Abstract
Antiangiogenic intervention is known to inhibit tumor growth and dissemination by attacking the tumor's vascular supply. Here, we report that this was achieved for the first time using an oral DNA minigene vaccine against murine vascular endothelial growth factor receptor 2 (FLK-1), a self-antigen overexpressed on proliferating endothelial cells in the tumor vasculature. Moreover, we identified the first H-2Db–restricted epitope, FLK400 (VILT-NPISM), specifically recognized by cytotoxic T lymphocytes (CTLs). Such CTLs were capable of killing FLK-1+ endothelial cells, resulting in suppression of angiogenesis and long-lived tumor protection. The specificity of this immune response was indicated because the DNA vaccine encoding the entire FLK-1 gene also induced a FLK400-specific CTL response. This minigene vaccine strategy provides a more flexible alternative to whole-gene vaccination and facilitates in-depth mechanism studies to tailor DNA vaccines for optimal T-cell activation and tumor protection.
Introduction
Antiangiogenic intervention, which inhibits tumor growth by attacking the tumor's vascular supply, was pioneered by Folkman and colleagues,1-3 who established that angiogenesis has a central role in the invasion, growth, and metastasis of solid tumors.2,4 In fact, angiogenesis is a rate-limiting step in the development of tumors because tumor growth is generally limited to 1 to 2 mm3 in the absence of a blood supply,5 and beyond this minimum size, tumors often become necrotic and apoptotic.6
Vascular endothelial growth factor (VEGF) and its receptor tyrosine kinases play vital roles in angiogenesis.7,8 Expression of murine VEGF receptor 2 (VEGFR2, also known as FLK-1), which binds the 5 isomers of murine VEGF, is restricted to endothelial cells and is up-regulated once these cells proliferate during angiogenesis in the tumor vasculature.4,7,8 In fact, several approaches have been used to block FLK-1, including dominant-negative receptor mutants, germline disruption of VEGFR genes, monoclonal antibodies against VEGF, and a series of synthetic receptor tyrosine kinase inhibitors.9,10
We first reported on an alternative strategy, namely, an oral DNA vaccine encoding the entire FLK-1 gene, which prevented effective angiogenesis and inhibited tumor growth largely by CD8+ T cell-mediated immune responses. CD8+ cytotoxic T lymphocytes (CTLs) have the ability to specifically detect and kill antigen-bearing cells. They recognize antigens in the form of 8 to 10 amino acid long peptides, presented to T-cell receptors (TCRs) on the cell surface as complexes with major histocompatibility complex (MHC) class I molecules. These peptides, usually referred to as CTL epitopes, are generated in the cytosol of cells after proteolytic processing of antigen by the proteasome.11 One of the primary aims of tumor vaccines is to induce CD8+ CTL responses against such epitopes to eradicate tumors and prevent their relapse. The induction of a more effective antigen-specific immune response by DNA vaccines requires optimization of the vaccine design, including novel approaches for vaccine delivery and effective antigen processing. Such strategies include the use of an oral carrier system with a double-attenuated strain of Salmonella typhimurium (dam-;AroA-), which delivers the DNA to secondary lymphoid organs for subsequent transcription, translation, and antigen processing.12,13
The application of minigene vaccines provides an attractive approach because of their ease of synthesis and manipulation. Moreover, in contrast to vaccines encoding entire genes, minigene vaccines can induce immune responses directed against specific antigen epitopes while avoiding the interference of nonrelevant antigen epitopes. Consequently, such vaccines lend themselves to in-depth studies of immunologic mechanisms far more readily than DNA vaccines encoding entire genes. In this regard, several minigene strategies were reported to induce effective antitumor responses by an HLA-A2–restricted melan-A peptide analog epitope,14 an HLA-A2–restricted carcinoembryonic antigen epitope,15 and H-2Db/Kb-restricted melanoma antigen epitopes.13 Here, we identify the first H-2Db–restricted FLK-1 epitope and demonstrate that a novel minigene DNA vaccine protects mice from tumors of different origins by inducing a T cell-mediated suppression of tumor angiogenesis.
Materials and methods
Animals, bacterial strains, and cell lines
Male or female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed according to the National Institutes of Health Guides for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of The Scripps Research Institute.
The murine lung carcinoma cell line D121 was provided by Dr L. Eisenbach (Weizmann Institute of Science, Rehovot, Israel). The murine prostate cancer cell line RM9 was obtained from Dr T. C. Thompson (Baylor College of Medicine, Houston, TX). The murine breast cancer cell line EO771 was kindly made available by Dr D. Ross (University of Kentucky, Louisville, KY). Murine endothelial cell line, MS1, was purchased from the American Type Culture Collection (ATCC; Rockville, MD). All cell lines were cultured in Dulbecco modified Eagle medium (Invitrogen, Grand Island, NY), supplemented with 10% (vol/vol) fetal bovine serum.
The double-attenuated S typhimurium (AroA-, dam-) strain RE88 was kindly provided by the Remedyne Corporation (Santa Barbara, CA) and was transformed with DNA vaccine plasmids as previously described.16
Construction of expression vectors
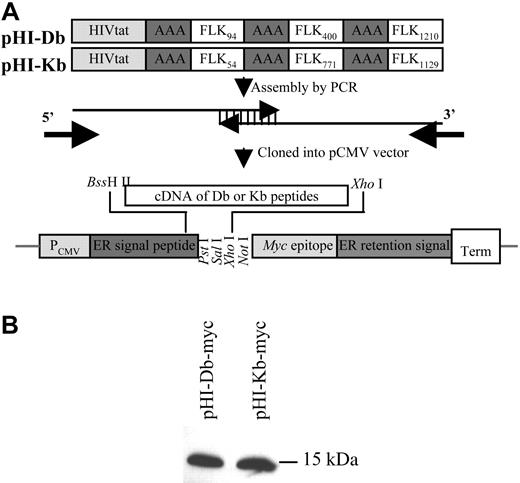
The expression vector pCMV/ER/Myc was purchased from Invitrogen (Carlsbad, CA). Vector construction is illustrated schematically in Figure 1A. The following expression vectors were constructed: pHI-myc, pHI-Db-myc, pHI-Kb-myc, where the HIVtat peptide (HI) represents RKKRRQRRR. The FLK94, FLK400, and FLK1210 peptides stand for RVVGNDTGA, VILTNPISM, and FHYDNTAGI, respectively. FLK54, FLK771, and FLK1129 peptides are designated for RGQRDLDWL, VIAMFFWLL, and TTPEMHYQTM, respectively. All peptides were engineered to be in-frame with the myc epitope. Constructs were confirmed by DNA sequencing at the Scripps Research Institute's Core Facility (La Jolla, CA). Peptide expression was demonstrated by Western blotting with monoclonal anti-myc antibody (Invitrogen, Carlsbad, CA). Once peptide expression was verified, a stop codon was introduced immediately in front of the myc epitope sequences. The resulting vectors, namely pHI, pHI-Db, and pHI-Kb, were verified by nucleotide sequencing and used to transform double-attenuated S typhimurium (dam-, AroA-) for immunization. The pCMV empty vector was also included in the experiments as a control.
Construction of the FLK-1 DNA minigene vaccine. (A) Minigenes encoding the HIVtat translocation peptide, a spacer (AAA), and murine FLK-1 H-2Db– and Kb-restricted epitopes (pHI-Db or pHI-Kb, respectively), were assembled by polymerase chain reaction (PCR) with overlapping oligonucleotides as templates. Db-restricted epitopes include FLK94: RVVGNDTGA; FLK400: VILTNPISM; FLK1210: FHYDNTAGI. Kb-restricted epitopes include FLK54: RGQRDLDWL; FLK771: VIAMFFWLL; FLK1129: TTPEMYQTM. The PCR fragments generated were cloned into a pCMV vector at C-terminal of ER signal peptide (endoplasmic reticulum) by using BssH II and XhoI restriction sites. (B) Proteins encoded by minigenes were expressed in mammalian cells. This was indicated when 293T cells were transfected with either pHI-Db-myc or pHI-Kb-myc for 24 hours, harvested, lysed, and analyzed by Western blotting with anti-myc monoclonal antibody.
Construction of the FLK-1 DNA minigene vaccine. (A) Minigenes encoding the HIVtat translocation peptide, a spacer (AAA), and murine FLK-1 H-2Db– and Kb-restricted epitopes (pHI-Db or pHI-Kb, respectively), were assembled by polymerase chain reaction (PCR) with overlapping oligonucleotides as templates. Db-restricted epitopes include FLK94: RVVGNDTGA; FLK400: VILTNPISM; FLK1210: FHYDNTAGI. Kb-restricted epitopes include FLK54: RGQRDLDWL; FLK771: VIAMFFWLL; FLK1129: TTPEMYQTM. The PCR fragments generated were cloned into a pCMV vector at C-terminal of ER signal peptide (endoplasmic reticulum) by using BssH II and XhoI restriction sites. (B) Proteins encoded by minigenes were expressed in mammalian cells. This was indicated when 293T cells were transfected with either pHI-Db-myc or pHI-Kb-myc for 24 hours, harvested, lysed, and analyzed by Western blotting with anti-myc monoclonal antibody.
Peptide synthesis
All peptides were synthesized with more than 95% purity by highperformance liquid chromatography (HPLC) by Multiple Peptide Systems (San Diego, CA).
Oral immunization and tumor-cell challenge
Groups of C57BL/6J mice were immunized 3 times at 1-week intervals by gavage with 100 μL phosphate-buffered saline (PBS) containing approximately 5 × 108 double-attenuated S typhimurium harboring either pCMV, pHI, pHI-Db, or pHI-Kb plasmids. Mice were challenged intravenously with different carcinoma cells 2 weeks after the last immunization.
Cytotoxicity and ELISPOT assays and in vivo depletion
Cytotoxicity was measured by a standard 51Cr-release assay as previously described.15 The percentage of specific target cell lysis was calculated by the formula [(E-S)/(T-S)] × 100, where E is the average experimental release, S the average spontaneous release, and T the average total release.
Enzyme-linked immunospot (ELISPOT) assays were performed with an ELISPOT kit (PharMingen, La Jolla, CA) according to the instructions provided by the manufacturer.
In vivo depletion was performed on vaccinated mice by intraperitoneal injection of anti-CD4 antibody (GK1.5, 0.4 mg/mouse) or anti-CD8 antibody (2.43, at 0.6 mg/mouse) 1 day before tumor challenge and repeated weekly.
Evaluation of antiangiogenic effects
Two weeks after the last vaccination, mice were given subcutaneous injections in the sternal region with 400 μL growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) containing 400 ng/mL basic fibroblast growth factor (PeproTech, Rocky Hill, NJ). In all mice, the endothelium was stained 6 days later by intravenous injection of 200 μL fluorescent Bandeiraea simplicifolia lectin I, isolectin B4 at 0.1 mg/mL (Vector Laboratories, Burlingame, CA). Fifteen minutes later, Matrigel plugs were excised and evaluated by confocal microscopy (Axiovert 100TV microscope; Carl Zeiss, Oberkochen, Germany; 40×/1.3 NA objective; and SPOT camera and software), and then lectin-fluorescein isothiocyanate (FITC) was extracted with RIPA lysis buffer (0.15 mM NaCl/0.05 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% sodium dodecyl sulfate) from 100-μg Matrigel plugs to be quantified by fluorometry at 490 nm.
Statistical analysis
The statistical significance of differential findings between experimental groups and controls was determined by the Student t test. Findings were regarded as significant when 2-tailed P was less than .05.
Results
Minigenes encoded by expression vectors are expressed in mammalian cells
We previously demonstrated that a DNA vaccine encoding the entire murine FLK-1 gene effectively induced CD8+ T cell-mediated antiangiogenesis that protected mice from tumor-cell challenge.17 Here, a minigene approach was adopted to identify the specific CTL epitopes involved to conduct in-depth mechanistic studies and to test our hypothesis that vaccination with such epitopes can induce similar antiangiogenic responses as the whole-gene vaccine. To this end, 3 peptides were included in H-2Db– or H-2Kb–restricted minigenes based on the binding predicted for these MHC class I molecules by the HLA Peptide Binding Predictions program provided by the BioInformatics & Molecular Analysis Section (BIMAS) of the National Institutes of Health (NIH), website: http://bimas.dcrt.nih.gov/molbio/hla_bind/.
Expression vectors were constructed based on the backbone of pCMV/ER/Myc (Figure 1A). A HIVtat peptide (RKKRRQRRR), one of the commonly used membrane-translocating peptides,18-20 is also included in our minigene vaccine to facilitate the delivery of the encoded peptides as previously demonstrated.15,20 After transfection of 293T cells with either pHI-myc, pHI-Db-myc, or pHI-Kb-myc, correct expression of these constructs was demonstrated by Western blotting, which revealed single bands with the expected molecular mass of 15 kDka (Figure 1B). The mature peptides did not contain the myc epitope because the vaccine vectors pHI, pHI-Db, and pHI-Kb were generated by introducing a stop codon immediately downstream from the peptide-coding sequences. The correct vector constructs were confirmed by DNA sequencing. The empty pCMV vector was also included for control purposes.
The pHI-Db minigene vaccine protects mice against tumors of different origin by inducing immune responses that suppress tumor angiogenesis
Initially, we tested the minigene DNA vaccines in a prophylactic lung cancer model, where mice were first vaccinated with the minigene vaccines and then challenged intravenously with D121 lung carcinoma cells. In this case, the pHI-Db minigene elicited the best tumor protection with 62.5% of mice surviving 75 days after tumor cell challenge (Figure 2A). In contrast, none of the mice in the pCMV control group survived and the pHI or pHI-Kb vaccines induced only minimal tumor protection with 25% of the mice surviving 75 days after tumor challenge (Figure 2A).
To verify that the minigene vaccine effectively protects mice from tumors of different origins because it was designed for antiangiogenesis purposes, the vaccine efficacy was also tested in a RM9 prostate carcinoma model. In this case, the pHI-Db minigene also protected the mice from RM9 tumor cell challenge (Figure 2B), suggesting that the pHI-Db vaccine induces suppression of metastases independent of the tumor type.
In vivo depletion assays were performed to identify the cell population responsible for the tumor protection effects. Depletion of CD8 cells completely abrogated the vaccine-induced protection, whereas the depletion of CD4 cells moderately enhanced the protection against tumor challenge (Figure 2C), suggesting the CD8 T cells are the major effectors.
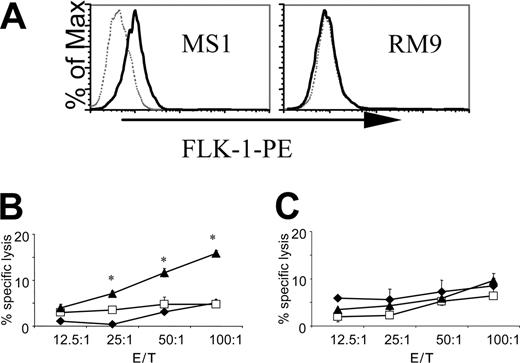
The specificity of the CTL responses was further investigated in 51Cr-release assays. The pHI-Db vaccine induced a specific cytotoxic response against a FLK-1+ (Figure 3A) endothelial cell line MS1 (Figure 3B), but not against FLK-1- (Figure 3A) RM9 prostate carcinoma cells (Figure 3C). These data suggest that the cytotoxic response induced by the pHI-Db vaccine was indeed directed against endothelial cells, presumably specific for FLK-1, rather than against tumor cells. This finding suggests that the pHI-Db minigene vaccine would induce an antiangiogenic response in vivo.
DNA minigene vaccine pHI-Db protects mice from tumor challenges. (A) Groups of C57BL/6 mice (n = 8) were immunized 3 times at 1-week intervals with attenuated S typhimurium harboring the vectors indicated. Empty diamonds indicate the pCMV control group; shaded squares show the pHI control group; red triangles depict the pHI-Db group; and green circles stand for the pHI-Kb groups. Mice were challenged intravenously 2 weeks after the last immunization with 1 × 105 D121 lung carcinoma cells and monitored for survival until 75 days after tumor challenge. *P < .02 compared to pCMV control group. (B) Vaccinated mice were challenged intravenously 2 weeks after the last immunization with 1 × 105 RM9 prostate carcinoma cells. Mice were killed 28 days after tumor cell challenge and lung weights assessed. The top panel depicts representative lungs and the bottom panel shows average lung weights. Normal lung weight is about 0.2 g. *P < .001 and .001 compared to pCMV and pHI, respectively. Experiments were repeated twice with similar results. (C) In vivo depletion was performed as described in “Materials and methods.” Mice were challenged intravenously with 2.5 × 105 tumor cells and killed 23 days thereafter. Error bars indicate standard deviation (SD).
DNA minigene vaccine pHI-Db protects mice from tumor challenges. (A) Groups of C57BL/6 mice (n = 8) were immunized 3 times at 1-week intervals with attenuated S typhimurium harboring the vectors indicated. Empty diamonds indicate the pCMV control group; shaded squares show the pHI control group; red triangles depict the pHI-Db group; and green circles stand for the pHI-Kb groups. Mice were challenged intravenously 2 weeks after the last immunization with 1 × 105 D121 lung carcinoma cells and monitored for survival until 75 days after tumor challenge. *P < .02 compared to pCMV control group. (B) Vaccinated mice were challenged intravenously 2 weeks after the last immunization with 1 × 105 RM9 prostate carcinoma cells. Mice were killed 28 days after tumor cell challenge and lung weights assessed. The top panel depicts representative lungs and the bottom panel shows average lung weights. Normal lung weight is about 0.2 g. *P < .001 and .001 compared to pCMV and pHI, respectively. Experiments were repeated twice with similar results. (C) In vivo depletion was performed as described in “Materials and methods.” Mice were challenged intravenously with 2.5 × 105 tumor cells and killed 23 days thereafter. Error bars indicate standard deviation (SD).
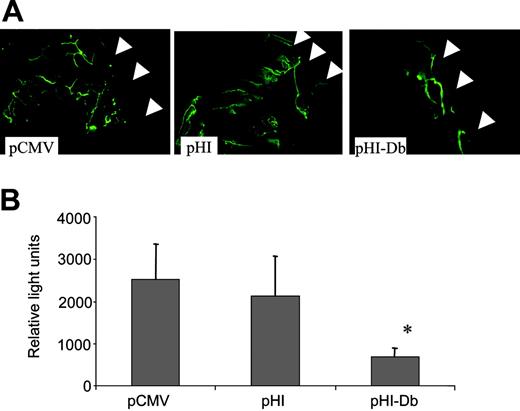
We further proved this hypothesis by performing Matrigel assays, which indicated that vaccination with minigene pHI-Db indeed suppressed vascularization. This was clearly demonstrated by reduced blood vessel formation observed in representative Matrigel plugs after in vivo staining of endothelium with FITC-conjugated lectin (Figure 4A). This difference in vessel formation was also demonstrated quantitatively by measuring the average relative fluorescence (Figure 4B). Taken together, these findings demonstrate that the pHI-Db minigene vaccine induced antiangiogenic effects, which protected mice from challenge with tumor cells of different origin.
The pHI-Db vaccine induces a FLK400-specific immune response
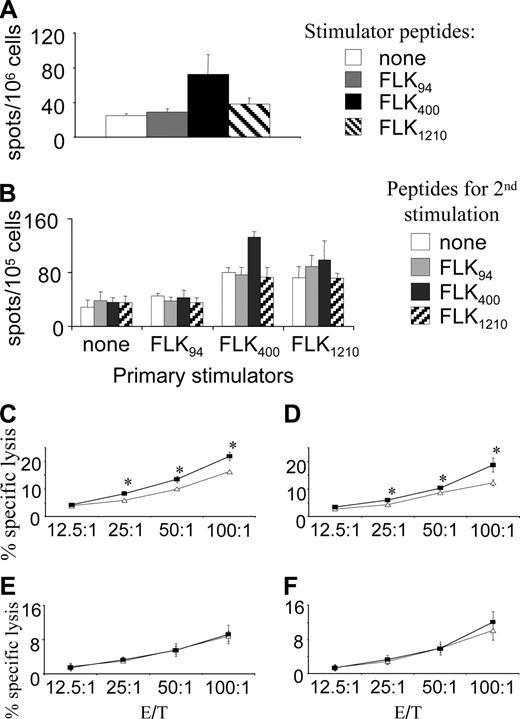
To evaluate each of the 3 peptides encoded by the pHI-Db minigene, splenocytes isolated from mice immunized with pHI-Db were analyzed by ELISPOT assays using individual synthetic peptides as stimulators. A specific FLK400 response was detected only in the pHI-Db–vaccinated group of mice (Figure 5A), whereas no significant FLK94- or FLK1210-specific responses were found in any of the experimental groups of mice, suggesting that FLK400 is the major epitope recognized by CTL effector cells.
The DNA minigene vaccine pHI-Db induces specific CTL killing of FLK-1+ endothelial cells but not of FLK-1- tumor cells. (A) Surface expression of FLK-1 by endothelial cell line MS1 and murine prostate carcinoma cell line RM9. Gray dotted lines indicate phycoerythrin (PE)–conjugated isotype control antibody; black solid lines, PE-conjugated anti–FLK-1. Groups of immunized C57BL/6J mice (n = 4) were killed 2 weeks after the last immunization and isolated splenocytes were stimulated with irradiated MS1 cells for 5 days. Thereafter, cytotoxicity assays were performed with MS1 (B) or RM9 (C) serving as target cells. ♦, pCMV control group;□, pHI group; ▴, pHI-Db group. Experiments were repeated 3 times with similar results. *P < 0.001 compared with pCMV or pHI control groups. E/T indicates the ratio of effector and target cells.
The DNA minigene vaccine pHI-Db induces specific CTL killing of FLK-1+ endothelial cells but not of FLK-1- tumor cells. (A) Surface expression of FLK-1 by endothelial cell line MS1 and murine prostate carcinoma cell line RM9. Gray dotted lines indicate phycoerythrin (PE)–conjugated isotype control antibody; black solid lines, PE-conjugated anti–FLK-1. Groups of immunized C57BL/6J mice (n = 4) were killed 2 weeks after the last immunization and isolated splenocytes were stimulated with irradiated MS1 cells for 5 days. Thereafter, cytotoxicity assays were performed with MS1 (B) or RM9 (C) serving as target cells. ♦, pCMV control group;□, pHI group; ▴, pHI-Db group. Experiments were repeated 3 times with similar results. *P < 0.001 compared with pCMV or pHI control groups. E/T indicates the ratio of effector and target cells.
To further strengthen this notion, splenocytes from vaccinated mice were stimulated with synthetic peptides for 5 days and tested against MS1 and RM9 target cells in cytotoxicity assays. Only FLK400-stimulated splenocytes exhibited specific cytotoxic killing against FLK-1+ MS1 target cells, but revealed almost no killing of FLK-1-RM9 tumor cells (Figure 5B). Splenocytes stimulated with FLK94 induced low levels of MS1-specific killing (Figure 5C), whereas FLK1210-stimulated splenocytes mainly displayed low levels of nonspecific killing (Figure 5D), confirming the dominance of the FLK400 epitope within the minigene vaccine.
When peptide-stimulated splenocytes isolated from pHI-Db–vaccinated mice were restimulated twice more in vitro with irradiated, peptide-loaded splenocytes every 7 days, and then tested again for their cytotoxicity, only those cells restimulated with FLK400-loaded splenocytes showed greatly enhanced cytotoxicity, resulting in a higher percent specific killing at a much lower effector-target (E/T) ratio (Figure 5E). In contrast, such restimulation with FLK94-loaded splenocytes resulted in a lower level of MS1-specific killing (Figure 5F), whereas cells restimulated with FLK1210-loaded splenocytes failed to induce any significant killing (Figure 5G). Taken together, these findings suggest that restimulation with FLK400-loaded splenocytes enriches the CTL population that specifically targets FLK-1+ endothelial cells.
The pHI-Db minigene vaccine induced suppression of angiogenesis determined by Matrigel assay. Quantification of vessel growth and staining of endothelium was determined by fluorometry and confocal microscopy, respectively, using FITC-labeled isolectin B4. (A) Representative Matrigel plugs were examined by confocal microscopy (original magnification × 200; 1.3 NA). The arrows indicate the borders of the Matrigel plug. (B) The average fluorescence of Matrigel plugs from each group of mice is depicted by the bar graph (n = 4; mean + SD). *P < .05 pCMV or pHI groups. The experiment was repeated once with similar results.
The pHI-Db minigene vaccine induced suppression of angiogenesis determined by Matrigel assay. Quantification of vessel growth and staining of endothelium was determined by fluorometry and confocal microscopy, respectively, using FITC-labeled isolectin B4. (A) Representative Matrigel plugs were examined by confocal microscopy (original magnification × 200; 1.3 NA). The arrows indicate the borders of the Matrigel plug. (B) The average fluorescence of Matrigel plugs from each group of mice is depicted by the bar graph (n = 4; mean + SD). *P < .05 pCMV or pHI groups. The experiment was repeated once with similar results.
The pHI-Db minigene vaccine induces an H-2Db–restricted, FLK400–specific response. Groups of vaccinated C57BL/6 mice (n = 4) were killed 2 weeks after the last immunization. (A) ELISPOT assays were performed on splenocytes isolated by using either no stimulator or synthetic peptides FLK94 (25 μg/mL), FLK400 (10 μg/mL), or FLK1210 (25 μg/mL) as stimulators. (B-D) Isolated splenocytes were stimulated with FLK400 (B), FLK94 (C), or FLK1210 (D) peptides for 5 days. Thereafter cytotoxicity assays were performed with MS1 (♦) or RM9 (○) serving as target cells. Experiment was repeated twice with 2 μg/mL or 10 μg/mL stimulator peptides and with similar results. (E-G) Splenocytes isolated from pHI-Db–vaccinated mice were stimulated with FLK400 (E), FLK94 (F), or FLK1210 (G) peptides for 7 days, and restimulated twice weekly with irradiated FLK400-loaded (E), FLK94-loaded (F), or FLK1210-loaded (G) splenocytes from normal C57BL/6 mice. Thereafter cytotoxicity assays were performed with MS1 (♦) or RM9 (○) serving as target cells. Error bars indicate SD.
The pHI-Db minigene vaccine induces an H-2Db–restricted, FLK400–specific response. Groups of vaccinated C57BL/6 mice (n = 4) were killed 2 weeks after the last immunization. (A) ELISPOT assays were performed on splenocytes isolated by using either no stimulator or synthetic peptides FLK94 (25 μg/mL), FLK400 (10 μg/mL), or FLK1210 (25 μg/mL) as stimulators. (B-D) Isolated splenocytes were stimulated with FLK400 (B), FLK94 (C), or FLK1210 (D) peptides for 5 days. Thereafter cytotoxicity assays were performed with MS1 (♦) or RM9 (○) serving as target cells. Experiment was repeated twice with 2 μg/mL or 10 μg/mL stimulator peptides and with similar results. (E-G) Splenocytes isolated from pHI-Db–vaccinated mice were stimulated with FLK400 (E), FLK94 (F), or FLK1210 (G) peptides for 7 days, and restimulated twice weekly with irradiated FLK400-loaded (E), FLK94-loaded (F), or FLK1210-loaded (G) splenocytes from normal C57BL/6 mice. Thereafter cytotoxicity assays were performed with MS1 (♦) or RM9 (○) serving as target cells. Error bars indicate SD.
To validate our hypothesis that the FLK400-specific immune response contributed to the antitumor effects elicited by the pHI-Db vaccine, we assessed the tumor protective ability of a minigene vaccine encoding only FLK400 in the absence of FLK94 and FLK1210 and compared it with the effect of the pHI-Db minigene vaccine in an EO771 breast carcinoma model. EO771 cells do not express FLK-1, but express surface H-2Db as detected by flow cytometry (Figure 6A). In fact, both pHI-Db and pHI-FLK400 minigene vaccines significantly protected the mice against EO771 tumor cell challenge and to an extent comparable to the protection induced by a DNA vaccine encoding the entire FLK-1 gene (Figure 6B). The pHI-Db vaccine also achieved similar efficacy as FLK-1 whole-gene vaccine in RM9 prostate and D121 lung carcinoma models (data not shown).
Long-term protection was established by the pHI-Db minigene vaccine; at 10 months after their last vaccination, pHI-Db–vaccinated mice showed significantly reduced lung metastases after intravenous challenging with EO771 breast carcinoma cells (Figure 6C), and FLK400-specific T cells could still be detected in the spleen of these mice (Figure 6D).
DNA minigene vaccine pHI-FLK400 suppresses tumor metastasis and the immunity induced by pHI-Db is long-lasting. (A) Surface expression of FLK-1 (black dotted line) or H-2Db (black solid line) of EO771 breast carcinoma cells. Isotype control is shown with gray shaded curve. (B) Groups of vaccinated mice (n = 8) were challenged intravenously with 2 × 105 EO771 breast carcinoma cells 2 weeks after the last vaccination. Mice were killed 21 days later and lung weights assessed. *P < .01 compared to pCMV and pHI; **P < .005 compared to pCMV and pHI; ***P < .05, P < .05, P > .05, and P > .05 compared to pCMV, pHI, pHI-Db, and pHI-FLK400, respectively. (C) Groups of vaccinated mice (n = 4) were challenged intravenously with 2 × 105 EO771 breast carcinoma cells 10 months after the last vaccination. *P < .01 compared to the pCMV control group. (D) ELISPOT assay performed with splenocytes isolated from mice (n = 4) 10 months after pHI-Db vaccination in the presence or absence of FLK400 peptide. *P < .02 compared to culture medium alone. Error bars indicate SD.
DNA minigene vaccine pHI-FLK400 suppresses tumor metastasis and the immunity induced by pHI-Db is long-lasting. (A) Surface expression of FLK-1 (black dotted line) or H-2Db (black solid line) of EO771 breast carcinoma cells. Isotype control is shown with gray shaded curve. (B) Groups of vaccinated mice (n = 8) were challenged intravenously with 2 × 105 EO771 breast carcinoma cells 2 weeks after the last vaccination. Mice were killed 21 days later and lung weights assessed. *P < .01 compared to pCMV and pHI; **P < .005 compared to pCMV and pHI; ***P < .05, P < .05, P > .05, and P > .05 compared to pCMV, pHI, pHI-Db, and pHI-FLK400, respectively. (C) Groups of vaccinated mice (n = 4) were challenged intravenously with 2 × 105 EO771 breast carcinoma cells 10 months after the last vaccination. *P < .01 compared to the pCMV control group. (D) ELISPOT assay performed with splenocytes isolated from mice (n = 4) 10 months after pHI-Db vaccination in the presence or absence of FLK400 peptide. *P < .02 compared to culture medium alone. Error bars indicate SD.
The DNA vaccine encoding the entire FLK-1 gene induces a FLK400-specific CTL response
We confirmed that FLK400 is indeed a true FLK-1 epitope by demonstrating that the FLK400-specific response was induced by a DNA vaccine encoding the entire FLK-1 gene. Such responses were detected in splenocytes freshly isolated from pFLK-1–vaccinated mice as demonstrated by ELISPOT assays (Figure 7A). These splenocytes maintained the specificity of the responses after in vitro stimulation with FLK400 peptides (Figure 7B). However, controls were negative because stimulation with FLK94 had no effect when compared to nonstimulated cells, and only nonspecific activation resulted from stimulation with FLK1210 (Figure 7B). Moreover, splenocytes isolated from pFLK-1–vaccinated mice also displayed preferential cytotoxic killing of EO771 tumor cells loaded with FLK400 as compared to the killing of unloaded EO771 cells (Figure 7C). Similar results were also observed in pHI-Db–vaccinated mice (Figure 7D), which were used as a positive control. The killing of EO771 or FLK400–loaded EO771 tumor cells was largely indistinguishable in pCMV or pHI control groups (Figure 7E-F). Taken together, these findings prove that the DNA vaccine encoding the entire FLK-1 gene was capable of inducing a FLK400-specific immune response.
Discussion
There are several advantages in targeting CD8+ T cells to proliferating endothelial cells in the tumor vasculature rather than directly to tumor cells. First, endothelial cells are genetically stable and do not down-regulate MHC class I antigen, an event that frequently occurs in solid human tumors and severely impairs T cell-mediated antitumor responses.21 Second, immune suppression triggered by tumor cells in the tumor microenvironment can also be avoided by this approach. Third, the therapeutic target is tumor-independent, thus killing of proliferating endothelial cells in the tumor microenvironment can be effective against a variety of malignancies. Finally, proliferating endothelial cells are readily available to lymphocytes in the bloodstream and consequently CD8+ T cells can reach the target tissues unimpaired by anatomic barriers such as the blood-brain barrier or encapsulation of tumor tissues.22
We took advantage of this approach as indicated by prior data from our laboratory, which demonstrated that an oral DNA vaccine encoding autologous FLK-1 prevents effective tumor angiogenesis and inhibits tumor growth and metastasis.17 However, the full-length FLK-1 gene used in these studies was about 4 kb, encoding for a protein of approximately 190 kDa,23 with its human counterpart being similar in size.24 Because of the large size of such genes, mutations are likely to be introduced during vaccine production and in the host once plasmids encoding these genes are delivered by S typhimurium to secondary lymphoid tissues such as Peyer patches. Thus, safety and quality control issues will be of great concern before such approaches become clinically applicable. For this and other reasons, minigene vaccine approaches were used to create a simpler and more defined vaccine, which also facilitates the identification of specific FLK-1 epitopes recognized by CTLs for in-depth studies on vaccine mechanisms and efficacy. Here, we demonstrated for the first time that a FLK-1–based minigene, pHI-Db, induced CD8+ T cell-mediated suppression of tumor angiogenesis and protected mice from carcinomas of different origins such as breast, prostate, and lung. Importantly, we identified FLK400 to be the major epitope recognized by CTLs, which mediated this tumor-protective effect. The pHI-Db vaccine-induced FLK400-specific response was long-lasting because we still detected such specific T cells in the spleen 10 months after the vaccination. These FLK400-specific T cells were very likely present in the periphery. In time of tumor challenge or relapse, on encounter of antigen, namely, FLK400 presented by a MHC class I molecule in the tumor microenvironment, these T cells were activated and proliferated and executed cytotoxic function. This can explain the long-term protection achieved by the minigene vaccine. Significantly, minigene vaccines pHI-Db and pHI-FLK400 showed an antitumor efficacy that was similar to that achieved by the DNA vaccine encoding the entire FLK-1 gene, thereby indicating that these minigene vaccines are promising alternatives.
DNA vaccine encoding full-length FLK-1 induces FLK400-specific responses. (A) ELISPOT assays performed with freshly isolated splenocytes from pFLK-1–vaccinated mice and stimulator with FLK94, FLK400, FLK1210, or no peptide. (B) Splenocytes isolated from pFLK-1–vaccinated mice were first stimulated in vitro for 5 days with peptides indicated by “primary stimulators,” then restimulated in ELISPOT assays. Stimulators used in such ELISPOT assays are unloaded, FLK94-loaded, FLK400-loaded, or FLK1210-loaded splenocytes from normal C57BL/6 mice. Splenocytes from pFLK-1 (C), pHI-Db (D), pCMV (E), and pHI (F) groups of mice were stimulated with irradiated MS1 cells for 5 days, and cytotoxicity assays were performed against unloaded (▵) or FLK400-loaded (▪) EO771 target cells. *P < .02 compared to unloaded EO771 target cells. The killing of FLK94-loaded or FLK1210-loaded EO771 cells was indistinguishable from that of unloaded EO771 cells (data not shown). This experiment was repeated once with similar results (data not shown). Error bars indicate SD.
DNA vaccine encoding full-length FLK-1 induces FLK400-specific responses. (A) ELISPOT assays performed with freshly isolated splenocytes from pFLK-1–vaccinated mice and stimulator with FLK94, FLK400, FLK1210, or no peptide. (B) Splenocytes isolated from pFLK-1–vaccinated mice were first stimulated in vitro for 5 days with peptides indicated by “primary stimulators,” then restimulated in ELISPOT assays. Stimulators used in such ELISPOT assays are unloaded, FLK94-loaded, FLK400-loaded, or FLK1210-loaded splenocytes from normal C57BL/6 mice. Splenocytes from pFLK-1 (C), pHI-Db (D), pCMV (E), and pHI (F) groups of mice were stimulated with irradiated MS1 cells for 5 days, and cytotoxicity assays were performed against unloaded (▵) or FLK400-loaded (▪) EO771 target cells. *P < .02 compared to unloaded EO771 target cells. The killing of FLK94-loaded or FLK1210-loaded EO771 cells was indistinguishable from that of unloaded EO771 cells (data not shown). This experiment was repeated once with similar results (data not shown). Error bars indicate SD.
It was previously reported that an orally delivered DNA minigene vaccine encoding murine melanoma peptide epitopes required poly-ubiquitination to lead to optimal antigen processing, which evoked a potent immune response.13 Likewise, in our current experiments, antigen processing proved to be important because a minigene vaccine encoding the FLK400 peptide was most effective in protecting mice from tumor cell challenges when it also encoded the HIVtat peptide (data not shown). The rationale for using this HIVtat peptide in our minigene vaccine is based on the fact that it is one of the commonly used membrane-translocating peptides. Such translocating peptides are able to transport antigen peptides into the endoplasmic reticulum, in a transport-associated protein (TAP)–independent manner, where they then can be effectively processed and trimmed to become CTL epitopes.18-20 Previously reported data from our laboratory also showed that the inclusion of this peptide in carcinoembryonic antigen-based DNA minigene vaccines induced effective CTL responses against the encoded CEA epitope.15
It is generally believed that CD4+ T-cell help is required to overcome tolerance to effectively generate immune responses against weak self antigen-like tumor-associated antigen, and in most cases CD4+ T cell help is required for the generation of long-lived, functional memory CD8+ T cells.25-28 In this regard, strategies such as fusion of tumor antigen to CD4+ stimulators to provide cognate CD4+ help in DNA vaccines have been reported.29 The aim of our minigene vaccine strategy was to specifically activate antigen-specific CD8+ T cells without providing a particular epitope for CD4 T cells. In fact, this strategy proved to be successful in inducing an effective CD8 immune response that efficiently induced long-term protection of mice from tumor cell challenges. It is possible that the administration of S typhimurium could induce activation of CD4+ T cells that are specific for epitopes on these bacteria and such CD4+ T cells could then provide the necessary help. In this regard, we found a slight up-regulation of activation markers on CD4+ T cells in Peyer patches after administration of attenuated S typhimurium harboring empty vector as compared to the PBS control group (data not shown). It is also possible that the attenuated S typhimurium could elicit danger signals,12 which directly activate antigen-presenting cells (APCs) and bypass the need for CD4+ T cell-mediated licensing of APCs.30 Moreover, it was recently suggested that a CD4+ T-cell population, without activation, can provide an antigen nonspecific maintenance function for CD8+ T-cell memory.30 Consequently, the antigen-specific activation of CD4+ T cells may not be crucial for the generation and maintenance of CD8+ T-cell memory. The exact nature of CD4+ T-cell help is difficult to demonstrate in our experimental system, because CD4+ T cells also contribute to the negative control. Such an effect is presumably mediated by regulatory T cells, which inhibit immune responses to ensure self-tolerance.31 In our experimental systems, the depletion of CD4+ T cells in vaccinated mice resulted in improved protection against tumor challenge.
In summary, we reported here the first antiangiogenic minigene vaccine and identified the initial H-2Db-restricted FLK-1 epitope-FLK400 (VILTNPISM). Importantly, the pHI-Db and pHI-FLK400 minigene vaccines achieved similar efficacy as the DNA vaccine encoding the entire FLK-1 gene, and thereby provided a safer, simpler, and more flexible alternative to the whole-gene vaccine, while adding a new dimension to antiangiogenic interventions in cancer immunotherapy.
Prepublished online as Blood First Edition Paper, May 26, 2005; DOI 10.1182/blood-2005-03-0969.
Supported by Department of Defense grants DAMD17-02-10562 and DAMD17-02-1-0137 (R.X.), grant 12RT-0002 from the California Tobacco-Related Disease Research Program (R.A.R.), and E. Merck, Darmstadt-Lexigen Research Center (Billerica, MA) grant SFP1330 (R.A.R.). H.Z. is currently a fellow of The Susan G. Komen Breast Cancer Foundation.
H.Z. designed and performed the research, analyzed data, and wrote this manuscript. Y.L., M.M., and N.M. contributed considerably to the design and performance of the study. The contributions of R.A.R. and R.X. included experimental designs and manuscript preparation.
R.A.R. is a consultant for E. Merck, Darmstadt-Lexigen Research Center, Billerica, MA, and received partial funding for this research from the company.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Margaret Hogan of the University of California San Diego, Carrie Dolman, and Dorothy Markowitz for excellent technical assistance, and Dr Charles D. Kaplan and Kathy Cairns for editorial assistance with manuscript preparation. This is manuscript no. 17266-IMM from The Scripps Research Institute.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal