Abstract
Although basophils are known to produce interleukin 4 (IL-4), the roles of these cells have been documented only in mice infected with parasites or in the effector phase of allergic inflammations. Here we show that naive mice lacking the transcription factor, interferon regulatory factor 2 (IRF-2), exhibited signal transducer and activator of transcription 6 (Stat6)–independent expansion of basophils in the periphery. IRF-2 appeared to act autonomously in the cells to negatively regulate the expansion of, but not cytokine production by, basophils. Spontaneous Th2 polarization of CD4+ T cells was observed in these mice and the genetic reduction of basophil numbers by mutating the Kit gene abolished such a polarization in vivo. We also found that both basophils and IL-4 derived from them were indeed essential for Th2 development under neutral conditions in vitro. Furthermore, neutralization of IL-3 abolished IL-4 production by basophils during Th1/Th2 differentiation cultures and subsequent Th2 development. These results indicated that basophils acted as a cellular converter to turn the neutral IL-3 into the Th2-inducing IL-4 during the initiation of Th1/Th2 differentiation. Thus, the negative regulatory role of IRF-2 on the basophil population size is critically important for preventing excess Th2 polarization and the Th1/Th2 balance in naive animals.
Introduction
One of the most important features of the atopic state underlying various allergic diseases is the tendency to initiate type 2 (Th2) rather than type 1 (Th1) helper T-cell responses on encounters with environmental allergens.1,2 Mechanisms underlying such a tendency remain unclear, and the key cell types initiating Th2 responses on antigen stimulation by producing interleukin 4 (IL-4) has not been clearly defined, although it is apparent that these cells would play critical roles in determining the subsequent direction of immune responses3,4 and possibly in the establishment of the atopic state. Basophils have been considered to be among several candidate cells for the initial IL-4 source because human and murine basophils produced IL-4 in vitro in response to various stimuli including IL-3 and high-affinity IgE receptor (FcϵRI) cross-linking5-9 and also in vivo on infection with nematodes.10-12 However, the roles of basophils related to type 2 immune responses known to date are restricted largely to those during allergic inflammation, in animals infected with Th2-inducing agents such as nematodes, or in memory responses to a type of T cell-dependent antigen.11-14 Thus, the impact of basophils on the homeostasis of the Th1/Th2 balances as well as the initiation of Th2 responses in steady-state conditions remains to be studied.
Interferon (IFN) regulatory factor 2 (IRF-2)15 is a transcription factor, belonging to the IRF family,16 that has been shown to attenuate the signals evoked by spontaneously produced IFN-α/β, thereby preventing a CD8+ T cell-mediated skin inflammation17 and allowing a CD4+ subset of dendritic cells (DCs) to develop normally.18,19 In terms of Th1/Th2 differentiation, the impaired ability of IRF-2–deficient macrophages to produce IL-1220,21 was suggested to be responsible for the Th2-dominant immune responses to Leishmania major.20 However, it was shown that deficiency in IL-12p40 expression did not result in spontaneous Th2 polarization in unimmunized animals.22 Moreover, we observed unimpaired IL-12p40 expression by cytosine phosphate guanosine (CpG) DNA-activated DCs,18 the cell type that is generally considered to be more important than macrophages in initiating Th1/Th2 differentiation.1 These observations prompted us to re-examine the mechanism for the preferential Th2 differentiation in mice lacking IRF-2 (IRF-2-/- mice).20
In this study, we found that the numbers of basophils were up-regulated in the spleen and peripheral blood in unmanipulated IRF-2-/- mice in a signal transducer and activator of transcription 6 (Stat6)–independent manner. IRF-2 appeared to act in a cell-autonomous manner to negatively regulate the signal induced by IL-3 that otherwise led to proliferation but not cytokine production. Not only infection-induced20 but also spontaneous Th2 polarization of CD4+ T cells was observed in these mice and we could correct such spontaneous polarization by reducing basophil numbers in vivo through introduction of a Kit mutation. Furthermore, IRF-2–deficient non-T spleen cells induced accelerated Th2 development of wild-type CD4+ T cells in vitro in a manner dependent on basophils. We also found that neutralization of endogenous IL-3, which was required for IL-4 production by basophils, abolished Th2 development in vitro. Our current study thus provided in vivo and in vitro evidence for the role of basophils in the initiation of Th2 responses and the regulation of the Th1/Th2 balance and demonstrated a novel mechanism for the suppression of excess Th2 polarization, mediated by IRF-2 via the negative control of basophil expansion.
Materials and methods
Mice
IRF-2-/- mice23 were kindly provided by Drs T. W. Mak (University of Toronto, Canada) and T. Taniguchi (University of Tokyo, Japan), backcrossed 10 times to C57BL/6, maintained, and used as described.18 KitW-v/+ mice on the C57BL/6 background and Stat6-/- mice24 backcrossed 10 times to C57BL/6 were purchased from SLC (Shizuoka, Japan) and kindly provided by Drs K. Takeda and S. Akira (Osaka University, Osaka, Japan), respectively. These mice were crossed to IRF-2-/- mice to generate IRF-2-/- KitW-v/W-v and IRF-2-/- Stat6-/- double-mutant mice. Double-mutant mice lacking IRF-2 and β2-microglobulin (β2-m) or the α-chain of the IFN-α/β receptor (IFNAR1) were described previously.17,18 B6-Ly5.1 mice were purchased from Sankyo (Tsukuba, Japan). OT-II T-cell receptor (TCR) transgenic (tg) mice were a kind gift from Dr W. R. Heath (WEHI, Melbourne, Australia). Control mice were the littermates heterozygous for the loci of interest that showed no measurable differences from wild-type mice. All mice were kept under specific pathogen-free conditions at the animal facility in Shinshu University and used according to the institutional guidelines for animal experimentation of Shinshu University.
Flow cytometry
All antibodies except for anti-CD4, CD8, CD11c, CD11b, NK1.1, and IgE antibodies (BD PharMingen, San Diego, CA) and fluorescein isothiocyanate (FITC)–anti-T1/ST2 antibody (MD Bioscience, Zurich, Switzerland) were purchased from e-Bioscience (San Diego, CA). Biotin-conjugated antibodies were developed using phycoerythrin (PE)–cyanin 7 (PC-7)–streptavidin (Beckman-Coulter, Hialeah, FL). Stained cells were analyzed on Cytomics FC500 flow cytometer (Beckman-Coulter), and data analysis was carried out with RXP software (Beckman-Coulter).
Cell separation
Splenic basophil enrichment was carried out through depletion of T, B, natural killer (NK), NKT, and erythroid cells by staining with biotin-conjugated antibodies against CD19, TCR-β, NK1.1, and TER119 followed by streptavidin magnetic beads on a magnetic-activated cell sorting (MACS) system (Miltenyi Biotech, Auburn, CA) or by IMag beads (BD PharMingen). For depletion of basophils, anti-IgE and anti–FcϵRI α-chain (FcϵRIα) antibodies were used together with these antibodies. In addition to the negative selection, positive selection for basophils was carried out on the AutoMACS (Miltenyi Biotec) using FITC–anti-Dx5 antibody and anti-FITC microbeads to prepare highly purified basophils (> 95% pure). CD4+ T cells were purified by depletion of CD8+, CD19+, CD11b+, TER119+, Ly-6G+, NK1.1+, CD11c+, c-kit+, sIgE+, and FcϵRIα+ cells from spleen cells as just described. Naive CD4+ T cells were purified further with PE–anti-CD62L antibody and anti-PE microbeads on the AutoMACS (Miltenyi Biotec). The purities of CD4+ or CD62L+CD4+ T cells were regularly higher than 90%. For examination of morphology, basophils were enriched as described and cells positive for FcϵRIα and negative for c-kit were further purified by sorting on the FACScalibur with the sorting option (BD Bioscience, San Jose, CA). Cytospins prepared with these sorted cells were stained with Wright stain. Images were taken with a digital camera (Spot RT color; Diagnostic Instruments, Sterling Heights, MI) mounted on an ECLIPSE E800 microscope (Nikon, Tokyo, Japan) equipped with 100 ×/1.40 oil objective lens. Image rewording was carried out using Spot version 3.4 for Windows (Diagnostic Instruments).
Cell cultures
Bone marrow (BM) cells (2 × 107) were cultured in 10 mL 10% fetal calf serum (FCS) containing RPMI 1640 medium with recombinant murine IL-3 (5 ng/mL) produced by a cell line transfected with murine IL-3 expression vector (kindly provided by Dr H. Karasuyama, Tokyo Medical and Dental University, Tokyo, Japan) for 13 days. In some experiments, BM cells derived from IRF-2-/- mice and those from B6-Ly5.1 mice were mixed 1:1 and cultured in the same manner. Freshly prepared CD4+ T cells were stimulated using plate-bound anti-CD3 antibody (10 μg/mL; e-Bioscience) in the presence of anti-CD28 antibody (1 μg/mL; BD PharMingen) for 48 hours. Basophil-containing or depleted spleen cells (1.25 × 106) or highly purified basophils (1.25 × 104) were stimulated with 0.5 mL recombinant murine IL-3 (5 ng/mL) for 24 hours for inducing IL-4 production.
Th1/Th2 differentiation in vitro
Th1/Th2 differentiation was induced in vitro using the standard 2-step culture method as described25 with slight modification. OT-II, instead of DO11.10, TCR tg CD4+ T cells were used. Briefly, CD4+ T cells (5 × 105) were cultured with T cell-depleted spleen cells (0.5-1 × 106) in the presence of 0.5 μM chicken ovalbumin (OVA) peptide (residues 323-339). In some experiments, supernatants were collected from the cultures on day 2 for measuring IL-3 and IL-4. T cells recovered on day 6 from these cultures were restimulated with plate-bound anti-CD3 antibody in the presence of anti-CD28 antibody for 5 hours for flow cytometry and for 24 hours for enzyme-linked immunosorbent assay (ELISA). Neutralizing antibodies to IL-3 (R&D Systems, Minneapolis, MN), IL-5 (BD PharMingen), and IL-4, purified from the culture supernatants of the hybridoma 11B11, were added in some cases at 1 μg/mL.
Detection of IRF-2 messages
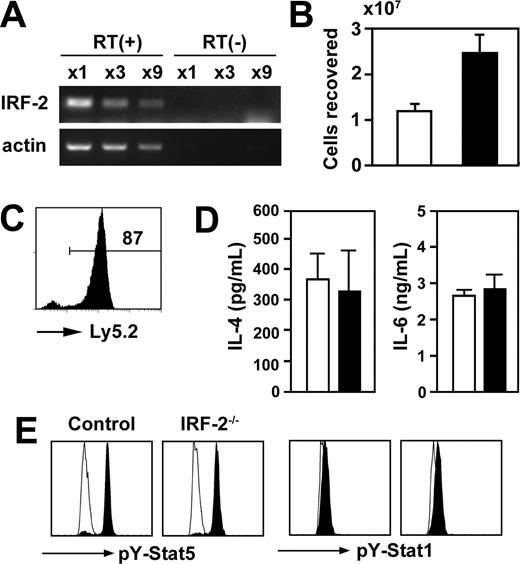
RNA was prepared from highly purified basophils (8 × 104), and reverse transcription was carried out using ImProm-II kit (Promega, Madison, WI) according to the supplier's instruction. For negative controls, reverse transcriptase was not added. One of 45-μL cDNA preparations was diluted serially and amplified using primers designed so as not to amplify the genomic Irf 2 gene: 5′-GATGGGACGTGGAAAAGGATG-3′ and 5′-TGGTCATCATCTCTCAGTGGT-3′.
Phosphorylation of Stat5 and Stat1
Basophil-enriched BM cells were stimulated with IL-3 (5 ng/mL) for 15 minutes and phosphorylated Stat5 and Stat1 were detected using antibodies purchased from BD PharMingen according to the protocol provided by the supplier with slight modification that fixed cells were treated with 90% methanol only for 20 minutes.
Detection of cytokines and serum IgE
Cytoplasmic staining with PE–anti-IL-4 and FITC–anti-IFN-γ antibodies (BD PharMingen) was carried out using the Cytoperm/Cytofix kit (BD PharMingen) according to the instructions of the supplier. The amounts of IL-4, IFN-γ, IL-3, and IL-12p40 in culture supernatants and serum IgE titers were measured using OptEIA kits (BD PharMingen).
Statistical analyses
Statistical analyses were done using the Mann-Whitney U test.
Results
Spontaneous Th2 polarization in unimmunized IRF-2-/- mice
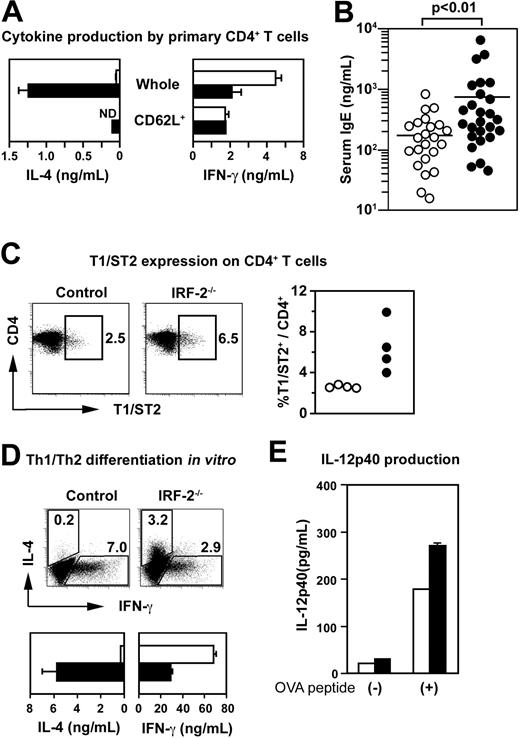
Splenic CD4+ T cells purified from naive IRF-2-/- mice23 produced substantially larger amounts of IL-4 within 2 days on CD3/CD28 stimulation than did those from control mice (Figure 1A), suggesting that CD4+ T cells in IRF-2-/- mice had spontaneously differentiated into Th2 cells in vivo. Accordingly, serum IgE levels were significantly up-regulated (Figure 1B) and CD4+ T cells expressing T1/ST2, an IL-1 receptor family molecule known to be a Th2 marker,26 were more abundant (Figure 1C) in IRF-2-/- mice compared with those in control mice. Because the amounts of IL-4 produced by naive CD62L+CD4+ T cells derived from IRF-2-/- mice were much less than those produced by unseparated CD4+ T cells (Figure 1A), it was suspected that the spontaneous Th2 polarization was due not to the IRF-2 deficiency in CD4+ T cells per se but to the abnormalities in the environment surrounding CD4+ T cells. In support of this view, T cell-depleted spleen cells prepared from IRF-2-/- mice induced Th2 polarization of wild-type OT-II TCR tg CD4+ T cells27 under the “neutral” conditions25 (Figure 1D).
Spontaneous Th2 polarization of CD4+ T cells in naive IRF-2-/- mice. (A) Cytokine production by freshly purified whole or CD62L+CD4+ T cells on CD3/CD28 stimulation. Means and SDs are shown for duplicate cultures with control (□) and IRF-2–deficient (▪) CD4+ T cell preparations, respectively. (B) Serum IgE titers of control (○) and IRF-2-/- mice (•). Each circle represents an individual mouse. (C) Expression of T1/ST2 was examined for gated CD4+ T cells. The percentages of T1/ST2+ cells within CD4+ T cells are shown in the dot plots. The percentages of T1/ST2+ cells among CD4+ T cells in individual mice were plotted for control (○) and IRF-2-/- mice (•) in the right panel. Values were significantly different between these 2 groups (P < .05). (D) In vitro differentiation of Th1 and Th2 cells from OT-II TCR tg CD4+ T cells in the presence of T cell-depleted spleen cells as examined by flow cytometry (top row) or by ELISA (bottom row). In the bottom panels, data obtained with control (□) and IRF-2–deficient (▪) cells are represented, respectively. (E) IL-12p40 production by T, B, NK, NKT and erythroid cell-depleted spleen cells from control (□) or IRF-2-/- mice (▪) in cultures with OT-II TCR tg CD4+ T cells in the absence (-) or presence (+) of OVA peptide. Data shown are means and SD of duplicate cultures. Representative of at least 3 independent experiments (A,D,E).
Spontaneous Th2 polarization of CD4+ T cells in naive IRF-2-/- mice. (A) Cytokine production by freshly purified whole or CD62L+CD4+ T cells on CD3/CD28 stimulation. Means and SDs are shown for duplicate cultures with control (□) and IRF-2–deficient (▪) CD4+ T cell preparations, respectively. (B) Serum IgE titers of control (○) and IRF-2-/- mice (•). Each circle represents an individual mouse. (C) Expression of T1/ST2 was examined for gated CD4+ T cells. The percentages of T1/ST2+ cells within CD4+ T cells are shown in the dot plots. The percentages of T1/ST2+ cells among CD4+ T cells in individual mice were plotted for control (○) and IRF-2-/- mice (•) in the right panel. Values were significantly different between these 2 groups (P < .05). (D) In vitro differentiation of Th1 and Th2 cells from OT-II TCR tg CD4+ T cells in the presence of T cell-depleted spleen cells as examined by flow cytometry (top row) or by ELISA (bottom row). In the bottom panels, data obtained with control (□) and IRF-2–deficient (▪) cells are represented, respectively. (E) IL-12p40 production by T, B, NK, NKT and erythroid cell-depleted spleen cells from control (□) or IRF-2-/- mice (▪) in cultures with OT-II TCR tg CD4+ T cells in the absence (-) or presence (+) of OVA peptide. Data shown are means and SD of duplicate cultures. Representative of at least 3 independent experiments (A,D,E).
Importantly, IL-12p40 production by T-, B-, NK-, and NKT-depleted spleen cells was not impaired in the cultures with wild-type OT-II TCR tg T cells and the specific OVA peptides (Figure 1E). Although we and others have reported the developmental defect in CD4+ subset of DCs in IRF-2-/-mice,18,19 this observation would not be surprising provided that CD8+ DCs were shown to be the major IL-12–producing DC subset.28 Thus, spleen cells other than DCs or T cells seemed to be responsible for the spontaneous Th2 polarization in IRF-2-/- mice.
Stat6 and IFN-α/β signal-independent expansion of basophils in the periphery in IRF-2-/- mice due to hyperproliferative responses to IL-3
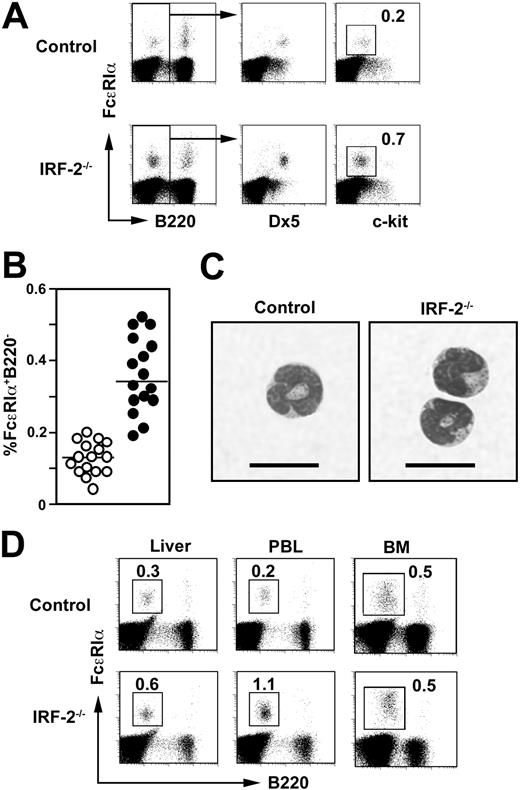
We noticed that basophils defined as c-kit-B220-Dx5+ cells with cell surface-associated IgE and bearing FcϵRIα10,11 were expanded 2- to 3-fold in the spleen in IRF-2-/- mice compared with those in control mice (Figure 2A-B), whereas mast cells (FcϵRIα+c-kit+) were virtually absent in the spleen both in control and IRF-2-/- mice (Figure 2A). Splenic basophils purified either from control or IRF-2-/- mice showed identical morphology with multilobular nuclei and a few cytoplasmic granules (Figure 2C) indistinguishable from that reported previously.10,11 Similar expansion of basophils was observed also in the peripheral blood and to a lesser extent in the liver in IRF-2-/- mice (Figure 2D). Notably, the numbers of basophils in the BM were not altered in IRF-2-/- mice compared with those in control mice (Figure 2D).
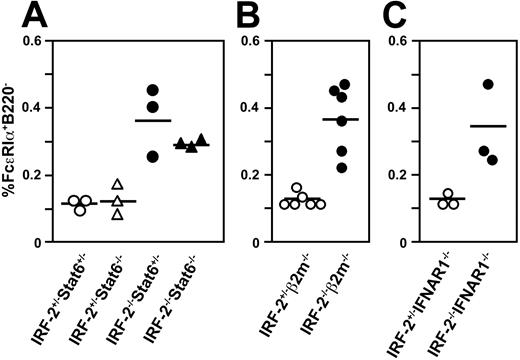
In IRF-2-/-Stat6-/- double-mutant mice, the basophil population was as large as that in IRF-2-/- mice (Figure 3A). In addition, we observed similar expansion of basophils in IRF-2-/- mice lacking β2-m or IFNAR1 to that in IRF-2-/- mice (Figure 3B-C). As shown previously by us, IRF-2-/- mice suffered from an inflammatory skin disease mediated by CD8+ T cells due to the uncontrolled IFN-α/β signals.17 These results indicated that the expansion of basophils in IRF-2-/- was a consequence of neither the spontaneous Th2 polarization nor the skin inflammation. Furthermore, it was clear that IFNAR1-dependent impaired development of the CD4+ DC subset18,19 did not contribute to the basophil expansion in IRF-2-/- mice.
Expansion of basophils in IRF-2-/- mice. (A) Flow cytometry for splenic basophils (FcϵRIα+Dx5+c-kit-B220-). Numbers represent the percentages of c-kit- cells within B220- cells. (B) Percentages of FcϵRIα+B220- cells in the spleen derived from control (○) and IRF-2-/- (•) mice, representing 16 litters. Values are significantly different between these 2 groups of mice (P < .001). (C) Morphology of freshly isolated basophils. Bars = 10 μm. (D) Mononuclear cells derived from the liver, peripheral blood (PBL), or BM were stained as in panel A. Numbers represent the percentages of FcϵRIα+B220- cells.
Expansion of basophils in IRF-2-/- mice. (A) Flow cytometry for splenic basophils (FcϵRIα+Dx5+c-kit-B220-). Numbers represent the percentages of c-kit- cells within B220- cells. (B) Percentages of FcϵRIα+B220- cells in the spleen derived from control (○) and IRF-2-/- (•) mice, representing 16 litters. Values are significantly different between these 2 groups of mice (P < .001). (C) Morphology of freshly isolated basophils. Bars = 10 μm. (D) Mononuclear cells derived from the liver, peripheral blood (PBL), or BM were stained as in panel A. Numbers represent the percentages of FcϵRIα+B220- cells.
Basophil expansion in IRF-2-/- mice was independent of Stat6, CD8+ T cell, or IFN-α/β signals. The percentages of FcϵRIα+B220- cells in the spleen in mice with the indicated genotypes are plotted. Each symbol represents the value of an individual animal and the horizontal bars, the means. In panel A, the values for IRF-2-/-Stat6-/- mice are significantly higher than those for IRF-2+/-Stat6+/- (P < .05) or for IRF-2+/-Stat6-/- (P < .01) mice but not significantly different compared to those for IRF-2-/-Stat6+/- mice (P > .5). In panels B and C, values for IRF-2-/- and IRF-2+/- mice are also significantly different (P < .005 and P < .05, respectively).
Basophil expansion in IRF-2-/- mice was independent of Stat6, CD8+ T cell, or IFN-α/β signals. The percentages of FcϵRIα+B220- cells in the spleen in mice with the indicated genotypes are plotted. Each symbol represents the value of an individual animal and the horizontal bars, the means. In panel A, the values for IRF-2-/-Stat6-/- mice are significantly higher than those for IRF-2+/-Stat6+/- (P < .05) or for IRF-2+/-Stat6-/- (P < .01) mice but not significantly different compared to those for IRF-2-/-Stat6+/- mice (P > .5). In panels B and C, values for IRF-2-/- and IRF-2+/- mice are also significantly different (P < .005 and P < .05, respectively).
Expansion of basophils resulted in the augmented IL-4 production by IRF-2–deficient spleen cells in vitro
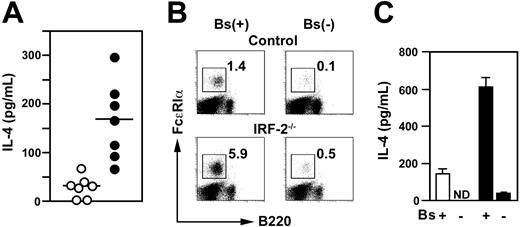
Spleen cells prepared from IRF-2-/- mice produced substantially larger amounts of IL-4 in response to IL-3 than did control spleen cells (Figure 4A). Moreover, enrichment and removal of basophils (Figure 4B) augmented and abrogated IL-3–induced IL-4 production, respectively (Figure 4C). These observations indicated that the vast majority of IL-4 produced by spleen cells derived from IRF-2-/- as well as control mice was attributable to basophils, consistent with previous reports.5,8,9 Thus, IRF-2 deficiency caused the accelerated expansion of basophils that led to the elevation of IL-4 production.
IRF-2 regulated proliferation, but not cytokine production, on IL-3 stimulation negatively in basophils
As depicted in Figure 5A, basophils freshly prepared from the BM indeed expressed IRF-2 messages constitutively. Importantly, basophils derived from IRF-2-/- mice proliferated in vitro more efficiently in response to IL-3 than did those from control mice (Figure 5B). This accelerated proliferation was not due to the abnormalities in other BM cells than basophils because IRF-2–deficient BM basophils outgrew control basophils even in cultures established with control and IRF-2–deficient BM cells mixed (Figure 5C). In contrast, purified basophils from control and IRF-2-/- mice produced similar amounts of IL-4 and IL-6 in response to IL-3 (Figure 5D). Furthermore, Stat5, but not Stat1, was phosphorylated to a similar extent on stimulation with IL-3 in control and IRF-2–deficient basophils (Figure 5E), indicating that IL-3 receptor expression and IL-3–induced immediate early signaling events were not affected by IRF-2 deficiency.
Basophils were responsible for the augmented IL-4 production by IRF-2–deficient spleen cells. (A) IL-3–induced IL-4 production by whole spleen cells isolated from control (○) or IRF-2-/- mice (•). Each symbol represents an individual mouse. Horizontal bars are the means. Amounts of IL-4 produced are significantly higher for IRF-2-/- mice than for controls (P < .005). (B) Flow cytometry for basophil-enriched (Bs(+)) or depleted (Bs(-)) spleen cells. Numbers represent the percentages of basophils. (C) IL-3–induced IL-4 production by the cell preparations shown in panel B. ND indicates not detectable (< 20 pg/mL). Repeated 3 times with similar results. Means and SD of duplicate cultures established with cells from control (□) or IRF-2-/- mice (▪) are shown.
Basophils were responsible for the augmented IL-4 production by IRF-2–deficient spleen cells. (A) IL-3–induced IL-4 production by whole spleen cells isolated from control (○) or IRF-2-/- mice (•). Each symbol represents an individual mouse. Horizontal bars are the means. Amounts of IL-4 produced are significantly higher for IRF-2-/- mice than for controls (P < .005). (B) Flow cytometry for basophil-enriched (Bs(+)) or depleted (Bs(-)) spleen cells. Numbers represent the percentages of basophils. (C) IL-3–induced IL-4 production by the cell preparations shown in panel B. ND indicates not detectable (< 20 pg/mL). Repeated 3 times with similar results. Means and SD of duplicate cultures established with cells from control (□) or IRF-2-/- mice (▪) are shown.
IRF-2 acted as a negative regulator of IL-3–induced proliferation on basophils. (A) IRF-2 messages in highly purified (> 95% pure) basophils from wild-type mice were examined by reverse transcription-polymerase chain reactions on serially diluted RNA preparations. Reverse transcriptase was not added in control amplifications (RT(-)). (B) BM cells from control (□) and IRF-2-/- (▪) mice were cultured in vitro in the presence of IL-3. The numbers of c-kit-FcϵRIα+ cells recovered on day 13 were enumerated. Data represent the means and SD of duplicated cultures. (C) BM cells prepared from B6-Ly5.1 and IRF-2-/- (Ly5.2) mice were mixed 1:1 and cultured as in panel B. On day 13, Ly5.2 expression was examined in c-kit-FcϵRI+ cells recovered from the cultures. Representative of 3 independent cultures. (D) Highly purified basophils (> 95%) prepared from control (□) or IRF-2-/- mice (▪) were stimulated with IL-3 for 24 hours, and the production of IL-4 and IL-6 was examined. Cumulative data were obtained from 2 independent trials, showing the means of 4 independent cultures and SD. (E) Basophils enriched from the BM were stimulated with IL-3 and basophils (Dx5+NK1.1-) were stained for phospho-Stat5 and phospho-Stat1. Open and filled histograms represent unstimulated and stimulated basophils, respectively.
IRF-2 acted as a negative regulator of IL-3–induced proliferation on basophils. (A) IRF-2 messages in highly purified (> 95% pure) basophils from wild-type mice were examined by reverse transcription-polymerase chain reactions on serially diluted RNA preparations. Reverse transcriptase was not added in control amplifications (RT(-)). (B) BM cells from control (□) and IRF-2-/- (▪) mice were cultured in vitro in the presence of IL-3. The numbers of c-kit-FcϵRIα+ cells recovered on day 13 were enumerated. Data represent the means and SD of duplicated cultures. (C) BM cells prepared from B6-Ly5.1 and IRF-2-/- (Ly5.2) mice were mixed 1:1 and cultured as in panel B. On day 13, Ly5.2 expression was examined in c-kit-FcϵRI+ cells recovered from the cultures. Representative of 3 independent cultures. (D) Highly purified basophils (> 95%) prepared from control (□) or IRF-2-/- mice (▪) were stimulated with IL-3 for 24 hours, and the production of IL-4 and IL-6 was examined. Cumulative data were obtained from 2 independent trials, showing the means of 4 independent cultures and SD. (E) Basophils enriched from the BM were stimulated with IL-3 and basophils (Dx5+NK1.1-) were stained for phospho-Stat5 and phospho-Stat1. Open and filled histograms represent unstimulated and stimulated basophils, respectively.
Basophil expansion was responsible for the spontaneous Th2 polarization in vivo in IRF-2-/- mice
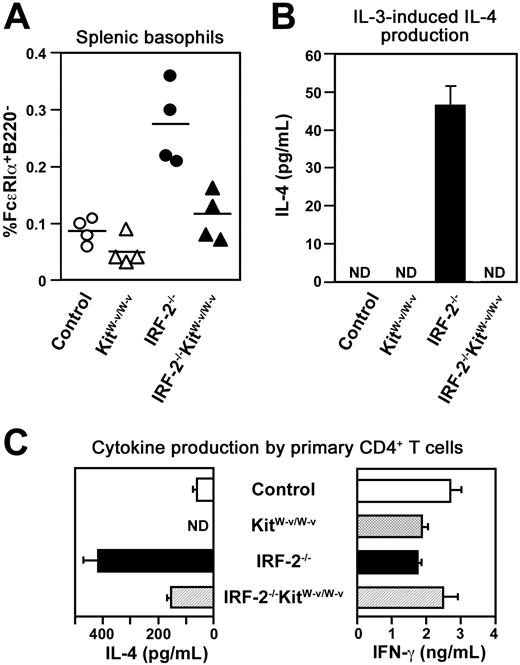
Was the basophil expansion responsible for the spontaneous Th2 polarization in vivo in IRF-2-/- mice? To down-modulate basophil numbers in vivo, we introduced a hypomorphic mutation in the Kit gene (KitW-v) that was previously shown to reduce the numbers of basophils as well as mast cells29 into IRF-2-/- mice. Because the percentages of basophils in the control spleen were much lower compared with those in IRF-2–deficient spleen, the effect of the Kit mutation on the numbers of basophils in control animals was not very apparent (compare control and KitW-v/W-v in Figure 6A). In contrast, we observed clear restoration in splenic basophil numbers in IRF-2-/-KitW-v/W-v mutant mice compared with IRF-2-/-KitW-v/+ mice (Figure 6A). Accordingly, IL-3–induced IL-4 production by spleen cells from these double-mutant mice was practically undetectable as in the cases of spleen cells from control mice (Figure 6B). Enhanced IL-4 production on CD3/CD28 stimulation by CD4+ T cells freshly purified from these double-mutant mice was no longer as prominent as that from IRF-2-/- mice (Figure 6C), suggesting that the spontaneous Th2 polarization in vivo occurred to a lesser extent in these double-mutant mice than in IRF-2-/- mice. In agreement with this conclusion, serum IgE levels were reduced significantly (P < .05) in IRF-2-/-KitW-v/W-v double-mutant mice (231 ± 129 ng/mL, n = 3) compared with those in IRF-2-/-KitW-v/+ mice (574 ± 97 ng/mL, n = 3). Thus, it seemed likely that the elevation of basophil numbers was the major, if not the sole, mechanism for the spontaneous Th2 polarization in vivo in IRF-2-/- mice.
Essential roles for basophils and IL-3 in Th2 differentiation in vitro under neutral conditions
To test directly if the enlarged basophil population was able to accelerate Th2 development, we next stimulated purified OT-II TCR tg CD4+ T cells with the specific OVA peptide in the presence of basophil-enriched or depleted spleen cells prepared as in Figure 4B as antigen-presenting cells (APCs). Notably, enrichment of basophils even in control APCs augmented the generation of Th2 cells from wild-type CD4+ T cells (compare open columns in Figure 7B with those in the lower panels of Figure 1D). On the contrary, depletion of basophils or neutralization of endogenously produced IL-4 greatly reduced the frequencies of Th2 cells as well as the amounts of T cell-derived IL-4 (Figure 7A-B). Th2 development was further accelerated, yet similarly dependent on both endogenous IL-4 and basophils, when basophil-enriched APCs prepared from IRF-2-/- mice were used (Figure 7B). Thus, the enlargement of the basophil population, but not the abnormalities in other cell types, including DCs, seemed to be responsible for the augmented Th2-inducing ability of APCs prepared from IRF-2-/- mice (Figure 1D).
IL-3 was indeed produced in these cultures, irrespective of the presence of basophils (Figure 7C), presumably by antigen-activated CD4+ T cells because IL-3 production was not observed when no antigen was added (S.H., unpublished data, September 2004). On the other hand, early IL-4 production (within the first 2 days) in these cultures was attributable to basophils because depletion of basophils reduced the levels of IL-4 produced (Figure 7D). Importantly, neutralization of IL-3 not only abrogated such an early IL-4 production (Figure 7D) but also strongly suppressed subsequent Th2 development induced with IRF-2–deficient APCs (Figure 7E-F). These results indicated that antigen-activated CD4+ T cells produced IL-3 and thereby induced bystander basophils to produce IL-4 that, in turn, drove the Th2 polarization of CD4+ T cells.
Genetic reduction of basophil numbers in vivo restored Th2 polarization in IRF-2-/- mice. (A) The percentages of FcϵRIα+B220- cells in the spleen in mice with the indicated genotypes. Control, KitW-v/W-v, and IRF-2-/- denote IRF-2+/-KitW-v/+, IRF-2+/-KitW-v/W-v, and IRF-2-/-KitW-v/+ mice, respectively. Each symbol represents the value of an individual animal. The values for IRF-2-/-KitW-v/W-v mice are significantly lower than those for IRF-2-/- mice (P < .01) and not significantly different from those for KitW-v/W-v mice (P > .05). (B) IL-3–induced IL-4 production by unseparated spleen cells prepared from mice of the indicated genotypes. Values are means and SD of duplicate cultures. ND indicates not detectable (< 10 pg/mL). (C) Cytokine production by fresh splenic CD4+ T cells on CD3/CD28 stimulation. Values are means and SD of duplicate cultures. ND indicates not detectable (< 20 pg/mL). Representative of experiments with 4 litters.
Genetic reduction of basophil numbers in vivo restored Th2 polarization in IRF-2-/- mice. (A) The percentages of FcϵRIα+B220- cells in the spleen in mice with the indicated genotypes. Control, KitW-v/W-v, and IRF-2-/- denote IRF-2+/-KitW-v/+, IRF-2+/-KitW-v/W-v, and IRF-2-/-KitW-v/+ mice, respectively. Each symbol represents the value of an individual animal. The values for IRF-2-/-KitW-v/W-v mice are significantly lower than those for IRF-2-/- mice (P < .01) and not significantly different from those for KitW-v/W-v mice (P > .05). (B) IL-3–induced IL-4 production by unseparated spleen cells prepared from mice of the indicated genotypes. Values are means and SD of duplicate cultures. ND indicates not detectable (< 10 pg/mL). (C) Cytokine production by fresh splenic CD4+ T cells on CD3/CD28 stimulation. Values are means and SD of duplicate cultures. ND indicates not detectable (< 20 pg/mL). Representative of experiments with 4 litters.
Essential roles for IL-3 in IL-4 production by basophils and in Th2 differentiation. (A-B) Cytokine production by CD4+ T cells differentiated in vitro in the presence of basophil-enriched (Bs[+]) or depleted (Bs[-]) spleen cells. Anti–IL-4 antibody was added in the indicated cultures. (C) IL-3 production on day 2 in the cultures established as in panel A with basophils from wild-type mice. (D) Indicated antibodies were added in the cultures established as in panel C, and IL-4 production on day 2 was examined. ND indicates not detectable (< 10 pg/mL). (E-F) Generation of Th1 and Th2 cells from wild-type CD4+ T cells induced by T cell-depleted spleen cells in the absence (-) or presence of the indicated antibodies. Examination of cytokine production was done by cytoplasmic staining (A,E) or by ELISA (B-D,F). In panels B and F, means and SDs of duplicate cultures with cells from control (□) and IRF-2-/- (▪) mice, respectively, are shown. Representative of 2 (A-B) or 3 (C-F) independent experiments.
Essential roles for IL-3 in IL-4 production by basophils and in Th2 differentiation. (A-B) Cytokine production by CD4+ T cells differentiated in vitro in the presence of basophil-enriched (Bs[+]) or depleted (Bs[-]) spleen cells. Anti–IL-4 antibody was added in the indicated cultures. (C) IL-3 production on day 2 in the cultures established as in panel A with basophils from wild-type mice. (D) Indicated antibodies were added in the cultures established as in panel C, and IL-4 production on day 2 was examined. ND indicates not detectable (< 10 pg/mL). (E-F) Generation of Th1 and Th2 cells from wild-type CD4+ T cells induced by T cell-depleted spleen cells in the absence (-) or presence of the indicated antibodies. Examination of cytokine production was done by cytoplasmic staining (A,E) or by ELISA (B-D,F). In panels B and F, means and SDs of duplicate cultures with cells from control (□) and IRF-2-/- (▪) mice, respectively, are shown. Representative of 2 (A-B) or 3 (C-F) independent experiments.
Discussion
IRF-2-/- mice were previously reported to mount Th2-biased immune responses on infection with L major.20 Even in uninfected, naive IRF-2-/- mice, we found in this study that splenic CD4+ T cells exhibited spontaneous Th2 polarization as indicated by augmented IL-4 production by freshly isolated CD4+ T cells, elevated serum IgE titers, and increased frequencies of CD4+ T cells bearing T1/ST2, a marker for Th2 cells (Figure 1A-C). Such a Th2 polarization seemed to be due, at least in part, to the abnormalities in non-T spleen cells (Figure 1D), suggesting that IRF-2 regulated Th1/Th2 balance of CD4+ T cells indirectly even in naive animals. It was unlikely, however, that the impaired IL-12 production by macrophages20,21 played roles in the accelerated Th2-inducing nature of IRF-2–deficient non-T spleen cells because IRF-2–deficient DCs produced as much IL-12p40 as did control DCs (Figure 1E). These observations led us to seek abnormalities in other cell types in the spleen. We then found that basophil numbers in the spleen and peripheral blood were elevated in IRF-2-/- mice in a manner independent of Stat6, hence, Th2 polarization itself or increased serum IgE concentrations (Figure 3A). Moreover, because IRF-2-/- mice lacking β2-m and IFNAR1 exhibited a similar elevation of basophil numbers (Figure 3B-C), the CD8+ T cell- and IFN-α/β–dependent skin inflammation developing spontaneously in these mice17 played no role in the phenotype. We envisage that the physiologic role of IRF-2, which was expressed constitutively in freshly isolated basophils (Figure 5A), is to limit the size of the basophil population in the peripheral blood and spleen by suppressing IL-3–induced expansion in a cell-autonomous manner (Figure 5B-C).
Curiously, basophil expansion in IRF-2-/- mice was observed in the spleen, peripheral blood, and, to a lesser extent, liver but not at all in the BM (Figure 2), suggesting that IRF-2 limited only the IL-3–dependent moiety of basophil expansion in the periphery but not the development of these cells in the BM. The observation that the numbers of basophils in the BM were controlled by an IL-3–independent mechanism in uninfected mice29 supported the notion. Interestingly, as shown in Figure 5D, IL-4 production by IRF-2–deficient basophils on a per cell basis was not altered, indicating that IRF-2 was not a general but a selective negative regulator of IL-3 response of basophils. Together with the unaltered Stat5 phosphorylation by IL-3 in IRF-2–deficient basophils (Figure 5E), these results suggested that IL-3 signals diverged into at least 2 distinct pathways leading either to proliferation or cytokine production in basophils and only the former was influenced by the presence of IRF-2. In this regard, understanding the molecular mechanism for the negative regulation of IL-3–mediated basophil expansion by IRF-2 may shed new light on the studies of IL-3–mediated signaling pathways.
With respect to the localization of basophils, our current observations contrasted to previous reports that basophils were recruited massively to the liver and BM on infection with Nippostrongylus brasiliensis and Strongyloides venezuelensis, respectively.10,11 Importantly, however, IL-3 was shown to contribute minimally to this process,11 indicating that factors other than IL-3 would be required for the migration of basophils to the liver and BM on nematode infection. These nematodes might possibly contain stimulants that induced basophil activation and mobilization in an IL-3–independent manner. The localization of basophils in vivo seemed, therefore, to be regulated in uninfected animals in a different way than that during infection.
Basophils were known to produce IL-4 in vitro on stimulation with various stimuli including IL-3 and FcϵRI cross-linking.5-9 Our genetic approaches to reduce basophil numbers by introducing a Kit mutation showed that the spontaneous Th2 polarization in vivo in IRF-2-/- mice was indeed greatly reduced concomitantly with the reduction of basophil numbers (Figure 6), suggesting strongly that IRF-2 was involved in the homeostatic regulation of Th1/Th2 balance in uninfected animals by keeping basophil expansion under control. Our study is the first to show that basophils can affect the homeostatic regulation of Th1/Th2 balance in uninfected mice. Furthermore, we examined directly the roles of basophils in Th2 development using a standard peptide-induced Th cell differentiation system in vitro with TCR tg CD4+ T cells.25 In agreement with the in vivo and ex vivo observations (Figure 1), the increase in basophil numbers was responsible for the augmented Th2 development induced by IRF-2–deficient splenic APCs in vitro under neutral conditions (Figure 7A-B). Furthermore, depletion of basophils from the APC populations diminished Th2 development observed in cultures even with splenic APCs prepared from control mice (Figure 7A-B). This observation was consistent with the disappearance of the weak spontaneous Th2 polarization in vivo even in control mice on introduction of the Kit mutation (Figure 6C, control and KitW-v/W-v mice). These results indicated that the Th2-inducing function of basophils was not acquired on IRF-2 deficiency but instead intrinsic to basophils and further supported the notion that IRF-2 deficiency altered only the population size of basophils without much affecting their properties (Figure 5D).
Another important observation was that neutralization of endogenous IL-3 during in vitro induction of Th1/Th2 differentiation resulted in the reduction of Th2 cell development (Figure 7E-F), likely by inhibiting IL-4 production by basophils (Figure 7D). This observation indicated that basophils themselves were incapable of promoting Th2 differentiation unless IL-3 was present. On the other hand, IL-3 was produced indeed during antigen-induced T-cell responses regardless of the presence of basophils (Figure 7C) but did not induce Th2 polarization if basophils were depleted (Figure 7A-B). Collectively, basophils and IL-3 were each able neither to directly induce Th2 cells nor to create Th2-inducing environment unless both of them were present concomitantly. Thus, basophils acted in these settings as a cellular converter that turned the neutral signals of IL-3 into Th2-inducing ones, namely, IL-4, and the production of IL-3 would have different immunologic outcomes depending on how many basophils were present nearby. Fine control of the numbers of basophils at the sites where Th1/Th2 differentiation was initiated would hence be critically important as to what extent Th2 cells were generated.
IL-3 production in vitro required antigen stimulation (Figure 7C and S.H., unpublished observation, September 2004). These observations seemed at first glance to mean that IL-3 was not produced in vivo in the absence of infection or immunization. However, considerable numbers of CD4+ T cells appeared to be activated spontaneously even in the absence of overt infections and extensive immunization presumably through interaction with enteric or other cross-reactive antigens as suggested by the presence of memory/activated phenotype CD4+ T cells. Therefore, IL-3 could be produced constantly in vivo, perhaps at low levels, even in naive mice, expand basophils, if not appropriately controlled, and stimulate their IL-4 production leading to spontaneous Th2 polarization of CD4+ T cells as seen here in IRF-2-/- mice. In this regard, it would be important to note that basophils spontaneously transcribe the green fluorescent protein (GFP) gene located in the IL4 locus in uninfected animals.11 Although the transcription of the IL4 gene did not necessarily result in the production of IL-4 proteins as shown very recently,30 such a basal transcription of the IL4 loci might be induced by IL-3 that was continuously produced in vivo.
It seems rather astonishing that the roles of IL-3 and basophils demonstrated here have not been noticed despite the standard in vitro Th1/2 differentiation system used here having been widely applied for more than a decade to examine the cellular and molecular components involved in Th1/Th2 differentiation.25,31-33 We envisage that this might be because the influence of basophils on Th2 development under neutral conditions was difficult to observe unless basophils were enriched or IRF-2–deficient APCs were used (Figures 1D and 7B,F). Studies of immunologic functions of basophils in vivo have been hampered by multiple difficulties, such as the paucity of these cells and the absence of appropriate animal models exhibiting basophil abnormalities.13 In this regard, IRF-2-/- mice exhibiting abnormal expansion of basophils that were apparently normal, at least in terms of their cytokine production, would be a valuable tool for analyzing basophil functions. It has been shown that once initiated, Th2 polarization was self-promoting and dominant over Th1 responses,34 and erroneous Th2 polarization would lead to inefficient host defense against pathogens, such as L major, to which type 1 immunity plays crucial roles.35,36 We hence infer that negative regulation of basophil numbers by IRF-2 would be critical for host defense against infection because it keeps the Th1/Th2 balance “neutral” in uninfected individuals so as to allow Th1 responses taking place efficiently. It would also be interesting to examine if basophils play roles in the initiation phase of allergic inflammation that have not been studied extensively in contrast to the effector phase.
Prepublished online as Blood First Edition Paper, May 24, 2005; DOI 10.1182/blood-2005-04-1344.
Supported by a Grant-in-Aid for Scientific Research on Priority Area (16043222) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grants-in-Aid (15790198) from the Japan Science Promotion Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Drs Tak W. Mak and Tadatsugu Taniguchi for IRF-2-/- mice, Kiyoshi Takeda and Shizuo Akira for Stat6-/- mice, William R. Hearth for OT-II TCR tg mice, Hajime Karasuyama for the IL-3–producing cell line, and Shin-Ichi Hayashi for the information on KitW-v/W-v mutants. Ms Namiko Azuta's technical assistance is also acknowledged.

![Figure 7. Essential roles for IL-3 in IL-4 production by basophils and in Th2 differentiation. (A-B) Cytokine production by CD4+ T cells differentiated in vitro in the presence of basophil-enriched (Bs[+]) or depleted (Bs[-]) spleen cells. Anti–IL-4 antibody was added in the indicated cultures. (C) IL-3 production on day 2 in the cultures established as in panel A with basophils from wild-type mice. (D) Indicated antibodies were added in the cultures established as in panel C, and IL-4 production on day 2 was examined. ND indicates not detectable (< 10 pg/mL). (E-F) Generation of Th1 and Th2 cells from wild-type CD4+ T cells induced by T cell-depleted spleen cells in the absence (-) or presence of the indicated antibodies. Examination of cytokine production was done by cytoplasmic staining (A,E) or by ELISA (B-D,F). In panels B and F, means and SDs of duplicate cultures with cells from control (□) and IRF-2-/- (▪) mice, respectively, are shown. Representative of 2 (A-B) or 3 (C-F) independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-04-1344/6/m_zh80180583980007.jpeg?Expires=1769936679&Signature=mROcXlDu1~vxh5IPS5YOG8o8P~oweo7i~ThZBajS2sA8IbQDRBSHKSBU4Sa-~lx7rm9-8wP4YzuBeEXvLkBLuX~UMj05wfipCemhSIyoEMTCOpYh-nD9j90O4cvVJUhkQR730Y6zzmeT1c5EWgUswJPscqP~y343uDzEMqGh7vnjLQTWF0hqd2xyNBMaoelP6G7emTKZAsbuEC7v4H~HU4TjLqVamnYYqtXh525dgpkbXSPksInN6MIZINGMMBg~CUfEZwY2HZtgEQbSIoQSJ4fGMw2QL985T7~2UhxkjSPf1xj~-AeXy6yb4N0eteilk8AscagPb6ld3rTlLcebmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal