Abstract
Globally suppressed T-cell function has been described in many patients with cancer to be a major hurdle for the development of clinically efficient cancer immunotherapy. Inhibition of antitumor immune responses has been mainly linked to inhibitory factors present in cancer patients. More recently, increased frequencies of CD4+CD25hi regulatory T cells (Treg cells) have been described as an additional mechanism reducing immunity. We assessed 73 patients with B-cell chronic lymphocytic leukemia (CLL) and 42 healthy controls and demonstrated significantly increased frequencies of cytotoxic T lymphocyte-associated protein 4 (CTLA4+)–, Forkhead box P3 (FOXP3+)–, glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR+)–, CD62L+–, transforming growth factor β1 (TGF-β1+)–, interleukin 10 (IL-10+)–Treg cells in patients with CLL, with highest frequencies in untreated or progressing patients presenting with extended disease. Most surprisingly, in the majority of patients with CLL treated with fludarabine-containing therapy regimens the inhibitory function of Treg cells was decreased or even abrogated. In addition, frequencies of Treg cells were significantly decreased after therapy with fludarabine. In light of similar findings for cyclophosphamide the combination of fludarabine and cyclophosphamide might be further exploited in strategies reducing immunosuppression prior to cancer immunotherapy.
Introduction
Human and murine CD4+CD25+ T cells contain cells that suppress antigen-specific T-cell immune responses.1-5 These naturally occurring regulatory CD4+CD25+ T cells originate from the thymus and play a central role in the maintenance of peripheral tolerance by suppression of autoreactive T-cell populations. In murine models, regulatory T cells (Treg cells) prevent autoimmune and inflammatory diseases1,6,7 and inhibit antitumor immune responses.8-12 Although a truly unique marker for Treg cells is still not available, several molecules have been associated with these cells including cytotoxic T lymphocyte-associated protein 4 (CTLA4),13-16 glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR, TNFRSF18),17,18 Forkhead box P3 (FOXP3),19-21 L-selectin (CD62L, SELL),22,23 and OX40 antigen (CD134, TNFRSF4).23,24
In humans, Treg cells are enriched within the CD4+CD25hi population, whereas CD4+CD25lo T cells represent mainly previously activated T helper cells.25 These CD4+CD25hi Treg cells inhibit proliferation and cytokine release by conventional CD4+CD25- T cells.26 Decrease of these cells was found in patients with autoimmune diseases,27-31 whereas an increase of Treg cells in patients after allogeneic bone marrow transplantation was associated with a reduced graft-versus-host disease.32-35 In patients with malignant melanoma,36 Hodgkin lymphoma,37 or ovarian,38,39 gastric,40,41 lung,39,42 breast,43,44 and pancreatic cancer43 inhibitory CD4+CD25+ T cells are also increased. In an elegant study, Curiel et al38 demonstrated that functional Treg cells were enriched in ascites from women with ovarian cancer, migrated toward CCL22 expressed by tumor cells and tumor-associated macrophages, and specifically inhibited antitumor immunity. Moreover, within this setting, the increase of Treg cells predicted poor survival.38 Only recently, studies assessing a potential influence of chemotherapy on Treg cells have been initiated. In mice, low-dose cyclophosphamide decreased the number of Treg cells.45
Based on these observations we were interested in understanding whether CD4+CD25hi T cells are also increased and possess inhibitory capacities in B-cell chronic lymphocytic leukemia (CLL) and, if so, to assess the frequency and function in the context of stage of disease and prior therapy. CLL, the most common type of leukemia in the Western hemisphere,46 is characterized by clonal proliferation and accumulation of neoplastic B lymphocytes.47-49 CLL is a particularly interesting model because it is frequently associated with clinically manifest immune defects, suggesting an underlying immune dysregulation.50-56 In fact, decreased T-cell responses to mitogenic and T-cell receptor-mediated stimulations have been described in patients with CLL57,58 ; however, the accounting cellular and molecular mechanisms are still unclear.59 Moreover, chemotherapy applied to patients with CLL includes drugs such as fludarabine, cyclophosphamide, or alemtuzumab, which are cytotoxic for T cells and have been shown to alter ratios of CD4+ to CD8+ T cells in vitro.60
Overall, we assessed Treg cells in 73 patients with CLL and 42 healthy individuals. In addition to presenting clear evidence of a stage-dependent increase of Treg cells in this leukemia, we observed, for the first time in humans, a significant impact of chemotherapy, particularly fludarabine-based therapy regimens, on the frequency and function of CD4+CD25hi T cells.
Patients, materials, and methods
Patients and clinical parameters
Peripheral blood from 42 healthy individuals and 73 patients with CLL was obtained following approval by our institutional review board (University Ethics Committee, Cologne, Germany), including 35 patients in whom we assessed blood samples at least at 2 different time points. Informed consent for blood donations was obtained, per the Declaration of Helsinki, from all volunteers. Peripheral blood mononuclear cells (PBMCs) from healthy donors (controls) and patients meeting diagnostic criteria for CLL were obtained from peripheral blood using Ficoll/Hypaque (Amersham, Uppsala, Sweden) density centrifugation and stored in liquid nitrogen until further use. Patients included for phenotypical or functional analysis were either untreated or had not received cytoreductive treatment for a period of at least 1 month before investigation. Staging was performed according to the Binet classification for CLL. The mean age of the patients with CLL at first analysis was 61.2 ± 10.4 years and that for the corresponding healthy controls, 43.0 ± 14.0 years. Clinical characteristics of the patients studied are summarized in Table S1, available on the Blood website (see the Supplemental Materials link at the top of the online article). Variance analysis to assess dependency of age and frequency of CD4+CD25hi T cells in healthy donors and patients with CLL did not reveal a correlation between these variables.
Antibodies and fluorescence-activated cell sorting analysis
Cell phenotype of T cells within PBMCs was defined by multicolor flow cytometry using the following antibodies: fluorescein isothiocyanate (FITC)–conjugated CD4; phycoerythrin (PE)–conjugated CTLA4 (CD152); PE-cyanin 5 (Cy-5)–conjugated CD25, –CD45RA, and –CTLA4; allophycocyanin (APC)–conjugated CD4; APC-Cy-7–conjugated CD4 (all from Becton Dickinson [BD] PharMingen, Heidelberg, Germany); PE-conjugated CD25, CD62L; peridinin chlorophyll protein (PerCP)–conjugated CD3; PE-Cy-7–conjugated CD25 (all from Becton Dickinson Biosciences, Heidelberg, Germany); and the corresponding isotype control antibodies (BD PharMingen). Cells were stained according to the manufacturer's recommendations. For intracellular staining cells were permeabilized using Cytofix/Cytoperm solution (BD PharMingen) after surface staining and incubated with interleukin 10 (IL-10)–FITC, GITR-FITC (both from R&D Systems, Wiesbaden, Germany), transforming growth factor β1 (TGF-β1)–PE (IQ-Products, Groningen, The Netherlands), and CTLA4-PE (BD PharMingen) or with the appropriate isotype controls (BD PharMingen). Samples were then washed and stored at 4°C until acquisition.
Samples were acquired either on a FACSCalibur or FACSCanto (both from BD Biosciences) and analyzed with CELLQuest or FACSDiva software (both from BD Biosciences) or WinMDI 2.8 (http://facs.scripps.edu/software.html). CD25lo and CD25hi T cells were gated as demonstrated in Figure 1A for all samples analyzed according to previously published data.25 The analysis was performed independently by 2 investigators (M.B. and J.L.S.) with similar results. Frequencies of CD4+CD25hi T cells in peripheral blood are shown as percent values of CD4+ T cells. To determine cells positive for additional Treg cell markers, we used stringent gating criteria, setting gates at the 1% level of the respective isotype control.
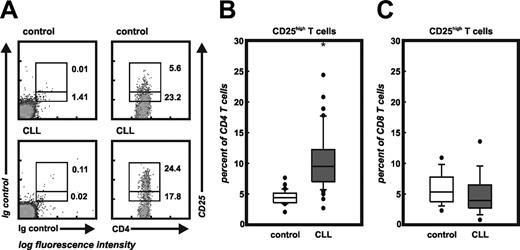
Frequency of CD4+CD25hi T cells. (A) Flow cytometric analysis of CD4 and CD25 on peripheral blood-derived T cells of a healthy individual and a patient with CLL. Cells were either stained with the appropriate isotype controls (left panel) or CD4 and CD25 mAbs (right panel). CD4+CD25+ T cells were divided into CD25lo and CD25hi cells according to previously published data.25 Numbers represent percentage of events within the respective rectangle. Settings shown here were used for the analysis of all samples under study. (B) Frequency of CD4+CD25hi T cells in 26 control and all 73 CLL samples. Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots) (*P < .001, Student t test). (C) Frequency of CD8+CD25hi T cells.
Frequency of CD4+CD25hi T cells. (A) Flow cytometric analysis of CD4 and CD25 on peripheral blood-derived T cells of a healthy individual and a patient with CLL. Cells were either stained with the appropriate isotype controls (left panel) or CD4 and CD25 mAbs (right panel). CD4+CD25+ T cells were divided into CD25lo and CD25hi cells according to previously published data.25 Numbers represent percentage of events within the respective rectangle. Settings shown here were used for the analysis of all samples under study. (B) Frequency of CD4+CD25hi T cells in 26 control and all 73 CLL samples. Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots) (*P < .001, Student t test). (C) Frequency of CD8+CD25hi T cells.
CD4+ T-cell isolation and culture
CD4+ T cells were purified from PBMCs using CD4 magnetic-activated cell sorting (MACS) beads (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.61 To assess polyclonal CD4+ T-cell activation 1 × 105 CD4+ T cells/well were activated in AIM-V (Gibco Invitrogen, Karlsruhe, Germany)/EX-Cell 610 (JRH Biosciences, Lenexa, KS) with anti-CD3 (0.2 μg/mL, OKT-3) and anti-CD28 monoclonal antibody (mAb; 0.2 μg/mL, kind gift of Dr L. M. Nadler, Dana-Faber Cancer Institute, Boston, MA) in 96-well plates. The proliferation of T cells was monitored by measuring incorporation of 5-bromo-2′-deoxyuridine (BrdU; Roche Diagnostics, Mannheim, Germany) on day 2 to 3 of culture. Cells were harvested 24 hours after the addition of BrdU. BrdU incorporation was assessed by absorbance at a wavelength of 450 nm using a multiwell enzyme-linked immunosorbent assay (ELISA) reader.
Isolation of CD4+CD25hi and CD4+CD25- T cells
In 18 patients with CLL we were able to obtain sufficient amounts of peripheral blood (> 50 mL) to isolate CD4+CD25- T cells and CD4+CD25hi T cells from PBMCs for functional analysis.14 Briefly, CD4 MACS multisort beads (Miltenyi Biotec) were used for isolation of CD4+ T cells. After detaching, cells were washed and CD4+CD25hi T cells were positively selected using CD25 microbeads (2 μL beads/107 CD4+ T cells). The described technique is optimized for the isolation of human CD4+CD25hi T cells with high purity.62 Use of higher concentrations of microbeads for isolation results in better recovery but decreases the purity. The negative fraction of CD4+CD25- T cells was used as effectors. Alternatively, CD4+ T cells were isolated with CD4 MACS beads as described earlier and stained with CD4-FITC and CD25-PE. CD4+CD25hi T cells were purified using a FACSDiVa cell sorter (BD Biosciences). Cells were reanalyzed after sorting and routinely showed more than 95% purity. For some experiments, CD4+CD25hi as well as CD4+CD25- T cells were preactivated with 0.5 μg/mL anti-CD3 mAb at 37°C for 20 hours in the presence of 10 U/mL IL-2 (Proleukin; Chiron, Munich, Germany) in X-VIVO 15 (BioWhittakker, Verviers, Belgium).
RNA extraction and real-time reverse transcription–PCR for FOXP3
Total RNA from purified CD4+CD25- and CD4+CD25hi T cells was isolated using TRIzol (Invitrogen, Karlsruhe, Germany). First-strand cDNA was synthesized from 100 ng total RNA using SuperScript III kit (Invitrogen) according to the manufacturer's recommendation. For FOXP3 and glyceraldehyde phosphate dehydrogenase (GAPDH) transcripts, real-time polymerase chain reaction (PCR) was performed with a LightCycler (Roche Diagnostics) based on specific primers and general fluorescence detection with SYBR Green. The following primer combinations were used: FOXP3 forward, 5′-CGG ACA CTC AAT GAG ATC TA-3′; FOXP3 reverse, 5′-ATC CTC CTT TCC TTG ATC TT-3′; GAPDH forward, 5′-TGA TGA CAT CAA GAA GGT GGT GAA-3′; and GAPDH reverse, 5′-TCC TTG GAG GCC ATG TGG GCC AT-3′. All PCRs were performed using LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics). cDNA from Jurkat cells was used as a standard and normalization to GAPDH was performed for each sample. Relative fold changes of FOXP3 expression in CD4+CD25hi T cells were normalized to GAPDH as described.38
Generation of dendritic cells
For T-cell stimulation we generated allogeneic dendritic cells (DCs) as described previously.63 Briefly, PBMCs were plated in Iscove modified Dulbecco medium (IMDM) with 5 mM glutamine and 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; all from PAA Laboratories, Coelbe, Germany) and 1% autologous plasma for 2 hours at 37°C. Subsequently, the adherent cell fraction was cultured for 20 hours in RPMI 1640 (PAA Laboratories) supplemented with 2 mM glutamine and 1% autologous plasma (DC medium). On day 1, new DC medium containing 800 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax; Novartis, Nuremberg, Germany) and 1000 U/mL IL-4 (Immunotools, Friesoythe, Germany) was added. Cytokines were added on day 3 in fresh DC medium. On day 5, nonadherent cells were replated in fresh DC medium with cytokines. On day 6, 10 ng/mL tumor necrosis factor α (TNF-α), 1 μg/mL prostaglandin E2 (PGE2; both from Sigma Aldrich, Taufkirchen, Germany), 1000 U/mL IL-6, and 10 ng/mL IL-1β (both from R&D Systems) were added and mature DCs were harvested on day 7.
Assessment of inhibitory function of CD4+CD25hi T cells
To assess the suppressive activity of CD4+CD25hi T cells on conventional T-cell proliferation, a modified allogeneic MLR was performed as previously described.14,64,65 Briefly, after magnetic separation both CD4+CD25- and CD4+CD25hi T cells were incubated for 20 hours with 10 U/mL IL-2 and 0.5 μg/mL anti-CD3 mAb in X-VIVO 15. Subsequently, these preactivated CD4+CD25- T cells (5 × 104/well) were cocultured with irradiated allogeneic PBMCs (2 × 105/well) or mature DCs (DCs/T cells, 1:20) in X-VIVO 15 supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin/streptomycin, and 2 mM glutamine (all from Gibco Invitrogen). Purified allogeneic CD4+CD25hi T cells were added at different concentrations as indicated. There was no influence on the inhibitory effect of CD4+CD25hi T cells when the CD4+CD25- T cells (effector T cells) were not preactivated prior to the inhibition assay. On day 4 the cells were pulsed with BrdU and BrdU incorporation was analyzed 20 hours later as described (see “CD4+ T-cell isolation and culture”).
Assessment of viability after in vitro incubation with fludarabine
To measure the effect of fludarabine, PBMCs from healthy controls were cultured in RPMI 1640 supplemented with 10% FCS, 100 U/mL penicillin/streptomycin, and 2 mM glutamine with or without 10 μM fludarabine (Fludara; MedacSchering Onkologie, Munich, Germany) for 48 hours. Cells were then harvested and stained using the following antibodies: FITC-conjugated CD3, APC-Cy-7–conjugated CD4, PE-Cy-7–conjugated CD25, 7-amino-actinomycin D (7-AAD), and PE-conjugated annexin V (all from BD Biosciences) according to the manufacturer's instructions. Viable cells were defined as annexin V and 7-AAD double-negative cells and listed as percent of parental CD4+CD25- or CD4+CD25+ T cells.
Cytometric bead array for chemokines
The concentration of interferon γ (IFN-γ) in cell culture supernatants was measured using the human Th1/Th2 cytokine kit II (BD PharMingen). In brief, capture beads were mixed with culture supernatants and PE detection reagent and incubated for 3 hours at room temperature. The beads were then washed with wash buffer and analyzed.
Statistical analysis
Comparison between paired or unpaired groups was performed using the appropriate Student t test. P values below .05 were defined as statistically significant. Due to the explorative nature of this study, no multiplicity adjustment procedures were performed. All statistical analyses were performed using the SPSS statistical software package (SPSS 12.0 for Windows, SPSS, Chicago, IL).
Online supplemental material
Figure S1 depicts proliferation of purified CD4+ T cells from healthy individuals and CLL patients activated with anti-CD3 and anti-CD28 mAbs. Table S1 contains information on the patients.
Results
Increased frequencies of CD4+CD25hi T cells in patients with CLL
Frequencies of CD4+CD25hi Treg cells were assessed using multicolor flow cytometry and following previously published data.25 As depicted in Figure 1A, healthy donors showed a significant number of CD4+CD25lo T cells with a smaller percentage of CD4+CD25hi T cells, whereas we found an increased frequency of CD4+CD25hi T cells in patients with CLL. Using these settings, we analyzed samples from 73 patients with CLL and 26 healthy individuals. The frequency of Treg cells in controls (4.5% ± 1.1%) was similar to previously published results (Figure 1B).25 In contrast, patients with CLL showed significantly increased frequencies of Treg cells (10.4% ± 4.4%, P < .001). Control experiments assessing cell surface expression of CD25 on CD8+ T cells did not reveal an overall activation of T cells in patients with CLL, supporting an increase in CD4+CD25hi Treg cells (Figure 1C).
CD4+CD25hi T cells from patients with CLL also express Treg cell-associated proteins
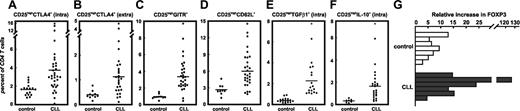
Before evaluating Treg cell function, we assessed expression of proteins that have been previously associated with Treg cells. These included CTLA4, GITR, CD62L, TGF-β1, IL-10, and FOXP3. The frequency of CD4+CD25hi CTLA4+ T cells was significantly increased in patients with CLL compared to healthy individuals both for intracellular and extracellular staining (Figure 2A-B; P < .01 and P < .05 for panels A and B, respectively). Similarly, CD4+CD25hi GITR+ cells from patients with CLL were significantly augmented (3.34% ± 1.67% in patients versus 0.96% ± 0.29% in controls, P < .001; Figure 2C). GITR expression in conventional CD4+CD25- T cells was similarly low in both populations (data not shown). In patients with CLL the CD4+CD25hi T-cell population also contained increased frequencies of CD62L+ cells (P < .01; Figure 2D).
Intracellular expression of TGF-β1 and IL-10 was assessed because these 2 cytokines have been associated with Treg cell function.66,67 Similarly to CTLA4, GITR, and CD62L, TGF-β1 and IL-10 were significantly increased in CD4+CD25hi T cells from patients with CLL (Figure 2E-F; P < .001 and P < .05 in panels E and F, respectively). We also observed IL-10+ as well as TGF-β1+ cells within conventional CD4+CD25- T cells in the patients but not in healthy controls (data not shown). Because FOXP3 has been shown to be a crucial molecule for murine CD4+CD25+ Treg cells, we also performed real-time PCR for FOXP3 in a subset of patients with CLL and healthy individuals. PCRs showed strong expression of FOXP3 mRNA in CD3+CD4+CD25hi T cells from healthy individuals (n = 7) and expression of FOXP3 was even higher in the CD3+CD4+CD25hi T cells from patients with CLL (n = 6). Overall, CD4+ T cells expressing high levels of CD25, FOXP3, CTLA4, GITR, CD62L, TGF-β1, and IL-10 are significantly increased in patients with CLL.
Expression of proteins associated with Treg cells. CTLA4, GITR, CD62L, TGF-β1, and IL-10 were assessed on CD4+ T cells coexpressing CD25 by either cell surface or intracellular multicolor flow cytometry. Percent positive cells were determined using stringent gating criteria with less than 1% of events within the positive gate when analyzing respective isotype controls. At least 25,000 events per analysis were acquired. Each dot represents a single individual assessed in the respective group; mean expression (line) of all samples in each group is also shown. Significant differences (P < .05, Student t test) between controls and CLL samples are marked by an asterisk: (A) intracellular CTLA4 (P < .01), (B) extracellular CTLA4 (P < .05), (C) intracellular GITR (P < .001), (D) extracellular CD62L (P < .01), (E) intracellular TGF-β1(P < .001), and (F) intracellular IL-10 (P < .05). (G) Expression of FOXP3 by human CD4+CD25hi T cells. CD4+CD25hi T cells and CD4+CD25- T cells were sorted by MACS from peripheral blood of healthy controls (n=7) and patients with CLL (n = 6). Real-time PCR for FOXP3 was performed and relative fold changes of CD4+CD25hi T cells to CD4+CD25- T cells were normalized to GAPDH as described.38
Expression of proteins associated with Treg cells. CTLA4, GITR, CD62L, TGF-β1, and IL-10 were assessed on CD4+ T cells coexpressing CD25 by either cell surface or intracellular multicolor flow cytometry. Percent positive cells were determined using stringent gating criteria with less than 1% of events within the positive gate when analyzing respective isotype controls. At least 25,000 events per analysis were acquired. Each dot represents a single individual assessed in the respective group; mean expression (line) of all samples in each group is also shown. Significant differences (P < .05, Student t test) between controls and CLL samples are marked by an asterisk: (A) intracellular CTLA4 (P < .01), (B) extracellular CTLA4 (P < .05), (C) intracellular GITR (P < .001), (D) extracellular CD62L (P < .01), (E) intracellular TGF-β1(P < .001), and (F) intracellular IL-10 (P < .05). (G) Expression of FOXP3 by human CD4+CD25hi T cells. CD4+CD25hi T cells and CD4+CD25- T cells were sorted by MACS from peripheral blood of healthy controls (n=7) and patients with CLL (n = 6). Real-time PCR for FOXP3 was performed and relative fold changes of CD4+CD25hi T cells to CD4+CD25- T cells were normalized to GAPDH as described.38
Reduced inhibitory function of CD4+CD25hi T cells from patients with CLL
In 18 patients with CLL, sufficient numbers of highly purified CD4+CD25hi T cells were isolated to analyze their inhibitory function in comparison to Treg cells from 16 healthy controls. Regulatory function of CD4+CD25hi T cells was assessed using an allogeneic MLR of CD4+CD25- T cells and allogeneic irradiated PBMCs65 or DCs as stimulators.14,64 Autologous conventional CD4+CD25- T cells from patients with CLL were not used in the MLR because these T cells might themselves be inhibitory.
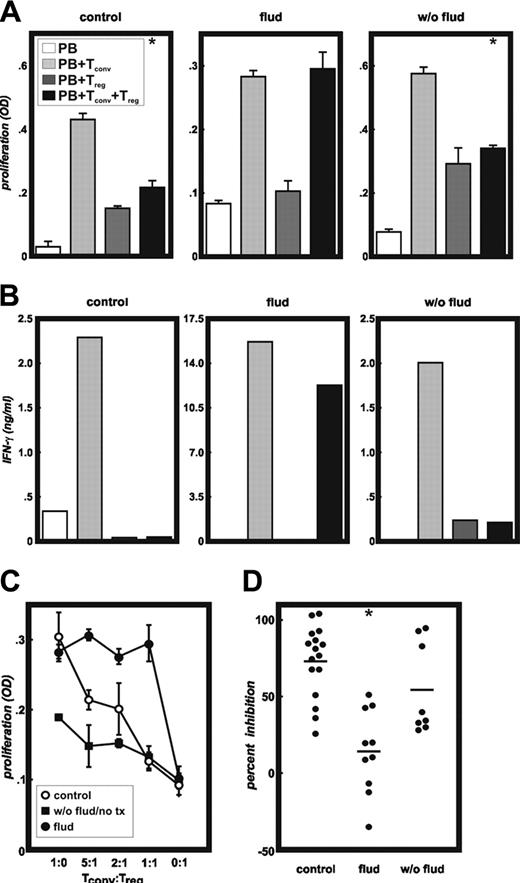
In controls, significant inhibition of allogeneic CD4+CD25- T cells as exemplified in Figure 3A (control, P < .01) was determined at 1:1 ratios of allogeneic conventional T cells to Treg cells. In contrast, patients with CLL pretreated with fludarabine-containing chemotherapy showed reduced or even abrogated Treg cell function (Figure 3A, flud). Treg cells from patients with CLL never treated with fludarabine-containing therapy regimens showed inhibitory function similar to healthy controls (Figure 3A, w/o flud). Assessment of IFN-γ production by the CD4+CD25- T cells confirmed the proliferation results. IFN-γ was greatly diminished in cultures inhibited by CD4+CD25hi T cells from healthy donors and patients with CLL never treated with fludarabine, but not in cultures derived from patients previously treated with fludarabine (Figure 3B). These data were further corroborated at lower numbers of Treg cells (Figure 3C). Healthy controls and patients with CLL never treated with fludarabine showed inhibition at lower ratios of Treg cells to conventional T cells, whereas patients with CLL pretreated with fludarabine did not show any inhibition at these conditions (Figure 3C). In Figure 3D inhibition of allogeneic T-cell proliferation by Treg cells is shown for all healthy controls and patients at a 1:1 ratio of Treg to conventional T cells. Overall, there was a significant reduction of Treg cell-induced inhibition within the fludarabine group (P < .001), whereas patients with CLL never treated with fludarabine showed inhibitory function of Treg cells that was not significantly different from healthy controls.
Sorting of CD4+CD25hi T cells by fluorescence-activated cell sorting (FACS) in some experiments did not reveal different results, further supporting that these cells had lost their inhibitory function (data not shown). Use of allogeneic mature DCs instead of PBMCs for T-cell stimulation led to similar results, suggesting that the observation was intrinsic to CD4+CD25hi T cells derived from patients with CLL (data not shown). Interestingly, when assessing proliferation of CD4+ T cells upon stimulation with anti-CD3 and anti-CD28 mAbs, we observed normal T-cell proliferation in 3 of 3 patients pretreated with fludarabine, whereas 7 of 11 patients untreated or not treated with fludarabine showed significantly reduced overall CD4+ T-cell proliferation (Figure S1).
CD4+CD25hi T cells are increased in patients with CLL with extended disease
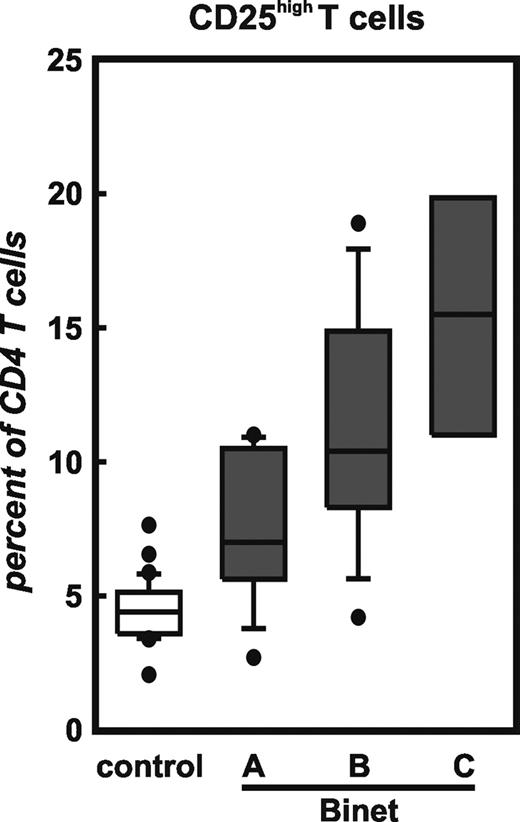
Next we assessed the frequency of Treg cells in context of stage of disease in previously untreated patients with CLL (Figure 4). Of all 73 patients, 26 were previously untreated. This analysis revealed a correlation between Treg cell frequency and stage of disease with highest numbers of Treg cells in patients with extended disease (Binet C) followed by patients with Binet B and with lowest but still increased numbers in Binet A. In patients in Binet stage B and especially in Binet stage C we also observed an increase of CD4+CD25hi T cells coexpressing GITR, CD62L, and intracellular CTLA4 compared to healthy controls (data not shown).
CD4+CD25hi T cells are reduced particularly after therapy with fludarabine
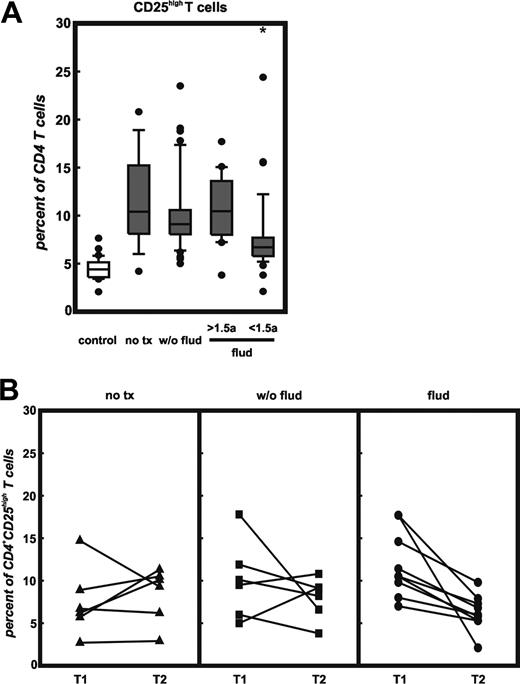
Next, we investigated the association of Treg cell frequencies and therapy (Figure 5). For this analysis, we only included samples from patients in Binet stages B and C because most patients with Binet stage A had not received therapy. Again, untreated patients (Binet B/C) showed an increased frequency of CD4+CD25hi T cells (Figure 5A, no tx) coexpressing GITR, CD62L, and intracellular CTLA4 (data not shown). Patients treated with chemotherapy excluding fludarabine (w/o flud) showed a similarly increased frequency of Treg cells and there was no correlation of frequency of Treg cells and time from therapy to phenotypic Treg-cell assessment (data not shown). In contrast, assessment of Treg cell counts in patients receiving fludarabine treatment showed significantly reduced Treg cell frequencies only in patients who had received last fludarabine treatment less than 18 months prior to Treg cell assessment (P < .01). The number of cells coexpressing GITR, CD62L, and intracellular CTLA4 was also lower than in patients never treated with fludarabine (data not shown). Those patients receiving their last fludarabine treatment more than 18 months prior to Treg cell measurement showed Treg cell frequencies comparable to patients treated without fludarabine-containing regimens or even untreated patients. It needs to be mentioned that the patient groups treated with fludarabine were enriched for patients with larger tumor burden and progressive disease, whereas many of the untreated patients showed a slower disease progression.
Functional analysis of CD4+CD25hi T cells. Highly purified CD4+CD25- T cells were stimulated by allogeneic irradiated PBMCs or DCs either in the presence or absence of highly purified CD4+CD25hi T cells derived from patients with CLL or healthy donors (both allogeneic). Treg cells from 16 controls, 10 patients pretreated with fludarabine, and 8 patients never treated with fludarabine were assessed in these MLRs. As a function of T-cell inhibition, proliferation (A) and IFN-γ production (B) were measured. Panel A shows representative experiments from a control, a CLL patient pretreated with fludarabine (flud), and a patient never treated with fludarabine (w/o flud). Open bars (PB) indicate background proliferation of irradiated allogeneic PBMCs; light gray bars (PB+Tconv), alloantigen induced proliferation of CD4+CD25- conventional T cells; dark gray bars (PB+Treg), background proliferation of CD4+CD25hi Treg cells; and black bars (PB+Tconv+Treg), proliferation of CD4+CD25- conventional T cells in the presence of CD4+CD25hi Treg cells at a 1:1 ratio (error bars represent SD; *P < .01 Student t test). (B) Measurement of IFN-γ by cytokine bead array in the supernatants from cultures described in panel A. (C) Inhibition of proliferation of CD4+CD25- conventional T cells by CD4+CD25hi Treg cells at different ratios (responders to suppressors) from a healthy donor (○), a fludarabine-treated CLL patient (•), and a CLL patient never treated with fludarabine (▪). Representative experiments are shown here, error bars represent SD. (D) Percentages of inhibition of proliferation of CD4+CD25- conventional T cells by CD4+CD25hi Treg cells at a 1:1 ratio from all healthy donors (n = 16), fludarabine-treated patients (n = 10), or patients with CLL never treated with fludarabine (n = 8) (*P < .001 Student t test).
Functional analysis of CD4+CD25hi T cells. Highly purified CD4+CD25- T cells were stimulated by allogeneic irradiated PBMCs or DCs either in the presence or absence of highly purified CD4+CD25hi T cells derived from patients with CLL or healthy donors (both allogeneic). Treg cells from 16 controls, 10 patients pretreated with fludarabine, and 8 patients never treated with fludarabine were assessed in these MLRs. As a function of T-cell inhibition, proliferation (A) and IFN-γ production (B) were measured. Panel A shows representative experiments from a control, a CLL patient pretreated with fludarabine (flud), and a patient never treated with fludarabine (w/o flud). Open bars (PB) indicate background proliferation of irradiated allogeneic PBMCs; light gray bars (PB+Tconv), alloantigen induced proliferation of CD4+CD25- conventional T cells; dark gray bars (PB+Treg), background proliferation of CD4+CD25hi Treg cells; and black bars (PB+Tconv+Treg), proliferation of CD4+CD25- conventional T cells in the presence of CD4+CD25hi Treg cells at a 1:1 ratio (error bars represent SD; *P < .01 Student t test). (B) Measurement of IFN-γ by cytokine bead array in the supernatants from cultures described in panel A. (C) Inhibition of proliferation of CD4+CD25- conventional T cells by CD4+CD25hi Treg cells at different ratios (responders to suppressors) from a healthy donor (○), a fludarabine-treated CLL patient (•), and a CLL patient never treated with fludarabine (▪). Representative experiments are shown here, error bars represent SD. (D) Percentages of inhibition of proliferation of CD4+CD25- conventional T cells by CD4+CD25hi Treg cells at a 1:1 ratio from all healthy donors (n = 16), fludarabine-treated patients (n = 10), or patients with CLL never treated with fludarabine (n = 8) (*P < .001 Student t test).
In 21 patients we obtained blood samples at least at 2 different time points more than 6 months apart (range, 7-128 months). At the second time point we observed in 5 of 6 untreated CLL patients slightly increased or similar frequencies of Treg cells (Figure 5B, no tx). In patients treated with non–fludarabine-based chemotherapy (w/o flud), there was a diverse response with 2 patients showing increased and 4 patients lower frequencies. In contrast, for 8 of 8 patients treated with fludarabine, frequencies were lower after fludarabine therapy (Figure 5B, flud). Overall, frequency of Treg cells in patients with CLL was associated with stage of disease and with significantly reduced frequencies after fludarabine-based chemotherapy.
Frequency of CD4+CD25hi T cells in context of stage of disease in previously untreated CLL patients. Patients were classified according to the Binet classification: Binet A (n = 9), Binet B (n = 13), and Binet C (n = 4). Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots) of data obtained by multicolor flow cytometry.
Frequency of CD4+CD25hi T cells in context of stage of disease in previously untreated CLL patients. Patients were classified according to the Binet classification: Binet A (n = 9), Binet B (n = 13), and Binet C (n = 4). Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots) of data obtained by multicolor flow cytometry.
To assess whether fludarabine might preferentially induce cell death in CD4+CD25+ T cells we incubated PBMCs with 10 μM fludarabine and measured apoptosis and cell death by flow cytometry using annexin V and 7-AAD. Whereas more than 50% of CD4+CD25- T cells were viable after exposure to 10 μM fludarabine for 48 hours, more than 70% of CD4+CD25+ T cells underwent apoptosis (Table 1). In contrast, more than two thirds of CD4+CD25+ T cells cultured in the absence of fludarabine were still alive at this time point.
Fludarabine-induced apoptosis in CD4+ CD25+ and CD4+ CD25– T cells
. | Experiment 1 . | . | Experiment 2 . | . | ||
|---|---|---|---|---|---|---|
. | Medium . | Medium plus fludarabine . | Medium . | Medium plus fludarabine . | ||
| CD4+ CD25–, % | 91 | 66 | 90 | 52 | ||
| CD4+ CD25+, % | 69 | 23 | 79 | 30 | ||
. | Experiment 1 . | . | Experiment 2 . | . | ||
|---|---|---|---|---|---|---|
. | Medium . | Medium plus fludarabine . | Medium . | Medium plus fludarabine . | ||
| CD4+ CD25–, % | 91 | 66 | 90 | 52 | ||
| CD4+ CD25+, % | 69 | 23 | 79 | 30 | ||
CD4+ CD25+ T cells are more susceptible to fludarabine-induced apoptosis than CD4+ CD25– T cells. PBMCs from 2 different donors were incubated in vitro with medium alone or with medium containing 10 μM fludarabine. After 48 hours cells were stained with 7-AAD and annexin V and viable cells were defined as 7-AAD and annexin-V double-negative cells. Viable cells are listed as percentage of the respective cell type.
Correlation of therapy and frequency of CD4+CD25hi T cells in patients with CLL. (A) Patients treated with fludarabine less than 18 months prior to T-cell analysis (flud < 1.5a) were compared with healthy controls (control), untreated (no tx), otherwise treated patients with CLL (w/o flud) or patients with CLL treated with fludarabine more than 18 month prior to analysis (flud > 1.5a) for the frequency of CD4+CD25hi T cells as assessed by multicolor flow cytometry. Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots); *P < .01 for flud < 1.5a versus flud > 1.5a by Student t test. (B) Serial analysis of CD4+CD25hi T cells for the 3 CLL treatment subgroups at 2 different time points separated by at least 6 months.
Correlation of therapy and frequency of CD4+CD25hi T cells in patients with CLL. (A) Patients treated with fludarabine less than 18 months prior to T-cell analysis (flud < 1.5a) were compared with healthy controls (control), untreated (no tx), otherwise treated patients with CLL (w/o flud) or patients with CLL treated with fludarabine more than 18 month prior to analysis (flud > 1.5a) for the frequency of CD4+CD25hi T cells as assessed by multicolor flow cytometry. Shown here are median, 75 percentile (box), SD (whiskers), and outliers (dots); *P < .01 for flud < 1.5a versus flud > 1.5a by Student t test. (B) Serial analysis of CD4+CD25hi T cells for the 3 CLL treatment subgroups at 2 different time points separated by at least 6 months.
Discussion
Most recently a loss of function of CD4+CD25hi T cells has been associated with autoimmune diseases,28 whereas a relative increase of CD4+CD25+ T cells was reported in patients with solid cancers.36,39-44 An analysis of 73 patients with CLL and 42 healthy controls revealed a significantly increased frequency of CD4+CD25hiFOXP3+CTLA4+ GITR+ CD62L+ TGFβ1+ IL-10+ Treg cells in the patients with CLL. Frequency of Treg cells was particularly increased in untreated patients with intermediate stage (Binet B) or extended disease (Binet C). However, when patients were treated with fludarabine-containing chemotherapy, the frequency of Treg cells was significantly reduced, which was also shown in individual patients assessed before and after treatment with fludarabine. Although the reduced frequency of Treg cells after therapy especially with fludarabine was surprising, the reduced inhibitory function of highly purified CD4+CD25hi T cells from these patients was an unexpected finding. First, in vitro experiments demonstrated a preferential induction of apoptosis in CD4+CD25+ T cells after incubation with fludarabine.
In ovarian cancer, a correlation between tumor stage and Treg cells within ascites but not with frequency in peripheral blood was recently established.38 In contrast to ovarian cancer, Treg cells in patients with CLL were even increased in peripheral blood most likely reflecting the disseminated character of this malignancy. A clear correlation between Treg cell frequency and tumor stage was only observed in untreated patients (Figure 4), whereas patients treated with fludarabine-containing regimens presented with significantly reduced Treg cells independent of stage of disease.
Within our data set it is not yet possible to determine whether Treg cell frequency is a predictive marker for survival because observation times for patients with CLL are still too short and most patients within this study are still alive. Nevertheless, our data strongly suggest that Treg cell numbers cannot serve as a predictive marker in cancer patients treated with chemotherapy, particularly with drugs that influence function and frequency of these cells. Whether the number of Treg cells in untreated patients with CLL is an independent predictor for survival needs further exploration.
The reduction of CD4+ T-cell counts has been described as a side effect of fludarabine.68 We have extended these findings, by demonstrating a preferential induction of apoptosis of CD4+CD25+ T cells. It will be interesting to determine whether the remaining cells are still inhibitory. Experiments addressing this important aspect are currently ongoing. In light of these findings, the successful use of adoptively transferred autologous tumor-specific T-cell clones in patients with malignant melanoma after induction therapy with cyclophosphamide and fludarabine is intriguing.69 Although recent data have established a role for cyclophosphamide in reducing Treg cells,11 the use of fludarabine is mainly associated with the reduction of cellularity prior to T-cell therapy. The reduced Treg cell frequencies and loss of inhibitory function in patients with CLL, however, would suggest a more specific effect of fludarabine by reducing particularly inhibitory circuits within the immune system. So far, our data set is still not large enough to determine with sufficient statistical power a potential correlation between the number of Treg cells and the clinical observation of autoimmune phenomena in CLL. Incorporating assessment of Treg cells within larger clinical trials will be necessary to answer this important question.
GITR is especially expressed at high levels on resting CD4+CD25hi Treg cells.18 So far, increased expression of GITR on Treg cells has not been reported for cancer patients.70 By flow cytometry we identified an increased expression of GITR on Treg cells in patients with CLL. In mice the ligand for GITR (GITR-L) has been mainly identified on immature DCs.71 In humans, GITR-L is expressed in a variety of tissues72 including PBMCs. Stimulation of GITR on murine CD4+CD25+ T cells abrogated Treg cell suppression, thereby breaking immune tolerance.18,73 An increased expression of GITR on Treg cells in cancer patients might be an interesting target for therapeutic exploitation. Whether GITR expression is elevated in other malignancies should therefore be studied in more detail.
In women with ovarian cancer, Treg cells uniformly expressed high levels of CCR4 and migrated to CCL22 expressed by tumor cells and macrophages within ascites.38 Because CLL cells also express CCL22 and attract CD4+CD40L+ T cells,74 we initiated a preliminary study on the expression of CCR4 on Treg cells in patients with CLL. In 17 patients, most of whom were pretreated with fludarabine, CCR4 expression varied in intensity and ranged from only 30% to 96% (mean, 67.7%) of the CD4+CD25hi Treg cells (M.B. and J.L.S., unpublished results, January 2005), which is slightly lower than previously published data.75 So far, there is no significant difference of CCR4 expression on Treg cells between untreated and chemotherapy-treated patients so that the reduced expression in patients with CLL is unlikely due to therapy.
The significantly increased expression of TGF-β1 and IL-10 in Treg cells from patients with CLL was also accompanied by a significant increase of conventional CD4+CD25- T cells expressing these cytokines (data not shown). Both cytokines play an important role for the inhibitory function of Treg cells.66 In contrast to CLL, IL-10 was reported not to be produced by Treg cells from patients with invasive breast, lung, or pancreatic cancer39,42 albeit other studies suggest that Treg cells might express IL-10 in some patients with solid tumors.43 CD4+CD25+ Treg cells in patients with lung, colorectal, and ovarian cancer produce larger amounts of TGF-β1 than conventional CD4+CD25- T cells.39,76 In light of TGF-β1 as an emerging target for antitumor therapy,77 it needs to be further evaluated if TGF-β1 production by Treg cells is also increased in patients with solid tumors in comparison to healthy controls as we have shown here for patients with CLL.
Taken together, we have elucidated an important effect of fludarabine-based treatment regimens on CD4+CD25hi Treg cells. It will be interesting to see if fludarabine in combination with cyclophosphamide is also reducing Treg cells after adoptive T-cell therapy69 and allogeneic nonmyeloablative stem cell transplantation.78 Monitoring frequency and function of Treg cells within such settings will help to define the significance of these cells for clinical efficacy of cancer immunotherapy.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-02-0642.
Supported by the Sofja Kovalevskaja Award of the Alexander von Humboldt-Foundation (J.L.S.) and the Wilhelm-Sander Stiftung (J.L.S. and M.K.). M.B.B. is supported by a Carreras Foundation Fellowship and a Max Eder Award from the Deutsche Krebshilfe. P.A.K. and E.E. are supported in part by grant HBFG-109-517.
M.B. and M.K. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to our patients for their commitment to this study. We thank A. Draube, E. Jäger, C. Schweighofer, and C. Pallasch for referral of patients; B. Gathof for providing us with blood samples from healthy individuals; and H. Abken and J. Chemnitz for critically reading the manuscript. We thank I. Büchmann, J. Claasen, and C. Hoyer for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal