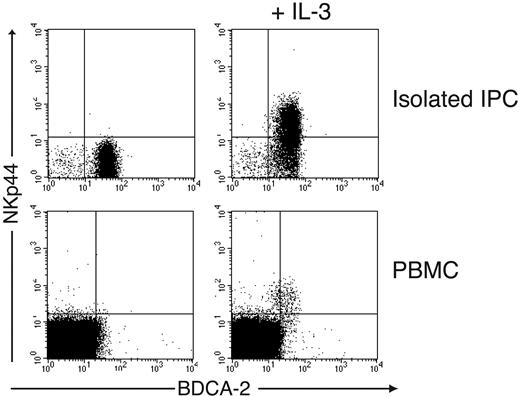
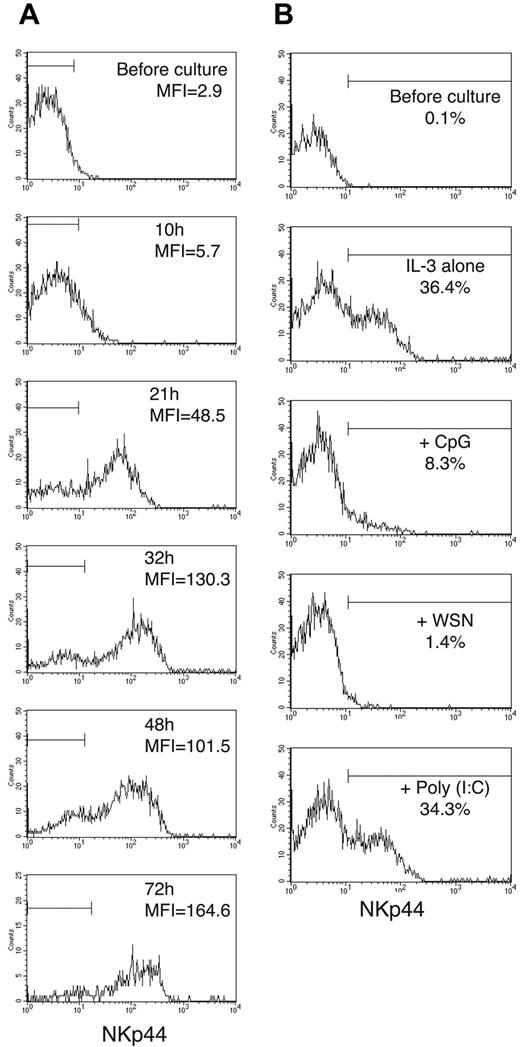
We investigated whether blood IPCs can be induced to express NKp44. Indeed, overnight incubation of IPCs purified from peripheral blood in medium containing IL-3 induced NKp44 on the surface of IPCs (Figure 2). Similarly, when whole PBMCs were cultured in IL-3, NKp44 became detectable on a small cell subset corresponding to IPCs but not on other cell types (Figure 2). These findings indicate that IL-3 acts specifically on IPCs to induce NKp44 expression. NKp44 was already detectable on IPCs at low levels after 10 hours in IL-3; it reached maximum levels by about 30 hours of culture and remained stable throughout the 72-hour period of culture (Figure 3A). We also cultured freshly isolated blood IPCs in the presence of IL-3 together with TLR9 and TLR7 agonists, such as CpG and influenza virus, which are known to induce IPC activation and IFN-α secretion. Remarkably, these stimuli inhibited the induction of NKp44 when added at the same time as IL-3 (Figure 3B). In contrast, when IPCs were first cultured with IL-3 to induce expression of NKp44 and then stimulated with CpG or influenza virus, expression of NKp44 did not significantly decrease (data not shown), indicating that TLR ligands can inhibit the induction of NKp44, but do not affect preexisting NKp44 expression.
IPCs express NKp44 in tonsil but not in peripheral blood. Mononuclear cells were prepared from tonsils and peripheral blood and stained with mAbs against BDCA-2 and NKp44.
IPCs express NKp44 in tonsil but not in peripheral blood. Mononuclear cells were prepared from tonsils and peripheral blood and stained with mAbs against BDCA-2 and NKp44.
IPCs from a DAP12-deficient patient do not express NKp44
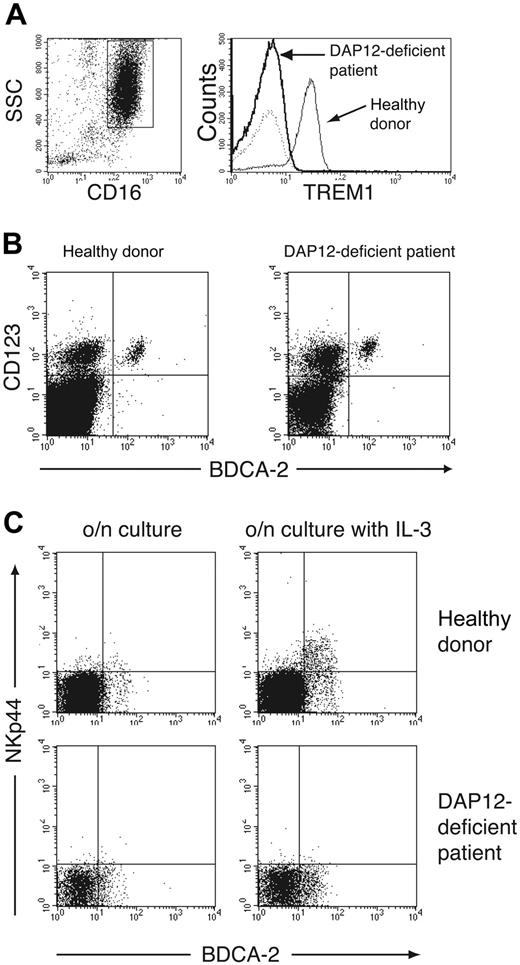
In NK cells, NKp44 associates with DAP12 (KARAP).32 This ITAM-bearing adaptor protein mediates downstream signaling events upon receptor engagement.42,43 We sought to determine whether NKp44 associates with DAP12 in IPCs. Human IPCs are rare blood cells and cannot be grown in vitro in sufficient numbers for biochemical demonstration of NKp44/DAP12 association. However, DAP12 is required for cell-surface expression of NKp44.32 Therefore, we analyzed cell-surface expression of NKp44 in IL-3-cultured PBMCs from a DAP12-deficient patient affected by the Nasu-Hakola disease.38,44 We stained granulocytes of this patient with a mAb against TREM-1, another known DAP12-associated receptor,40 and found no TREM-1 expression, confirming the absence of functional DAP12 (Figure 4A). Staining of the DAP12-deficient PBMCs with mAbs against BDCA-2 and CD123 revealed the presence of blood IPCs as well as their expression of the IL-3 receptor α chain (Figure 4B). Following overnight culture of PBMCs with IL-3, however, DAP12-deficient IPCs failed to express NKp44 (Figure 4C). We conclude that NKp44 associates with DAP12 in IPCs as it does in NK cells.
IL-3 induces expression of NKp44 in peripheral blood IPCs. (Top) IPCs isolated from peripheral blood were stained with mAbs against BDCA-2 and NKp44 before (left) and after (right) overnight culture in IL-3. (Bottom) Total PBMCs were cultured overnight in the absence (left) or presence (right) of IL-3 and analyzed for expression of BDCA-2 and NKp44.
IL-3 induces expression of NKp44 in peripheral blood IPCs. (Top) IPCs isolated from peripheral blood were stained with mAbs against BDCA-2 and NKp44 before (left) and after (right) overnight culture in IL-3. (Bottom) Total PBMCs were cultured overnight in the absence (left) or presence (right) of IL-3 and analyzed for expression of BDCA-2 and NKp44.
IL-3-induced NKp44 expression persists during IPC culture, whereas CpG and virus block NKp44 induction. (A) Peripheral blood IPCs were cultured in the presence of IL-3. The mean fluorescence intensity (MFI) of anti-NKp44 staining was measured at 10, 21, 32, 48, and 72 hours of culture. Cells stained with control antibody fell within the horizontal bars. (B) Freshly isolated peripheral blood IPCs were cultured in IL-3-containing medium in the presence of CpG, influenza virus (WSN), or poly(I:C) (polyinosinic-polycytidylic acid). NKp44 expression was evaluated after 16 hours of culture. CpG and WSN blocked NKp44 induction, whereas the TLR3 agonist poly(I:C) did not influence NKp44 expression, consistent with the lack of TLR3 in IPCs. Cells stained with control antibodies fell outside the horizontal bars.
IL-3-induced NKp44 expression persists during IPC culture, whereas CpG and virus block NKp44 induction. (A) Peripheral blood IPCs were cultured in the presence of IL-3. The mean fluorescence intensity (MFI) of anti-NKp44 staining was measured at 10, 21, 32, 48, and 72 hours of culture. Cells stained with control antibody fell within the horizontal bars. (B) Freshly isolated peripheral blood IPCs were cultured in IL-3-containing medium in the presence of CpG, influenza virus (WSN), or poly(I:C) (polyinosinic-polycytidylic acid). NKp44 expression was evaluated after 16 hours of culture. CpG and WSN blocked NKp44 induction, whereas the TLR3 agonist poly(I:C) did not influence NKp44 expression, consistent with the lack of TLR3 in IPCs. Cells stained with control antibodies fell outside the horizontal bars.
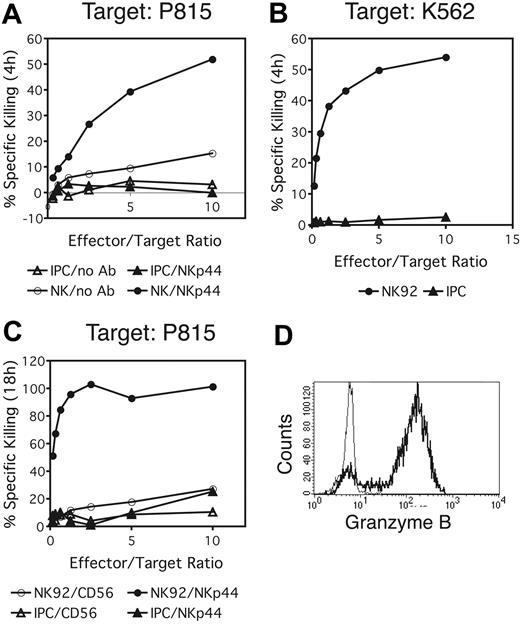
NKp44 triggering does not activate IPC-mediated killing of target cells
Crosslinking of NKp44 on NK cells triggers cytotoxicity.30 IPCs have not been reported to function as cytotoxic cells, despite their abundant expression of granzyme B.45,46 We tested the possibility of IPC-mediated target-cell lysis upon NKp44 crosslinking. NKp44+ IPCs were cultured with the Fc receptor (FcR)-bearing cell line P815 in the presence of anti-NKp44 antibody to induce receptor crosslinking. Activated NK cells were used as controls. Cytotoxicity was measured in a 4-hour chromium release assay. No target-cell lysis was observed when IPCs were used as effector cells, whereas activated NK cells efficiently killed P815 in the presence of NKp44 antibody (Figure 5A). We also examined the ability of IPCs to directly kill the target cell line K562, which is highly susceptible for NK-cell-mediated killing. K562 was lysed efficiently by NK cells but not by IPCs (Figure 5B). To investigate whether IPCs use other mechanisms of cytotoxicity, such as those mediated by members of the TNF receptor family (TNF-related apoptosis-inducing ligand [TRAIL], CD95L), which require longer time spans to induce target-cell death, we cocultured IPCs with P815 in the presence of crosslinking anti-NKp44 antibody for 18 hours. However, no significant target-cell lysis was detected in these settings (Figure 5C). In conclusion, we did not observe any significant IPC-mediated cytotoxicity, neither by triggering the NKp44 receptor nor by coculturing IPCs with the typical NK-cell target K562, although IPCs did contain intracellular granzyme B (Figure 5D).
DAP12-deficient IPCs do not express NKp44. (A) To confirm DAP12 deficiency, we evaluated granulocytes for expression of TREM-1, a DAP12-associated receptor (right). TREM-1 expression was determined by gating on CD16+ side scatter (SSC) high granulocytes (left). (B) The presence of IPCs in DAP12-deficient PBMCs was confirmed by analyzing PBMCs for expression of BDCA-2 and CD123. Lineage-positive cells (CD3+, CD14+, CD16+, CD20+, CD56+) were excluded from the analysis. (C) Total PBMCs from a healthy donor and the DAP12-deficient patient were cultured overnight in the presence (right) or absence (left) of IL-3. Selective expression of NKp44 on IPCs was evaluated by counterstaining with BDCA-2. CD19+, CD14+, and CD56+ cells were excluded from the analysis.
DAP12-deficient IPCs do not express NKp44. (A) To confirm DAP12 deficiency, we evaluated granulocytes for expression of TREM-1, a DAP12-associated receptor (right). TREM-1 expression was determined by gating on CD16+ side scatter (SSC) high granulocytes (left). (B) The presence of IPCs in DAP12-deficient PBMCs was confirmed by analyzing PBMCs for expression of BDCA-2 and CD123. Lineage-positive cells (CD3+, CD14+, CD16+, CD20+, CD56+) were excluded from the analysis. (C) Total PBMCs from a healthy donor and the DAP12-deficient patient were cultured overnight in the presence (right) or absence (left) of IL-3. Selective expression of NKp44 on IPCs was evaluated by counterstaining with BDCA-2. CD19+, CD14+, and CD56+ cells were excluded from the analysis.
NKp44 does not elicit IPC-mediated cytotoxicity. (A,C) Redirected lysis of the FcR-bearing cell line P815 in the presence of an anti-NKp44 antibody was determined by a 4-hour (A) or 18-hour (C) chromium release assay. Activated NK cells (A) or the NK cell line NK92 (C) were included as controls. (B) Direct killing of K562 cells by IPCs or NK92 was tested in a 4-hour chromium release assay. (D) Intracellular granzyme B expression in IPCs. Bold profile shows anti-granzyme-B staining; thin profile represents an isotype-matched control antibody.
NKp44 does not elicit IPC-mediated cytotoxicity. (A,C) Redirected lysis of the FcR-bearing cell line P815 in the presence of an anti-NKp44 antibody was determined by a 4-hour (A) or 18-hour (C) chromium release assay. Activated NK cells (A) or the NK cell line NK92 (C) were included as controls. (B) Direct killing of K562 cells by IPCs or NK92 was tested in a 4-hour chromium release assay. (D) Intracellular granzyme B expression in IPCs. Bold profile shows anti-granzyme-B staining; thin profile represents an isotype-matched control antibody.
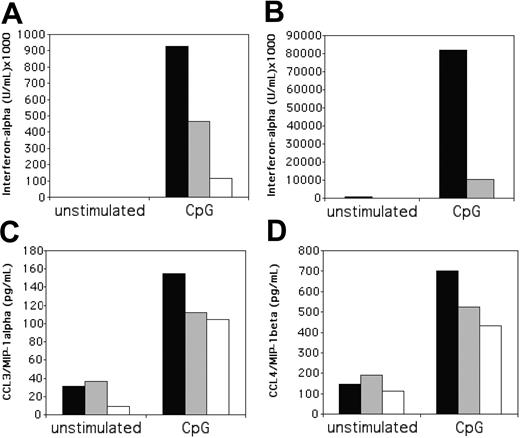
Engagement of NKp44 inhibits IFN-α secretion in response to CpG
To further evaluate the function of NKp44 in IPCs, we tested the effects of NKp44 engagement on cytokine production. We incubated IPCs with IL-3 to induce NKp44 and then cultured NKp44+ IPCs in plates coated with anti-NKp44 antibody in the presence of CpG, which induces IFN-α and chemokine secretion. Remarkably, NKp44 crosslinking consistently inhibited CpG-induced IFN-α secretion (Figure 6A); the degree of inhibition varied between IPC preparation by 2- to 16-fold. This ability of NKp44 to inhibit the IFN-α response of IPCs to CpG was reminiscent of the inhibitory function previously reported for the C-type lectin BDCA-2.25 However, comparison of NKp44 and BDCA-2 effects on IFN-α secretion revealed that BDCA-2 mediates stronger inhibition than NKp44 (Figure 6A). IPCs express the Fcγ receptor FcγRII,47,48 and IgG-Fc can inhibit IFN-α response of IPCs to viruses.49 Additionally, engagement of FcγRs can drastically modulate the cytokine profile of other hematopoietic cells such as monocytes or macrophages.50,51 To address whether the anti-NKp44 mAb inhibits IFN-α response of IPCs by engaging an FcγR, we performed experiments with anti-NKp44 F(ab′)2 fragments, which do not engage Fc receptors. Plate-bound anti-NKp44 F(ab′)2 fragments induced an inhibition of IFN-α response to CpG similar to that seen with complete antibody (Figure 6B), demonstrating that inhibition is not mediated by interaction of the antibody with the FcγRII. In contrast to CpG-induced IFN-α, NKp44 crosslinking did not significantly inhibit CpG-induced secretion of proinflammatory chemokines such as MIP-1α and MIP-1β (Figure 6C-D). We conclude that ligation of NKp44 selectively inhibits IFN-α response to CpG.
Crosslinking of NKp44 on IL-3-cultured IPCs reduces IFN-α responses to CpG. Peripheral blood IPCs were cultured overnight in IL-3 and then transferred to wells containing plate-bound anti-NKp44 antibody, in the presence or absence of CpG as IFN-α-inducing stimulant. After 20 hours, culture supernatants were tested for the presence of IFN-α (A-B), MIP-1α (C), and MIP-1β (D). For IFN-α, 2 representative experiments are shown, which were carried out with whole antibody (A), or with F(ab′)2 fragments (B) to crosslink NKp44. In some experiments, IPCs were also stimulated on anti-BDCA-2 mAb-coated plates for comparison. ▪ indicate control antibody; ▦, anti-NKp44 mAb; □, anti-BDCA-2 mAb.
Crosslinking of NKp44 on IL-3-cultured IPCs reduces IFN-α responses to CpG. Peripheral blood IPCs were cultured overnight in IL-3 and then transferred to wells containing plate-bound anti-NKp44 antibody, in the presence or absence of CpG as IFN-α-inducing stimulant. After 20 hours, culture supernatants were tested for the presence of IFN-α (A-B), MIP-1α (C), and MIP-1β (D). For IFN-α, 2 representative experiments are shown, which were carried out with whole antibody (A), or with F(ab′)2 fragments (B) to crosslink NKp44. In some experiments, IPCs were also stimulated on anti-BDCA-2 mAb-coated plates for comparison. ▪ indicate control antibody; ▦, anti-NKp44 mAb; □, anti-BDCA-2 mAb.
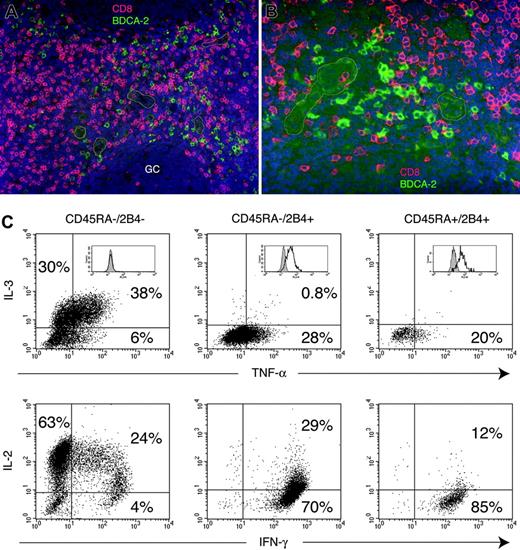
CD8 T cells are in close proximity of IPCs in tonsil and provide a source of IL-3. (A-B) Colocalization of CD8 T cells and IPCs in the T-cell areas of tonsil. Cryosections from tonsils were stained with anti-BDCA-2 and anti-CD8, followed by Alexa 488-conjugated and Texas Red-conjugated secondary reagents. BDCA-2 is shown in green, CD8 in red, and DAPI nuclear stain in blue. Some of the HEVs are marked by white profiles. GC indicates germinal center. (For panel A, objective was 20 × /0.50 NA; for panel B, objective was 40 × /0.75 NA). (C) CD8 T cells were magnetically enriched from peripheral blood and sorted into CD45RA-/2B4- central memory cells (left column), CD45RA-/2B4+ effector-memory cells (middle column), CD45RA+/2B4+ effector cells (right column), and CD45RA+/2B4- naive cells (not shown). Cytokine production of the sorted cell subsets was assessed by intracellular staining with anti-IL-2, IL-3, IFN-γ, and TNF-α following 6 hours of stimulation with PMA and ionomycin. Percentages of cytokine-secreting cells are indicated in each quadrant. Inserts in top panel represents fluorescence-activated cell sorting (FACS) profiles of individual CD8 T-cell subsets after intracellular staining with an antiperforin antibody.
CD8 T cells are in close proximity of IPCs in tonsil and provide a source of IL-3. (A-B) Colocalization of CD8 T cells and IPCs in the T-cell areas of tonsil. Cryosections from tonsils were stained with anti-BDCA-2 and anti-CD8, followed by Alexa 488-conjugated and Texas Red-conjugated secondary reagents. BDCA-2 is shown in green, CD8 in red, and DAPI nuclear stain in blue. Some of the HEVs are marked by white profiles. GC indicates germinal center. (For panel A, objective was 20 × /0.50 NA; for panel B, objective was 40 × /0.75 NA). (C) CD8 T cells were magnetically enriched from peripheral blood and sorted into CD45RA-/2B4- central memory cells (left column), CD45RA-/2B4+ effector-memory cells (middle column), CD45RA+/2B4+ effector cells (right column), and CD45RA+/2B4- naive cells (not shown). Cytokine production of the sorted cell subsets was assessed by intracellular staining with anti-IL-2, IL-3, IFN-γ, and TNF-α following 6 hours of stimulation with PMA and ionomycin. Percentages of cytokine-secreting cells are indicated in each quadrant. Inserts in top panel represents fluorescence-activated cell sorting (FACS) profiles of individual CD8 T-cell subsets after intracellular staining with an antiperforin antibody.
CD8 T cells with memory phenotype produce IL-3
Because IL-3 is required for NKp44 expression on IPCs, we asked which cells may provide IL-3 to IPCs in vivo. Mast cells and eosinophils are a well-established source of IL-3. However, these cell types are unlikely to be present in the T-cell area and around HEVs of secondary lymphoid organs, where IPCs are mainly located. In fact, analysis of tonsil sections by immunofluorescence with antibodies specific for IPCs and other cell types revealed that IPCs are often positioned in close contact to CD8 T cells (Figure 7A-B). However, whether CD8 T cells produce IL-3 is not known. We previously demonstrated that CD8 T cells can be divided into 4 distinct subsets, based on the expression of CD45RA and the cell-surface receptor 2B452 : (1) CD45RA+/2B4- cells correspond to naive T cells; (2) CD45RA+/2B4high cells are effector T cells; (3) CD45RA-/2B4- cells represent memory T cells; (4) CD45RA-/2B4+ cells are effector-memory T cells. Because most of the CD45RA-/2B4- cells express CC chemokine receptor 7 (CCR7; not shown), they largely overlap with the well-described subset of central memory cells.53 We sorted these 4 subsets of CD8 T cells from peripheral blood and assessed their capacity to produce IL-3 by intracellular staining. Remarkably, we found that only memory CD8 T cells produce IL-3 (approximately 20%-70% of the cells in different individuals) (Figure 7C). IL-3 production correlated with IL-2 production and lack of intracellular perforin. In contrast, effector or effector-memory subsets produced high levels of IFN-γ and perforin but not IL-3 (Figure 7C). The naive subset solely produced IL-2 (not shown). This result suggests that memory CD8 T cells activated by or in close proximity to IPCs may provide the IL-3 required for IPC survival and NKp44 up-regulation.
Discussion
In this report, we demonstrate that a subset of tonsil IPCs and IL-3-cultured blood IPCs express the NK-cell receptor NKp44 and that engagement of NKp44 inhibits IFN-α responses of IPCs to CpG. NKp44 was originally considered a specific marker for activated NK cells and some TCR-γδ T-cell clones.30,31 NKp44 was then detected on some tonsil NK cells.36,37 This is the first report to describe the presence of an activating NK-cell receptor on human IPCs. Because IPCs in a DAP12-deficient patient lacked NKp44, it is likely that in normal IPCs NKp44 reaches the cell surface and signals through DAP12, as in the case of NK cells. Blood IPCs expressed NKp44 upon in vitro culture with IL-3. None of the other cell types that express the receptor for IL-3, such as basophils, monocytes, and monocyte-derived DCs, expressed NKp44 upon culture with IL-3. Moreover, IL-3 did not induce NKp44 expression on NK cells, consistent with their lack of IL-3R (data not shown). Although IL-2 can induce NKp44 on NK cells,30 it was unable to do so on IPCs, which also express the IL-2R-α chain (CD25) (data not shown).
In NK cells, NKp44 ligation triggers exocytosis of lytic granules and secretion of IFN-γ against tumor cells expressing yet unknown NKp44 ligands.30,31,33,34 Because IPCs express high levels of granzyme B,45,46 we evaluated whether IPC triggering through NKp44 induces cytotoxicity. However, our experiments showed no evidence for NKp44-mediated killing of FcR+ cells coated with an anti-NKp44 antibody. In addition, IPCs were unable to directly kill classic NK target cells, such as K562. These results are consistent with the original characterization of IPCs as a cell type unambiguously distinct from NK cells.7 Despite its ability to deliver activating signals, NKp44 paradoxically reduced IFN-α responses of IPCs to CpG. This inhibition was consistent throughout many experiments, although the degree of inhibition varied depending on the donor, the expression level of NKp44, and the activation state of IPCs after isolation from PBMCs. The signaling mechanism of this NKp44-mediated inhibition of CpG-induced secretion of IFN-α is yet unknown. It has been shown that the C-type lectin BDCA-2, which also inhibits IFN-α responses to CpG, induces a strong Ca2+ mobilization.25 Thus, NKp44 may inhibit IPC function through DAP12 by triggering a cascade of protein tyrosine phosphorylation, which leads to activation of PLCγ and Ca2+ mobilization.42,43 This activating pathway may trigger the secretion of yet unidentified cytokines that inhibit IFN-α secretion. This possibility is corroborated by previous studies showing that crosslinking of certain activating receptors in monocytes or macrophages, such as the FcRs and immunoglobulin-like transcript receptor 1 (ILT1), promote secretion of IL-10, which inhibits release of IL-12.50,51,54 NKp44 and/or DAP12 may also inhibit IPCs by recruiting cytoplasmic protein tyrosine phosphatases or other inhibitory mediators. Tyrosine phosphatases are typically recruited through immunoreceptor tyrosine-based inhibitory motifs (ITIMs).55 Campbell et al32 have recently reported the presence of an ITIM-like sequence in the cytoplasmic region of NKp44, although they were unable to demonstrate an inhibitory function of NKp44 in NK cells. DAP12 does not contain ITIMs, but an ITAM that recruits protein tyrosine kinases.42,43 However, recent evidence indicates that in certain conditions, ITAMs can recruit tyrosine phosphatases instead of tyrosine kinases and mediate inhibition.56
In our study, we demonstrated that tonsil IPCs frequently colocalize with CD8 T cells and that, within CD8 T cells, a subset with a memory phenotype produces IL-3, which is required to induce NKp44 expression in IPCs. Thus, CD8 T cells may provide an important source of IL-3 for induction of NKp44 in IPCs in vivo. On the other hand, TLR ligands inhibited IL-3-mediated induction of NKp44. From these observations, we can propose the following model. Upon viral infection, NKp44- blood IPCs enter secondary lymphoid organs presumably through high endothelial venules.18,19 Here, activation of IPCs through TLRs prevents expression of NKp44 and induces secretion of type I IFNs, activating innate responses. The development of adaptive immunity reduces the viral load and, consequently, IPC stimulation through TLRs. Moreover, activated memory CD8 T cells secrete IL-3 and induce NKp44 on IPCs, thus effectively down-modulating the innate immune response. As NKp44 can bind viral components, such as hemagglutinins,35 ligation of NKp44 may further down-regulate innate responses by IPCs. Moreover, tumor microenvironment was shown to induce functional paralysis of tumor-associated IPCs.23,24 Thus, expression of yet unknown NKp44 ligands by tumor cells may provide a mechanism for inhibition of tumor-associated IPCs.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-12-4802.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Julia Klesney-Tait and Susan Gilfillan for helpful comments. We also thank Jackie Hughes and Bill Eades (High Speed Cell Sorter Core, Alvin J. Siteman Cancer Center, Washington University School of Medicine) for expert cell sorting and Dr Randall Clary (Children's Hospital, Washington University School of Medicine, St Louis) for providing tonsil specimens.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal